Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (9): 1738-1751.doi: 10.12122/j.issn.1673-4254.2024.09.14
Previous Articles Next Articles
Weiyang FANG1,2( ), Hui XIAO1, Shuang WANG2, Xiaoming LIN2(
), Hui XIAO1, Shuang WANG2, Xiaoming LIN2( ), Chaomin CHEN1(
), Chaomin CHEN1( )
)
Received:2024-06-06
Online:2024-09-20
Published:2024-09-30
Contact:
Xiaoming LIN, Chaomin CHEN
E-mail:506486730@qq.com;xiaominglin@gidichina.org;15773839131@163.com
Weiyang FANG, Hui XIAO, Shuang WANG, Xiaoming LIN, Chaomin CHEN. A deep learning model based on magnetic resonance imaging and clinical feature fusion for predicting preoperative cytokeratin 19 status in hepatocellular carcinoma[J]. Journal of Southern Medical University, 2024, 44(9): 1738-1751.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.09.14
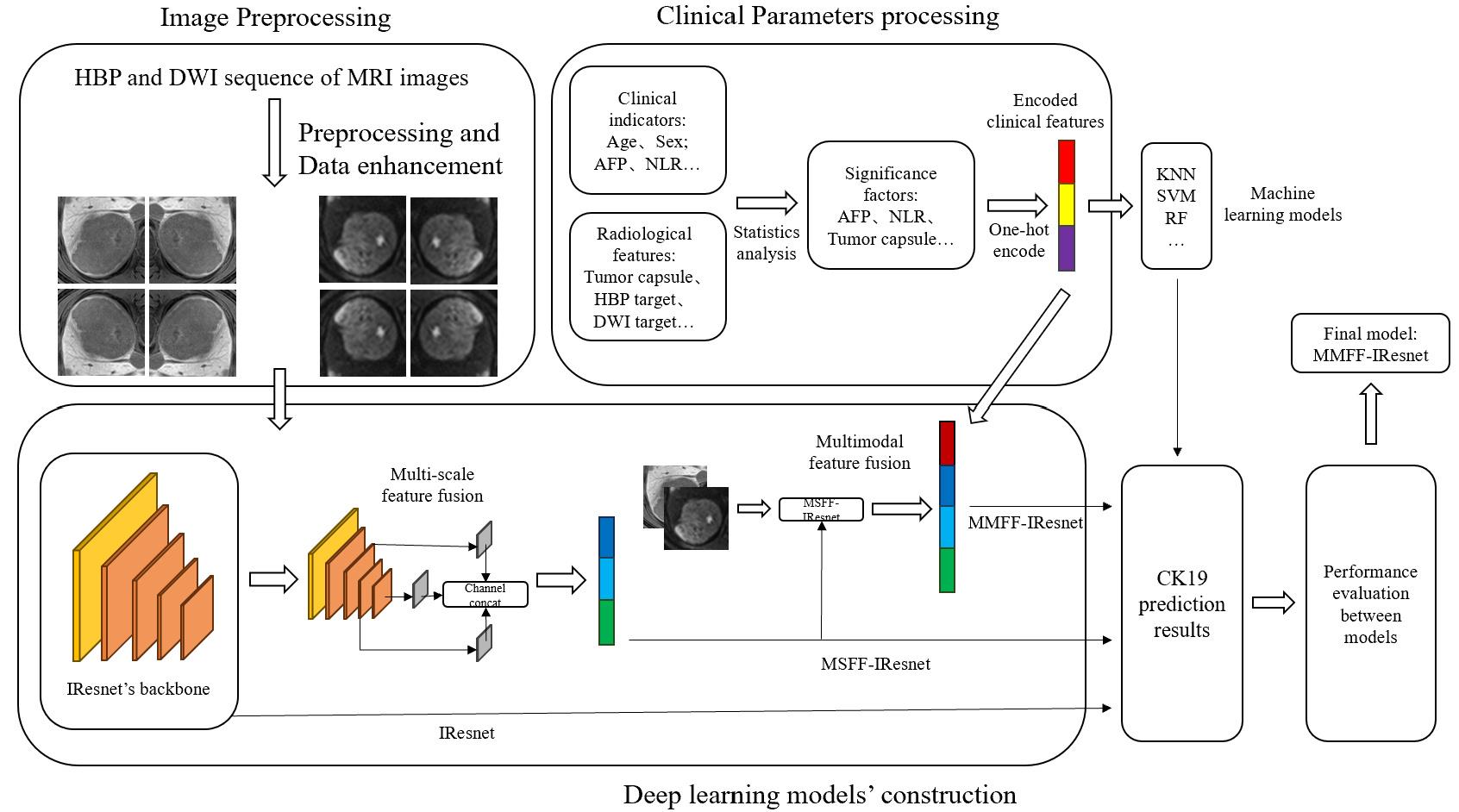
Fig.2 Technical framework of the multi-scale and multi-modality feature fusion model. IResnet: The baseline model. MSFF-IResnet: The multi-scale feature fusion model based on IResnet and multi-scale feature fusion methods. MMFF-IResnet: The multi-scale and multi-modality feature fusion model based on MSFF-IResnet and multi-modality data.
| Item | CK19- cases | CK19+ cases | Number before augmentation | Number after augmentation |
|---|---|---|---|---|
| HBP sequence | ||||
| Training group | 62 | 30 | 740 | 3000 |
| Validation group | 16 | 8 | 190 | 190 |
| DWI sequence | ||||
| Training group | 62 | 30 | 710 | 3000 |
| Validation group | 16 | 8 | 180 | 180 |
Tab.1 Number of MRI samples before and after data augmentation
| Item | CK19- cases | CK19+ cases | Number before augmentation | Number after augmentation |
|---|---|---|---|---|
| HBP sequence | ||||
| Training group | 62 | 30 | 740 | 3000 |
| Validation group | 16 | 8 | 190 | 190 |
| DWI sequence | ||||
| Training group | 62 | 30 | 710 | 3000 |
| Validation group | 16 | 8 | 180 | 180 |
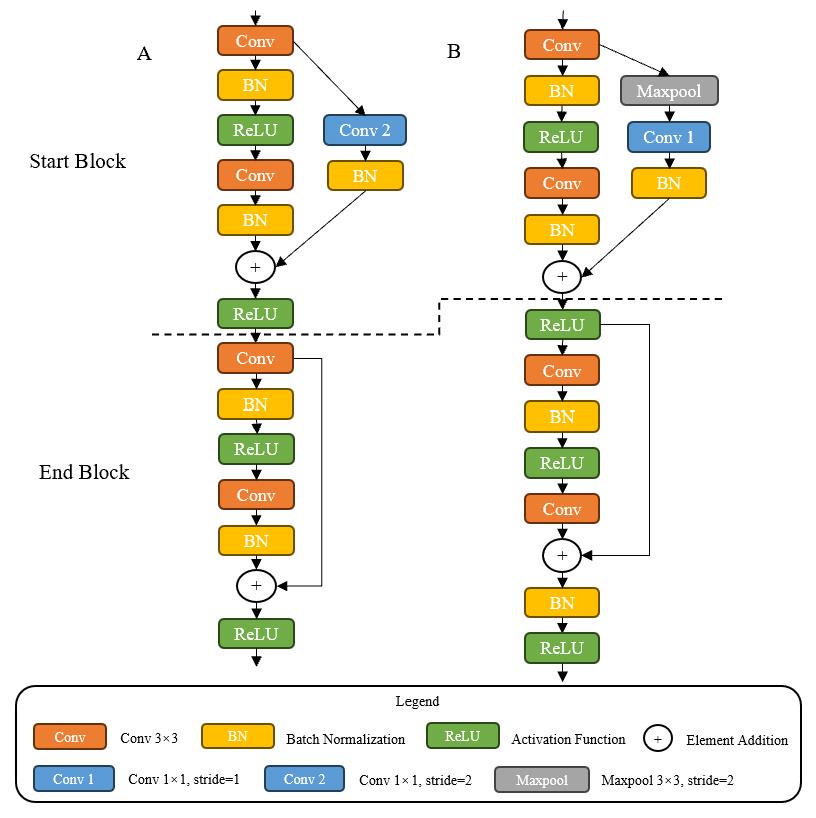
Fig.3 Comparison of residual blocks. A: Residual blocks of Resnet. B: Residual blocks of IResnet18. Start Block is the first block and End Block the last block in residual blocks.
