Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (8): 1589-1598.doi: 10.12122/j.issn.1673-4254.2025.08.03
Qingge WANG1( ), Xiaohui ZHAO2, Yuxuan HE2, Feixiang LIU3, Yunke ZHANG3(
), Xiaohui ZHAO2, Yuxuan HE2, Feixiang LIU3, Yunke ZHANG3( )
)
Received:2025-03-01
Online:2025-08-20
Published:2025-09-05
Contact:
Yunke ZHANG
E-mail:826955610@qq.com;henanzyk@126.com
Supported by:Qingge WANG, Xiaohui ZHAO, Yuxuan HE, Feixiang LIU, Yunke ZHANG. Qixiong Zuogui Granules enhance synaptic plasticity in aging rats by regulating the BDNF/TrkB signaling pathway[J]. Journal of Southern Medical University, 2025, 45(8): 1589-1598.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.08.03
| Group | SOD (U/mL) | MDA (nmol/mL) | GSH-Px (U/mL) |
|---|---|---|---|
| Control | 142.91±9.22 | 3.88±0.32 | 1317.86±33.34 |
| Model | 115.30±6.801) | 5.63±0.561) | 1038.23±69.171) |
| Don | 131.94±6.502) | 4.48±0.613) | 1212.83±56.603) |
| QXZG | 134.53±12.542) | 4.40±0.523) | 1244.88±83.564) |
Tab.1 Comparison of serum oxidative stress indexes of the rats among the 4 groups
| Group | SOD (U/mL) | MDA (nmol/mL) | GSH-Px (U/mL) |
|---|---|---|---|
| Control | 142.91±9.22 | 3.88±0.32 | 1317.86±33.34 |
| Model | 115.30±6.801) | 5.63±0.561) | 1038.23±69.171) |
| Don | 131.94±6.502) | 4.48±0.613) | 1212.83±56.603) |
| QXZG | 134.53±12.542) | 4.40±0.523) | 1244.88±83.564) |

Fig.7 Comparison of SYN and GAP43 proteins in the hippocampus of the rats in the 4 groups. A: Expression of SYN proteins in the hippocampal CA1 region (×200). B: Expression of GAP43 proteins in the hippocampal CA1 region (×200). C, D: Mean fluorescence intensity of SYN and GAP43 proteins. ***P<0.001 vs Control group; ##P<0.01, ###P<0.001 vs Model group.
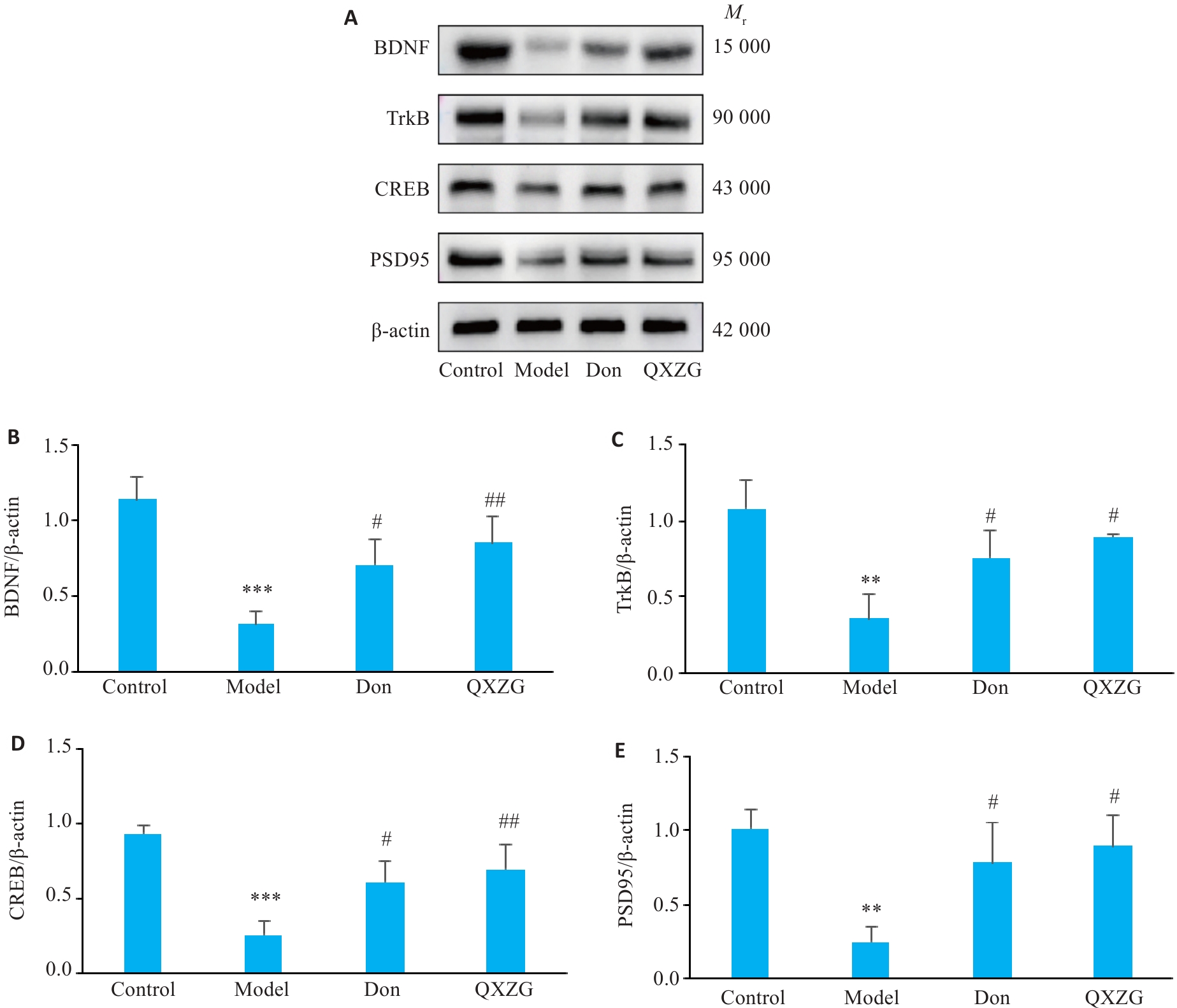
Fig.8 Comparison of BDNF/TrkB pathway proteins and PSD95 proteins in the hippocampus of the rats among the 4 groups. A: Protein bands in Western blotting. B-E: Relative expression levels of BDNF, TrkB, CREB, and PSD95 proteins. **P<0.01, ***P<0.001 vs Control group; #P<0.05, ##P<0.01 vs Model group.
| [1] | Guerville F, De Souto Barreto P, Ader I, et al. Revisiting the hallmarks of aging to identify markers of biological age[J]. J Prev Alzheimers Dis, 2020, 7(1): 56-64. doi:10.14283/jpad.2019.50 |