| Input | Operator | Stride | Output |
|---|---|---|---|
| 1×224×224 | Conv2d 7×7 | 2 | 32×112×112 |
| 32×112×112 | Maxpool2d 2×2 | 32×56×56 | |
| 32×56×56 | Ires Stage1 (Ires block ×2) | 1 | 32×56×56 |
| 32×56×56 | Ires Stage2 (Ires block ×2) | 2 | 64×28×28 |
| 64×28×28 | Ires Stage3 (Ires block ×2) | 2 | 128×14×14 |
| 128×14×14 | Ires Stage4 (Ires block ×2) | 2 | 256×7×7 |
| 256×7×7 | GAP 1×1 | 256 | |
| 256 | FC | 2 |
Tab.2 Network architecture of IResnet18
| Input | Operator | Stride | Output |
|---|---|---|---|
| 1×224×224 | Conv2d 7×7 | 2 | 32×112×112 |
| 32×112×112 | Maxpool2d 2×2 | 32×56×56 | |
| 32×56×56 | Ires Stage1 (Ires block ×2) | 1 | 32×56×56 |
| 32×56×56 | Ires Stage2 (Ires block ×2) | 2 | 64×28×28 |
| 64×28×28 | Ires Stage3 (Ires block ×2) | 2 | 128×14×14 |
| 128×14×14 | Ires Stage4 (Ires block ×2) | 2 | 256×7×7 |
| 256×7×7 | GAP 1×1 | 256 | |
| 256 | FC | 2 |
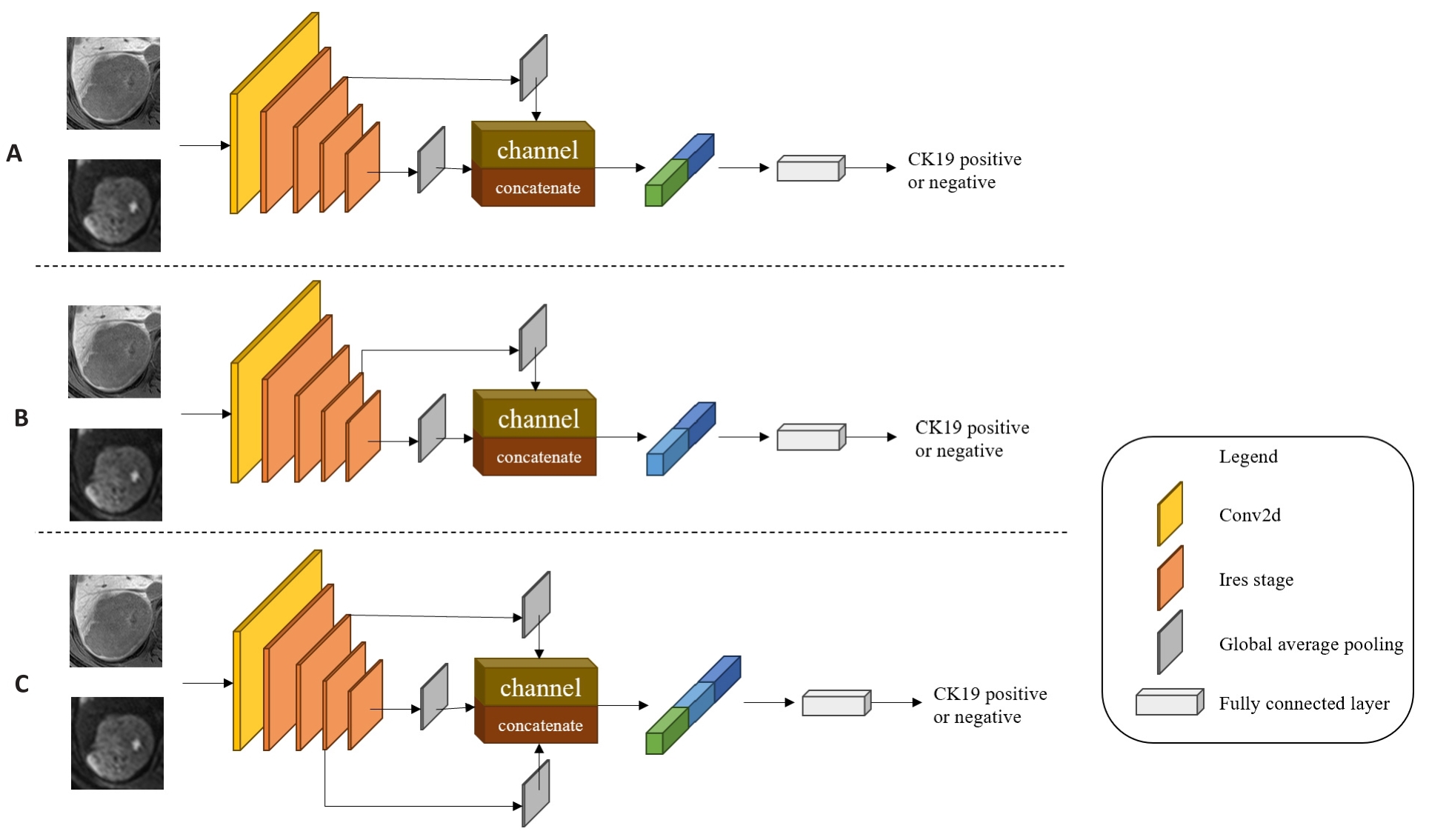
Fig.4 Multi-scale feature fusion models. A: MSFF-IResnet-a, which concatenates the second Ires Stage's shallow level feature map with the fourth Ires Stage's deep level feature map by channel dimension in IResnet18. B: MSFF-IResnet-b, which concatenates the third Ires Stage's middle level feature map with the fourth Ires Stage's deep level feature map by channel dimension in IResnet18. C: MSFF-IResnet-c, which concatenates the second Ires Stage's shallow level feature map and the third Ires Stage's middle level feature map with the fourth Ires Stage's deep level feature map by channel dimension in IResnet18.
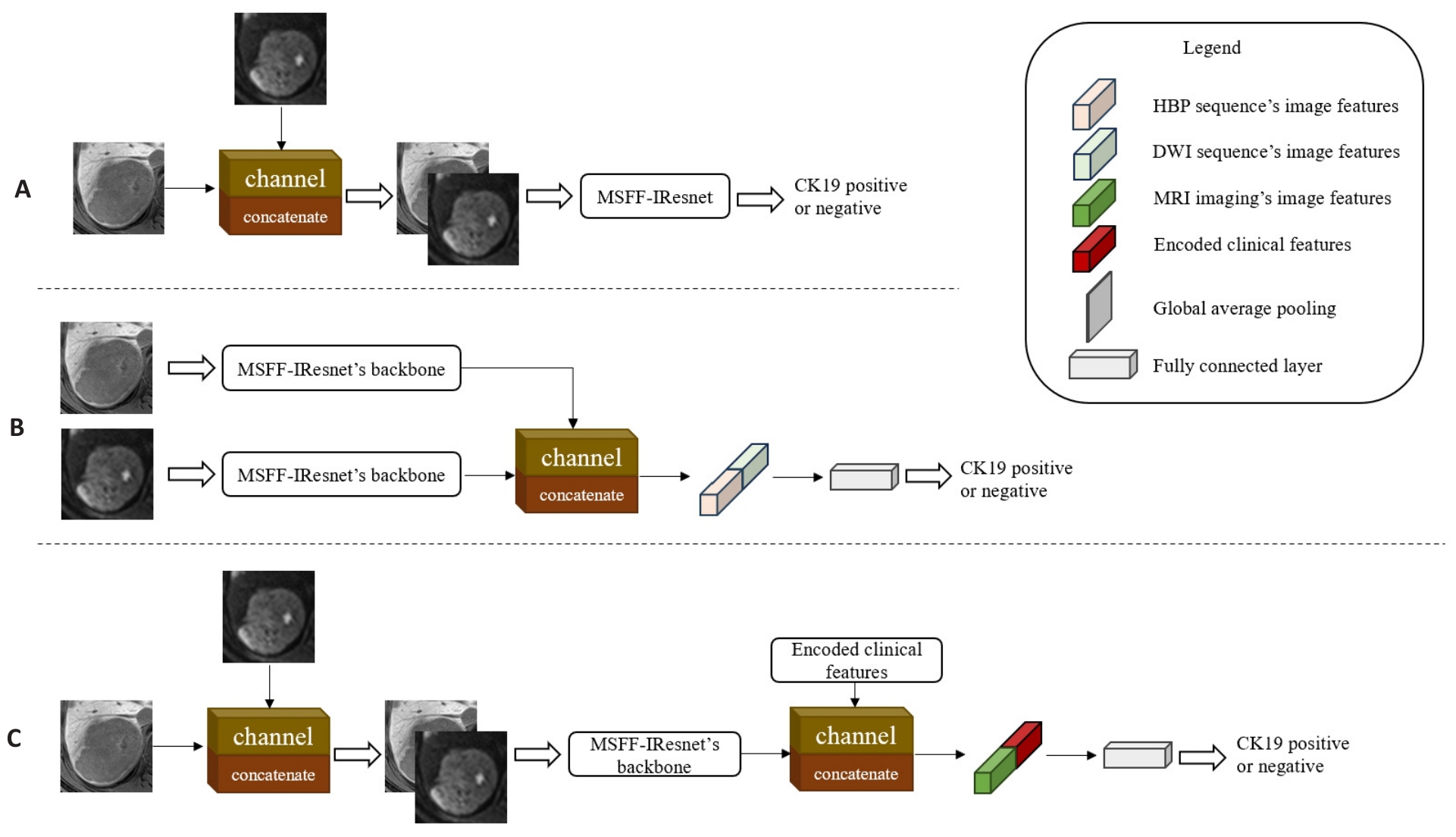
Fig.5 Multi-scale and multi-modality feature fusion models at different stages. A: MMFF-IResnet-a, which fuses two sequences' images by channel dimension in the input stage of MSFF-IResnet. B: MMFF-IResnet-b with fusion of two sequences' feature maps by channel dimension in the feature extraction stage of two independent MSFF-IResnet. C: MMFF-IResnet-c, which concatenates the encoded clinical features by channel dimension in the feature extraction stage of MMFF-IResnet-a.
| Machine learning Model | Parameters |
|---|---|
| KNN | n_neighbors=15, weights=distance |
| SVM | kernel=poly, C=2 |
| LR | class_weight=balanced, random_state=20 |
| DTC | Criterion=entropy, max_depth=6, random_state=20 |
| RF | n_estimators=6, criterion=entropy, random_state=20, class_weight=balanced |
Tab.3 Machine learning models and specific parameter settings
| Machine learning Model | Parameters |
|---|---|
| KNN | n_neighbors=15, weights=distance |
| SVM | kernel=poly, C=2 |
| LR | class_weight=balanced, random_state=20 |
| DTC | Criterion=entropy, max_depth=6, random_state=20 |
| RF | n_estimators=6, criterion=entropy, random_state=20, class_weight=balanced |
| Parameters | Value |
|---|---|
| Batch size | 128 |
| Learning rate | 0.01 |
| Weight decay | 1e-4、1e-5 |
| Warm up epoch | 5 |
| Total epoch | 100 |
| Early stopping patience | 10 |
Tab.4 Special parameter settings of the deep learning models
| Parameters | Value |
|---|---|
| Batch size | 128 |
| Learning rate | 0.01 |
| Weight decay | 1e-4、1e-5 |
| Warm up epoch | 5 |
| Total epoch | 100 |
| Early stopping patience | 10 |
| Characteristics | Clinical Data of HCC Patients (n=116) | Qualitative or quantitative analysis | |
|---|---|---|---|
| CK19- (n=78, 67.2%) | CK19+ (n=38, 32.8%) | P | |
| Age (year, Mean±SD) | 57.7±12.4 | 59.4±11.1 | 0.451 |
| Gender | 0.485 | ||
| Male | 68 (87.2%) | 35 (92.1%) | |
| Female | 10 (12.8%) | 3 (7.9%) | |
| Hepatitis | 0.815 | ||
| Absent | 7 (9.0%) | 3 (7.9%) | |
| Present | 71 (91.0%) | 35 (92.1%) | |
| AFP (ng/mL) | 0.017* | ||
| ≤20 | 45 (57.7%) | 11 (28.9%) | |
| 20<, ≤400 | 25 (32.1%) | 14 (36.8%) | |
| >400 | 8 (10.3%) | 13 (34.2%) | |
| ALT[U/L, Median (Min, Max)] | 34.0 (9.0, 113.0) | 37.0 (15.0, 129.0) | 0.276 |
| AST [U/L, Median (Min, Max)] | 37.0 (15.0, 139.0) | 37.0 (18.0, 205.0) | 0.537 |
| GGT [U/L, Median (Min, Max)] | 52.5 (14.0, 1055.0) | 72.0 (13.0, 1013.0) | 0.648 |
| LY [109/L, Median (Min, Max)] | 1.54 (0.42, 3.95) | 1.36 (0.42, 2.35) | 0.521 |
| NLR [Median (Min, Max)] | 1.78 (0.48, 12.13) | 2.58 (1.14, 8.17) | 0.004* |
| ALB [g/L, Median (Min, Max)] | 41.2 (24.9, 74.3) | 41.7 (26.0, 49.9) | 0.239 |
| Tbil [μmol/L, Median (Min, Max)] | 14.37 (4.46, 35.12) | 15.29 (5.16, 93.30) | 0.466 |
| MVI | 0.099 | ||
| Negative | 60 (76.9%) | 23 (60.5%) | |
| Positive | 18 (23.1%) | 15 (39.5%) | |
| Tumor size[mm, Median (Min, Max)] | 3.50 (1.20, 11.70) | 3.80 (1.58, 14.79) | 0.553 |
| Tumor capsule | 0.016* | ||
| None | 13 (16.7%) | 0 (0%) | |
| Absent | 22 (28.1%) | 21 (55.3%) | |
| Present | 43 (55.1%) | 17 (44.7%) | |
| Tumor margin | 0.254 | ||