| [2] | Case SL, Frazier HN, Anderson KL, et al. Falling short: the contribution of central insulin receptors to gait dysregulation in brain aging[J]. Biomedicines, 2022, 10(8): 1923. doi:10.3390/biomedicines10081923 |
| [3] | Hajam YA, Rani R, Ganie SY, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives[J]. Cells, 2022, 11(3): 552. doi:10.3390/cells11030552 |
| [4] | You H, Lu B. Diverse functions of multiple Bdnf transcripts driven by distinct Bdnf promoters[J]. Biomolecules, 2023, 13(4): 655. doi:10.3390/biom13040655 |
| [5] | Numakawa T, Odaka H. The role of neurotrophin signaling in age-related cognitive decline and cognitive diseases[J]. Int J Mol Sci, 2022, 23(14): 7726. doi:10.3390/ijms23147726 |
| [6] | Magee JC, Grienberger C. Synaptic plasticity forms and functions[J]. Annu Rev Neurosci, 2020, 43: 95-117. doi:10.1146/annurev-neuro-090919-022842 |
| [7] | Fernández de Sevilla D, Nuñez A, Araque A, et al. Metabotropic regulation of synaptic plasticity[J]. Neuroscience, 2021, 456: 1-3. doi:10.1016/j.neuroscience.2020.10.006 |
| [8] | 张欣蕾, 王家传, 赵 红. 中医药抗衰老的研究进展[J]. 深圳中西医结合杂志, 2023, 33(2): 129-33. |
| [9] | 薄文集, 石和元, 王 平. 《本草纲目》对衰老的认识及其防治老年病的思想特点[J]. 湖北中医药大学学报, 2018, 20(2): 47-50. doi:10.3969/j.issn.1008-987x.2018.02.11 |
| [10] | 魏智慧. 芪芎左归饮治疗老年颈动脉粥样硬化患者(肾虚血瘀型)的临床观察及对血清P53、P21的影响[D]. 郑州: 河南中医药大学, 2023. |
| [11] | 孙世标. 芪芎左归饮治疗脑动脉粥样硬化的临床疗效观察及其对NF-κB相关级联因子的影响[D]. 郑州: 河南中医药大学, 2023. |
| [12] | 李少康. 衰老后脑卒中血瘀证与神经血管单元SIRT1/NF-κB通路相关性研究[D]. 郑州: 河南中医药大学, 2023. |
| [13] | 要莹莹, 樊飞燕, 李少康, 等. 芪芎左归复方干预骨髓间充质干细胞外泌体对缺血性脑卒中大鼠血脑屏障通透性的影响及抗衰老作用[J]. 中国中药杂志, 2024, 49(18): 5016-24. doi:10.19540/j.cnki.cjcmm.20240524.501 |
| [14] | 魏 伟, 吴希美, 李元建. 药理实验方法学[M]. 4版. 北京: 人民卫生出版社, 2010. |
| [15] | 韩 诚, 张俊龙. 从“肾脑相关” 论衰老学习记忆功能障碍[J]. 中华中医药杂志, 2020, 35(10): 5112-6. |
| [16] | Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice[J]. Nat Med, 2014, 20(6): 659-63. doi:10.1038/nm.3569 |
| [17] | 江晶晶, 江励华, 黄 臻, 等. 补肾活血法在延缓大脑衰老过程中的应用[J]. 长春中医药大学学报, 2014, 30(6): 1064-7. doi:10.13463/j.cnki.cczyy.2014.06.039 |
| [18] | 陈珞珈. 黄帝内经[M]. 长春: 吉林大学出版社, 2009. |
| [19] | 尹佳婷. 黄芪对自然衰老小鼠肠道功能及菌群稳态的调控作用研究[D]. 南京: 南京中医药大学, 2022. |
| [20] | 董 昱, 葛 伟. 中药菟丝子抗衰老作用研究进展[J]. 实用老年医学, 2024, 38(3): 219-22. doi:10.3969/j.issn.1003-9198.2024.03.002 |
| [21] | 张继雅. 山桃仁水提物延缓线虫衰老及其机制研究[D]. 太原: 山西大学, 2023. |