| Absent | 10 (12.8%) | 8 (21.1%) | |
| Present | 68 (87.2%) | 30 (78.9%) | |
| Cystic or necrosis portion or fat deposition | 0.776 | ||
| Absent | 49 (63.3%) | 23 (60.5%) | |
| Present | 29 (36.7%) | 15 (39.5%) | |
| Target sign of DWI | 0.844 | ||
| Absent | 47 (60.3%) | 24 (63.2%) | |
| Present | 31 (39.7%) | 14 (36.8%) | |
| Arterial rim enhancement | 0.097 | ||
| Absent | 67 (85.9%) | 27 (71.1%) | |
| Present | 11 (14.1%) | 11 (28.9%) | |
| Peritumoral eEnhancement | 0.538 | ||
| Absent | 57 (73.1%) | 25 (65.8%) | |
| Present | 21 (26.9%) | 13 (34.2%) | |
| Target sign of HBP | 0.278 | ||
| Absent | 45 (57.7%) | 17 (44.7%) | |
| Present | 33 (42.3%) | 21 (55.3%) | |
| Peritumoral hypo-intensity of HBP | 0.187 | ||
| Absent | 60 (76.9%) | 24 (63.2%) | |
| Present | 18 (23.1%) | 14 (36.8%) | |
| Satellite nodules | 0.155 | ||
| Absent | 72 (92.3%) | 38 (100.0%) | |
| Present | 6 (7.7%) | 0 (0%) | |
Tab.5 Statistical analysis of clinical parameters of HCC patients with CK19 expression
| Characteristics | Clinical Data of HCC Patients (n=116) | Qualitative or quantitative analysis | |
|---|---|---|---|
| CK19- (n=78, 67.2%) | CK19+ (n=38, 32.8%) | P | |
| Age (year, Mean±SD) | 57.7±12.4 | 59.4±11.1 | 0.451 |
| Gender | 0.485 | ||
| Male | 68 (87.2%) | 35 (92.1%) | |
| Female | 10 (12.8%) | 3 (7.9%) | |
| Hepatitis | 0.815 | ||
| Absent | 7 (9.0%) | 3 (7.9%) | |
| Present | 71 (91.0%) | 35 (92.1%) | |
| AFP (ng/mL) | 0.017* | ||
| ≤20 | 45 (57.7%) | 11 (28.9%) | |
| 20<, ≤400 | 25 (32.1%) | 14 (36.8%) | |
| >400 | 8 (10.3%) | 13 (34.2%) | |
| ALT[U/L, Median (Min, Max)] | 34.0 (9.0, 113.0) | 37.0 (15.0, 129.0) | 0.276 |
| AST [U/L, Median (Min, Max)] | 37.0 (15.0, 139.0) | 37.0 (18.0, 205.0) | 0.537 |
| GGT [U/L, Median (Min, Max)] | 52.5 (14.0, 1055.0) | 72.0 (13.0, 1013.0) | 0.648 |
| LY [109/L, Median (Min, Max)] | 1.54 (0.42, 3.95) | 1.36 (0.42, 2.35) | 0.521 |
| NLR [Median (Min, Max)] | 1.78 (0.48, 12.13) | 2.58 (1.14, 8.17) | 0.004* |
| ALB [g/L, Median (Min, Max)] | 41.2 (24.9, 74.3) | 41.7 (26.0, 49.9) | 0.239 |
| Tbil [μmol/L, Median (Min, Max)] | 14.37 (4.46, 35.12) | 15.29 (5.16, 93.30) | 0.466 |
| MVI | 0.099 | ||
| Negative | 60 (76.9%) | 23 (60.5%) | |
| Positive | 18 (23.1%) | 15 (39.5%) | |
| Tumor size[mm, Median (Min, Max)] | 3.50 (1.20, 11.70) | 3.80 (1.58, 14.79) | 0.553 |
| Tumor capsule | 0.016* | ||
| None | 13 (16.7%) | 0 (0%) | |
| Absent | 22 (28.1%) | 21 (55.3%) | |
| Present | 43 (55.1%) | 17 (44.7%) | |
| Tumor margin | 0.254 | ||
| Absent | 10 (12.8%) | 8 (21.1%) | |
| Present | 68 (87.2%) | 30 (78.9%) | |
| Cystic or necrosis portion or fat deposition | 0.776 | ||
| Absent | 49 (63.3%) | 23 (60.5%) | |
| Present | 29 (36.7%) | 15 (39.5%) | |
| Target sign of DWI | 0.844 | ||
| Absent | 47 (60.3%) | 24 (63.2%) | |
| Present | 31 (39.7%) | 14 (36.8%) | |
| Arterial rim enhancement | 0.097 | ||
| Absent | 67 (85.9%) | 27 (71.1%) | |
| Present | 11 (14.1%) | 11 (28.9%) | |
| Peritumoral eEnhancement | 0.538 | ||
| Absent | 57 (73.1%) | 25 (65.8%) | |
| Present | 21 (26.9%) | 13 (34.2%) | |
| Target sign of HBP | 0.278 | ||
| Absent | 45 (57.7%) | 17 (44.7%) | |
| Present | 33 (42.3%) | 21 (55.3%) | |
| Peritumoral hypo-intensity of HBP | 0.187 | ||
| Absent | 60 (76.9%) | 24 (63.2%) | |
| Present | 18 (23.1%) | 14 (36.8%) | |
| Satellite nodules | 0.155 | ||
| Absent | 72 (92.3%) | 38 (100.0%) | |
| Present | 6 (7.7%) | 0 (0%) | |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Lower | Upper | Lower | Upper | |||||
| AFP (ng/mL) | ||||||||
| ≤20 | 0.024 | 0.189 | ||||||
| 20<, ≤400 | 1 | 1 | ||||||
| >400 | 6.000 | 1.650 | 21.824 | 0.007 | 0.121 | |||
| NLR | 1.259 | 0.977 | 1.622 | 0.075 | 1.417 | 1.036 | 1.938 | 0.029 |
| MVI | 0.493 | |||||||
| Negetive | 1 | 1 | ||||||
| Positive | 2.274 | 0.848 | 6.102 | 0.103 | ||||
| Tumor Capsule | 0.202 | 0.089 | ||||||
| None | ||||||||
| Absent | 2.422 | 0.918 | 6.387 | 0.074 | 3.250 | 1.136 | 9.301 | 0.028 |
| Present | 1 | 1 | ||||||
| Arterial rim enhancement | 0.229 | |||||||
| Absent | 1 | 1 | ||||||
| Present | 2.526 | 0.829 | 7.702 | 0.103 | ||||
Tab.6 Results of univariate and multivariate logistic regression analysis
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Lower | Upper | Lower | Upper | |||||
| AFP (ng/mL) | ||||||||
| ≤20 | 0.024 | 0.189 | ||||||
| 20<, ≤400 | 1 | 1 | ||||||
| >400 | 6.000 | 1.650 | 21.824 | 0.007 | 0.121 | |||
| NLR | 1.259 | 0.977 | 1.622 | 0.075 | 1.417 | 1.036 | 1.938 | 0.029 |
| MVI | 0.493 | |||||||
| Negetive | 1 | 1 | ||||||
| Positive | 2.274 | 0.848 | 6.102 | 0.103 | ||||
| Tumor Capsule | 0.202 | 0.089 | ||||||
| None | ||||||||
| Absent | 2.422 | 0.918 | 6.387 | 0.074 | 3.250 | 1.136 | 9.301 | 0.028 |
| Present | 1 | 1 | ||||||
| Arterial rim enhancement | 0.229 | |||||||
| Absent | 1 | 1 | ||||||
| Present | 2.526 | 0.829 | 7.702 | 0.103 | ||||
| Model | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| KNN | 0.810±0.084 | 0.850±0.200 | 0.567±0.152 | 0.932±0.097 | 0.747±0.084 |
| SVM | 0.810±0.084 | 0.810±0.185 | 0.607±0.179 | 0.914±0.091 | 0.759±0.098 |
| LR | 0.749±0.111 | 0.628±0.168 | 0.673±0.116 | 0.789±0.123 | 0.731±0.108 |
| DTC | 0.798±0.098 | 0.743±0.164 | 0.607±0.179 | 0.895±0.081 | 0.750±0.107 |
| RF | 0.762±0.109 | 0.669±0.201 | 0.633±0.099 | 0.824±0.120 | 0.728±0.103 |
Tab.7 Classification performance of the machine learning models
| Model | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| KNN | 0.810±0.084 | 0.850±0.200 | 0.567±0.152 | 0.932±0.097 | 0.747±0.084 |
| SVM | 0.810±0.084 | 0.810±0.185 | 0.607±0.179 | 0.914±0.091 | 0.759±0.098 |
| LR | 0.749±0.111 | 0.628±0.168 | 0.673±0.116 | 0.789±0.123 | 0.731±0.108 |
| DTC | 0.798±0.098 | 0.743±0.164 | 0.607±0.179 | 0.895±0.081 | 0.750±0.107 |
| RF | 0.762±0.109 | 0.669±0.201 | 0.633±0.099 | 0.824±0.120 | 0.728±0.103 |
| Item | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| HBP sequence | |||||
| IResnet | 0.651±0.098 | 0.526±0.141 | 0.700±0.177 | 0.627±0.203 | 0.718±0.080 |
| MSFF-IResnet-a | 0.707±0.089 | 0.549±0.086 | 0.846±0.054 | 0.639±0.153 | 0.780±0.068 |
| MSFF-IResnet-b | 0.738±0.038 | 0.611±0.078 | 0.620±0.180 | 0.795±0.102 | 0.738±0.077 |
| MSFF-IResnet-c | 0.782±0.068 | 0.643±0.079 | 0.673±0.066 | 0.785±0.084 | 0.819±0.083 |
| DWI sequence | |||||