| [22] | 杨芊芊. 牛膝拮抗衰老小鼠及衰老神经干细胞的药效物质基础研究[D]. 北京: 北京中医药大学, 2020. |
| [23] | 袁荣献, 代 欣, 封 帆, 等. 新工艺炮制的熟地黄对D-半乳糖致衰老小鼠学习记忆障碍的作用及机制研究[J]. 江苏中医药, 2024, 56(11): 72-7. |
| [24] | 杨 雁, 孙羽灵, 孙建梅, 等. 山药活性成分药理作用研究进展[J]. 中国野生植物资源, 2022, 41(12): 55-60. doi:10.3969/j.issn.1006-9690.2022.12.011 |
| [25] | Qin XD, Hua J, Lin SJ, et al. Astragalus polysaccharide alleviates cognitive impairment and β-amyloid accumulation in APP/PS1 mice via Nrf2 pathway[J]. Biochem Biophys Res Commun, 2020, 531(3): 431-7. doi:10.1016/j.bbrc.2020.07.122 |
| [26] | 紫 仰, 吴慧珍, 李安哲, 等. 山茱萸化学成分及药理作用研究[J]. 广州化工, 2023, 51, 3: 20-2. |
| [27] | 朱紫悦, 金美玲, 许翔月, 等. D-半乳糖诱导脑老化模型机制的研究进展[J]. 中国比较医学杂志, 2024, 34(10): 104-10, 117. |
| [28] | 刘建亚, 冯文静, 王仁萍, 等. D-半乳糖致衰老动物模型及其机制研究进展[J]. 中华老年多器官疾病杂志, 2018, 17(3): 224-7. doi:10.11915/j.issn.1671-5403.2018.03.049 |
| [29] | Azman KF, Zakaria R. D-Galactose-induced accelerated aging model: an overview[J]. Biogerontology, 2019, 20(6): 763-82. doi:10.1007/s10522-019-09837-y |
| [30] | Shwe T, Pratchayasakul W, Chattipakorn N, et al. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions[J]. Exp Gerontol, 2018, 101: 13-36. doi:10.1016/j.exger.2017.10.029 |
| [31] | Wang BS, Han J, Elisseeff JH, et al. The senescence-associated secretory phenotype and its physiological and pathological implications[J]. Nat Rev Mol Cell Biol, 2024, 25(12): 958-78. doi:10.1038/s41580-024-00727-x |
| [32] | Theleritis C, Siarkos K, Politis A, et al. A systematic review of pharmacological interventions for apathy in aging neurocognitive disorders[J]. Brain Sci, 2023, 13(7): 1061. doi:10.3390/brainsci13071061 |
| [33] | Rao YL, Ganaraja B, Murlimanju BV, et al. Hippocampus and its involvement in Alzheimer's disease: a review[J]. 3 Biotech, 2022, 12(2): 55. doi:10.1007/s13205-022-03123-4 |
| [34] | Chidambaram SB, Anand N, Varma SR, et al. Superoxide dismutase and neurological disorders[J]. IBRO Neurosci Rep, 2024, 16: 373-94. doi:10.1016/j.ibneur.2023.11.007 |
| [35] | Militello R, Luti S, Gamberi T, et al. Physical activity and oxidative stress in aging[J]. Antioxidants (Basel), 2024, 13(5): 557. doi:10.3390/antiox13050557 |
| [36] | Fan AP, An HY, Moradi F, et al. Quantification of brain oxygen extraction and metabolism with [15O]-gas PET: a technical review in the era of PET/MRI[J]. Neuroimage, 2020, 220: 117136. doi:10.1016/j.neuroimage.2020.117136 |