| IResnet | 0.745±0.056 | 0.606±0.043 | 0.679±0.150 | 0.776±0.060 | 0.752±0.091 |
| MSFF-IResnet-a | 0.714±0.067 | 0.551±0.085 | 0.833±0.139 | 0.651±0.107 | 0.764±0.075 |
| MSFF-IResnet-b | 0.749±0.059 | 0.605±0.103 | 0.819±0.102 | 0.707±0.117 | 0.781±0.098 |
| MSFF-IResnet-c | 0.735±0.111 | 0.578±0.133 | 0.917±0.092 | 0.642±0.136 | 0.796±0.118 |
Tab.8 Classification results of the ablation experiment using multi-scale feature fusion methods
| Item | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| HBP sequence | |||||
| IResnet | 0.651±0.098 | 0.526±0.141 | 0.700±0.177 | 0.627±0.203 | 0.718±0.080 |
| MSFF-IResnet-a | 0.707±0.089 | 0.549±0.086 | 0.846±0.054 | 0.639±0.153 | 0.780±0.068 |
| MSFF-IResnet-b | 0.738±0.038 | 0.611±0.078 | 0.620±0.180 | 0.795±0.102 | 0.738±0.077 |
| MSFF-IResnet-c | 0.782±0.068 | 0.643±0.079 | 0.673±0.066 | 0.785±0.084 | 0.819±0.083 |
| DWI sequence | |||||
| IResnet | 0.745±0.056 | 0.606±0.043 | 0.679±0.150 | 0.776±0.060 | 0.752±0.091 |
| MSFF-IResnet-a | 0.714±0.067 | 0.551±0.085 | 0.833±0.139 | 0.651±0.107 | 0.764±0.075 |
| MSFF-IResnet-b | 0.749±0.059 | 0.605±0.103 | 0.819±0.102 | 0.707±0.117 | 0.781±0.098 |
| MSFF-IResnet-c | 0.735±0.111 | 0.578±0.133 | 0.917±0.092 | 0.642±0.136 | 0.796±0.118 |
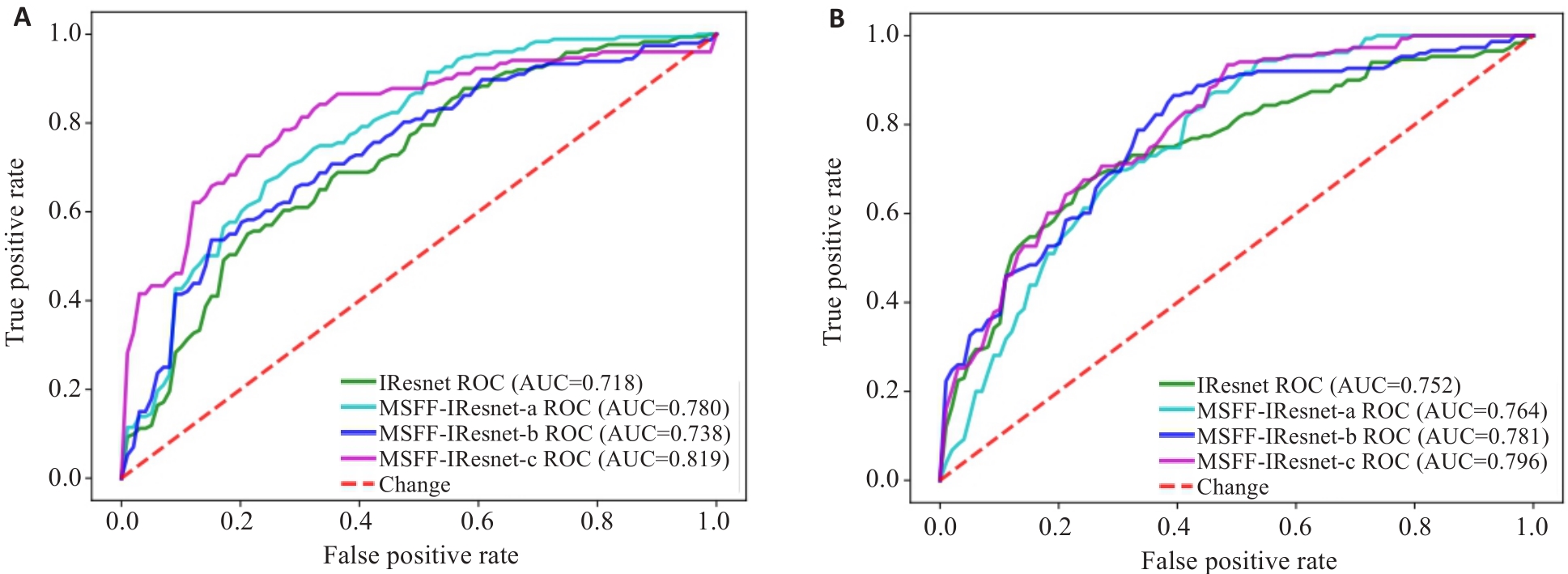
Fig.6 ROC charts of the ablation experiment for multi-scale feature fusion methods. A: ROC of models based on HBP sequence. B: ROC of models based on DWI sequence.
| Method | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| MSFF-IResnet | 0.782±0.068 | 0.643±0.079 | 0.673±0.066 | 0.785±0.084 | 0.819±0.083 |
| MMFF-IResnet-a | 0.796±0.085 | 0.702±0.182 | 0.773±0.118 | 0.804±0.136 | 0.829±0.122 |
| MMFF-IResnet-b | 0.790±0.061 | 0.648±0.097 | 0.778±0.173 | 0.787±0.079 | 0.823±0.089 |
| MMFF-IResnet-c | 0.806±0.080 | 0.708±0.148 | 0.801±0.128 | 0.812±0.131 | 0.842±0.100 |
Tab.9 Classification results of the ablation experiment using multi-modality feature fusion methods
| Method | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| MSFF-IResnet | 0.782±0.068 | 0.643±0.079 | 0.673±0.066 | 0.785±0.084 | 0.819±0.083 |
| MMFF-IResnet-a | 0.796±0.085 | 0.702±0.182 | 0.773±0.118 | 0.804±0.136 | 0.829±0.122 |
| MMFF-IResnet-b | 0.790±0.061 | 0.648±0.097 | 0.778±0.173 | 0.787±0.079 | 0.823±0.089 |
| MMFF-IResnet-c | 0.806±0.080 | 0.708±0.148 | 0.801±0.128 | 0.812±0.131 | 0.842±0.100 |
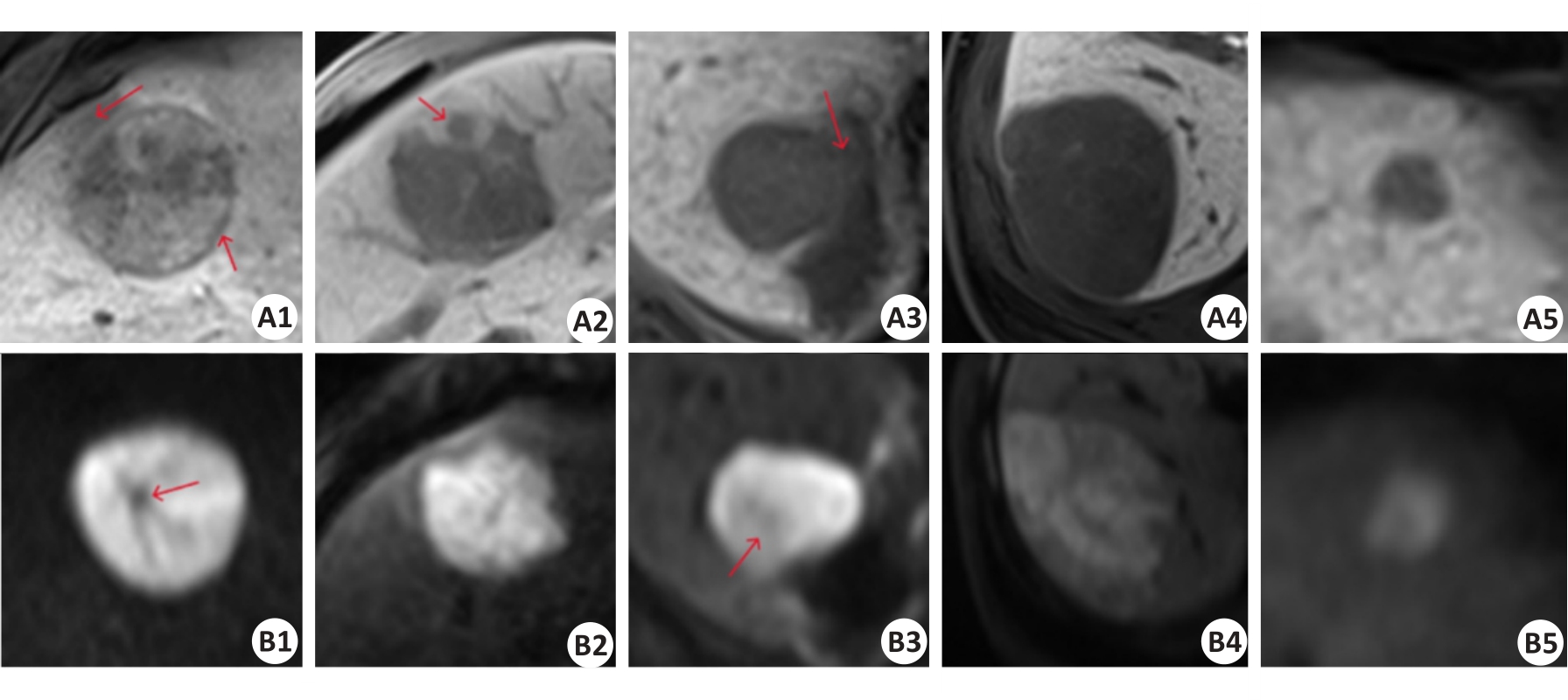
Fig.8 Comparison of positive and negative sample images of HBP sequence (A1-A5) and DWI sequence (B1-B5). A1-A2 and B1-B2: True CK19-positive cases predicted by the baseline model and multi-scale feature fusion models. A3 and B3: CK19-positive cases predicted as negative by the baseline model but corrected to be positive by multi-scale feature fusion models. A4-A5 and B4-B5: true CK19-negative cases predicted by the baseline model and multi-scale feature fusion models. The red arrows in A1, A2, A3, B1, and B3 sequentially represent the imaging features of peritumoral hypo-intensity, HBP target sign, irregular tumor margin, peritumoral hypo-intensity, fat deposition, and lower high signal tumor area.
| Research | Method | Year | Cases | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Peng [ | Machine Learning | 2024 | 226 | 0.706 | - | - | 0.706 |
| Lu [ | Machine Learning | 2024 | 220 | 0.758 | 0.625 | 0.8 | 0.748 |
| Wang [ | Radiomics | 2024 | 137 | - | 0.724 | 0.759 | 0.75 |
| Zhang [ | Radiomics | 2023 | 311 | - | 0.769 | 0.818 | 0.795 |
| Hu [ | Radiomics | 2023 | 110 | 0.88 | 0.84 | 0.89 | 0.92 |
| Ours | Deep Learning | 2024 | 116 | 0.806 | 0.801 | 0.812 | 0.842 |
Tab.10 Comparison with the results of other research
| Research | Method | Year | Cases | Accuracy | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| Peng [ | Machine Learning | 2024 | 226 | 0.706 | - | - | 0.706 |
| Lu [ | Machine Learning | 2024 | 220 | 0.758 | 0.625 | 0.8 | 0.748 |
| Wang [ | Radiomics | 2024 | 137 | - | 0.724 | 0.759 | 0.75 |
| Zhang [ | Radiomics | 2023 | 311 | - | 0.769 | 0.818 | 0.795 |
| Hu [ | Radiomics | 2023 | 110 | 0.88 | 0.84 | 0.89 | 0.92 |
| Ours | Deep Learning | 2024 | 116 | 0.806 | 0.801 | 0.812 | 0.842 |
| 1 | Forner A, Reig M, Bruix J. Hepatocellular carcinoma[J]. Lancet, 2018, 391(10127): 1301-14. |
| 2 | Wu MS, Zhong JH, Chen K, et al. Association of CK19 expression with the efficacy of adjuvant transarterial chemoembolization after hepatic resection in hepatocellular carcinoma patients at high risk of recurrence[J]. J Clin Transl Res, 2022, 8(1): 71-9. |
| 3 | Zhuo JY, Lu D, Tan WY, et al. CK19-positive hepatocellular carcinoma is a characteristic subtype[J]. J Cancer, 2020, 11(17): 5069-77. |
| 4 | Guo YX, Chen JJ, Zhang YF, et al. Differentiating Cytokeratin 19 expression of hepatocellular carcinoma by using multi-b-value diffusion-weighted MR imaging with mono-exponential, stretched exponential, intravoxel incoherent motion, diffusion kurtosis imaging and fractional order calculus models[J]. Eur J Radiol, 2022, 150: 110237. |
| 5 | 肝癌新辅助治疗中国专家共识协作组, 中国研究型医院学会消化外科专业委员会, 中国抗癌协会肝癌专业委员会. 肝癌新辅助治疗中国专家共识(2023版)[J]. 中华外科杂志, 2023, 61(12): 1035-45. |
| 6 | Choi SY, Kim SH, Park CK, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a retrospective observational study[J]. Radiology, 2018, 286(3): 897-908. |
| 7 | Chen YY, Chen J, Zhang Y, et al. Preoperative prediction of cytokeratin 19 expression for hepatocellular carcinoma with deep learning radiomics based on gadoxetic acid-enhanced magnetic resonance imaging[J]. J Hepatocell Carcinoma, 2021, 8: 795-808. |
| 8 | Yoneda N, Sato Y, Kitao A, et al. Epidermal growth factor induces cytokeratin 19 expression accompanied by increased growth abilities in human hepatocellular carcinoma[J]. Lab Invest, 2011, 91(2): 262-72. |
| 9 | Yoon JK, Choi JY, Rhee H, et al. MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification[J]. Eur Radiol, 2022, 32(8): 5119-33. |
| 10 | Hu XX, Wang WT, Yang L, et al. MR features based on LI-RADS identify cytokeratin 19 status of hepatocellular carcinomas[J]. Eur J Radiol, 2019, 113: 7-14. |
| 11 | Wang WT, Gu DS, Wei JW, et al. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI[J]. Eur Radiol, 2020, 30(5): 3004-14. |
| 12 | Lu XY, Xi T, Lau WY, et al. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior[J]. Ann Surg Oncol, 2011, 18(8): 2210-7. |
| 13 | Lee CW, Lin SE, Yu MC, et al. Does neutrophil to lymphocyte ratio have a role in identifying cytokeratin 19-expressing hepatocellular carcinoma?[J]. J Pers Med, 2021, 11(11): 1078. |
| 14 | Govaere O, Komuta M, Berkers J, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas[J]. Gut, 2014, 63(4): 674-85. |
| 15 | Sun DW, Zhang YY, Sun XD, et al. Prognostic value of cytokeratin 19 in hepatocellular carcinoma: a meta-analysis[J]. Clin Chim Acta, 2015, 448: 161-9. |
| 16 | Tsuchiya K, Komuta M, Yasui Y, et al. Expression of keratin 19 is related to high recurrence of hepatocellular carcinoma after radiofrequency ablation[J]. Oncology, 2011, 80(3/4): 278-88. |
| 17 | Qin SD, Zhang J, Qi YP, et al. Individual and joint influence of cytokeratin 19 and microvascular invasion on the prognosis of patients with hepatocellular carcinoma after hepatectomy[J]. World J Surg Oncol, 2022, 20(1): 209. |
| 18 | Yang F, Wan YD, Xu L, et al. MRI-radiomics prediction for cytokeratin 19-positive hepatocellular carcinoma: a multicenter study[J]. Front Oncol, 2021, 11: 672126. |
| 19 | Caviglia GP, Ciruolo M, Olivero A, et al. Prognostic role of serum cytokeratin-19 fragment (CYFRA 21-1) in patients with hepatocellular carcinoma[J]. Cancers, 2020, 12(10): 2776. |
| 20 | Zhao Y, Tan XL, Chen JM, et al. Preoperative prediction of cytokeratin-19 expression for hepatocellular carcinoma using T1 mapping on gadoxetic acid-enhanced MRI combined with diffusion-weighted imaging and clinical indicators[J]. Front Oncol, 2022, 12: 1068231. |
| 21 | 关星群, 刘子蔚, 郭保亮, 等. 钆塞酸二钠增强MRI结合T1mapping术前预测肝细胞癌CK19表达及列线图构建[J]. 中国CT和MRI杂志, 2023, 21(6): 103-6. |
| 22 | Lv J, Yin HY, Yu HJ, et al. The added value of 18F-FDG PET/MRI multimodal imaging in hepatocellular carcinoma for identifying cytokeratin 19 status[J]. Abdom Radiol, 2023, 48(7): 2331-9. |
| 23 | 鲁梦恬, 瞿 琦, 徐 磊, 等. 基于LI-RADS v2018及MRI特征对肝细胞癌CK19表达的风险预测及预后评估[J]. 磁共振成像, 2024, 15(3): 107-13, 121. |
| 24 | 彭超群, 杨 燕, 杨程羽, 等. 基于钆塞酸二钠增强MRI特征构建肝细胞癌CK19表达术前预测模型[J]. 中国医学计算机成像杂志, 2024, 30(2): 197-204. |