| [37] | Li YY, Li F, Qin DD, et al. The role of brain derived neurotrophic factor in central nervous system[J]. Front Aging Neurosci, 2022, 14: 986443. doi:10.3389/fnagi.2022.986443 |
| [38] | Johnstone A, Mobley W. Local TrkB signaling: themes in development and neural plasticity[J]. Cell Tissue Res, 2020, 382(1): 101-11. doi:10.1007/s00441-020-03278-7 |
| [39] | Arévalo JC, Deogracias R. Mechanisms controlling the expression and secretion of BDNF[J]. Biomolecules, 2023, 13(5): 789. doi:10.3390/biom13050789 |
| [40] | Wang CS, Kavalali ET, Monteggia LM. BDNF signaling in context: From synaptic regulation to psychiatric disorders[J]. Cell, 2022, 185(1): 62-76. doi:10.1016/j.cell.2021.12.003 |
| [41] | Guo CP, Liu Y, Fang MS, et al. ω-3PUFAs improve cognitive impairments through Ser133 phosphorylation of CREB upregulating BDNF/TrkB signal in schizophrenia[J]. Neurotherapeutics, 2020, 17(3): 1271-86. doi:10.1007/s13311-020-00859-w |
| [42] | Cai CY, Wang LF, Li SX, et al. Ras inhibitor lonafarnib rescues structural and functional impairments of synapses of Aβ1-42 mice via α7nAChR-dependent BDNF upregulation[J]. J Neurosci, 2022, 42(31): 6090-107. doi:10.1523/jneurosci.1989-21.2022 |
| [43] | Mirza FJ, Zahid S. The role of synapsins in neurological disorders[J]. Neurosci Bull, 2018, 34(2): 349-58. doi:10.1007/s12264-017-0201-7 |
| [44] | Schmitt U, Tanimoto N, Seeliger M, et al. Detection of behavioral alterations and learning deficits in mice lacking synaptophysin[J]. Neuroscience, 2009, 162(2): 234-43. doi:10.1016/j.neuroscience.2009.04.046 |
| [45] | Mardones MD, Jorquera PV, Herrera-Soto A, et al. PSD95 regulates morphological development of adult-born granule neurons in the mouse hippocampus[J]. J Chem Neuroanat, 2019, 98: 117-23. doi:10.1016/j.jchemneu.2019.04.009 |
| [46] | VanGuilder HD, Farley JA, Yan H, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline[J]. Neurobiol Dis, 2011, 43(1): 201-12. doi:10.1016/j.nbd.2011.03.012 |
| [47] | Li B, Wang ZJ, Yu M, et al. miR-22-3p enhances the intrinsic regenerative abilities of primary sensory neurons via the CBL/p-EGFR/p-STAT3/GAP43/p-GAP43 axis[J]. J Cell Physiol, 2020, 235(5): 4605-17. doi:10.1002/jcp.29338 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||