| 25 | Huang XL, Long LL, Wei JQ, et al. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis[J]. J Cancer Res Clin Oncol, 2019, 145(12): 2995-3003. |
| 26 | Hu XJ, Wang Q, Huang GB, et al. Gadoxetic acid-enhanced MRI-based radiomics signature: a potential imaging biomarker for identifying cytokeratin 19-positive hepatocellular carcinoma[J]. Comput Math Methods Med, 2023, 2023: 5424204. |
| 27 | Liu JQ, Wang J, Huang XL, et al. A radiomics model based on magnetic resonance imaging to predict cytokeratin 7/19 expression and liver fluke infection of hepatocellular carcinoma[J]. Sci Rep, 2023, 13(1): 17553. |
| 28 | Zhang LQ, Zhou HS, Zhang XQ, et al. A radiomics nomogram for predicting cytokeratin 19-positive hepatocellular carcinoma: a two-center study[J]. Front Oncol, 2023, 13: 1174069. |
| 29 | 王 倩, 黎金葵, 徐俊霞, 等. Gd-EOB-DTPA增强MRI预测CK19阳性肝细胞癌及其预后的临床价值[J]. 临床放射学杂志, 2024, 43(3): 388-93. |
| 30 | Shen L, Lin ZC, Huang QM. Relay backpropagation for effective learning of deep convolutional neural networks[M]//Lecture Notes in Computer Science. Cham: Springer International Publishing, 2016: 467-82. |
| 31 | Duta IC, Liu L, Zhu F, et al. Improved residual networks for image and video recognition[C]//2020 25th International Conference on Pattern Recognition (ICPR). Milan, Italy. IEEE, 2021: 9415-22. |
| 32 | Jeong HT, Kim MJ, Kim YE, et al. MRI features of hepatocellular carcinoma expressing progenitor cell markers[J]. Liver Int, 2012, 32(3): 430-40. |
| 33 | An C, Kim DW, Park YN, et al. Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection[J]. Radiology, 2015, 276(2): 433-43. |
| 34 | Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy[J]. World J Surg Oncol, 2013, 11: 212. |
| [1] | Jiazhi OU, Chang'an ZHAN, Feng YANG. An autoencoder model based on one-dimensional neural network for epileptic EEG anomaly detection [J]. Journal of Southern Medical University, 2024, 44(9): 1796-1804. |
| [2] | Chen WANG, Mingqiang MENG, Mingqiang LI, Yongbo WANG, Dong ZENG, Zhaoying BIAN, Jianhua MA. Reconstruction from CT truncated data based on dual-domain transformer coupled feature learning [J]. Journal of Southern Medical University, 2024, 44(5): 950-959. |
| [3] | LONG Kaixing, WENG Danyi, GENG Jian, LU Yanmeng, ZHOU Zhitao, CAO Lei. Automatic classification of immune-mediated glomerular diseases based on multi-modal multi-instance learning [J]. Journal of Southern Medical University, 2024, 44(3): 585-593. |
| [4] | XIAO Hui, FANG Weiyang, LIN Mingjun, ZHOU Zhenzhong, FEI Hongwen, CHEN Chaomin. A multiscale carotid plaque detection method based on two-stage analysis [J]. Journal of Southern Medical University, 2024, 44(2): 387-396. |
| [5] | HE Huishan, GUO Erjia, MENG Wenyi, WANG Yu, WANG Wen, HE Wenle, WU Yuankui, YANG Wei. Predicting cerebral glioma enhancement pattern using a machine learning-based magnetic resonance imaging radiomics model [J]. Journal of Southern Medical University, 2024, 44(1): 194-200. |
| [6] | MI Jia, ZHOU Yujia, FENG Qianjin. A 3D/2D registration method based on reconstruction of orthogonal-view Xray images [J]. Journal of Southern Medical University, 2023, 43(9): 1636-1643. |
| [7] | CHU Zhiqin, QU Yaoming, ZHONG Tao, LIANG Shujun, WEN Zhibo, ZHANG Yu. A Dual-Aware deep learning framework for identification of glioma isocitrate dehydrogenase genotype using magnetic resonance amide proton transfer modalities [J]. Journal of Southern Medical University, 2023, 43(8): 1379-1387. |
| [8] | LIU Yuxuan, CHU Zhiqin, ZHANG Yu. Physical model-based cascaded generative adversarial networks for accelerating quantitative multi-parametric magnetic resonance imaging [J]. Journal of Southern Medical University, 2023, 43(8): 1402-1409. |
| [9] | YU Jiahong, ZHANG Kunpeng, JIN Shuang, SU Zhe, XU Xiaotong, ZHANG Hua. Sinogram interpolation combined with unsupervised image-to-image translation network for CT metal artifact correction [J]. Journal of Southern Medical University, 2023, 43(7): 1214-1223. |
| [10] | LU Mingjun, QU Yaoming, MA Andong, ZHU Jianbin, ZOU Xia, LIN Gengyun, LI Yuxin, LIU Xinzi, WEN Zhibo. Prediction of 1p/19q codeletion status in diffuse lower-grade glioma using multimodal MRI radiomics [J]. Journal of Southern Medical University, 2023, 43(6): 1023-1028. |
| [11] | TENG Lin, WANG Bin, FENG Qianjin. Deep learning-based dose prediction in radiotherapy planning for head and neck cancer [J]. Journal of Southern Medical University, 2023, 43(6): 1010-1016. |
| [12] | ZHOU Hao, ZENG Dong, BIAN Zhaoying, MA Jianhua. A semi-supervised network-based tissue-aware contrast enhancement method for CT images [J]. Journal of Southern Medical University, 2023, 43(6): 985-993. |
| [13] | GONG Gao, CAO Shi, XIAO Hui, FANG Weiyang, QUE Yuqing, LIU Ziwei, CHEN Chaomin. Prediction of microvascular invasion in hepatocellular carcinoma with magnetic resonance imaging using models combining deep attention mechanism with clinical features [J]. Journal of Southern Medical University, 2023, 43(5): 839-851. |
| [14] | HAO Yuwei, GAO Sheng, ZHANG Xiaoyue, CUI Mengqiu, DING Xiaohui, WANG He, YANG Dawei, YE Huiyi, WANG Haiyi. Comparison of diagnostic performance of Clear Cell Likelihood Score v1.0 and v2.0 for clear renal cell carcinoma [J]. Journal of Southern Medical University, 2023, 43(5): 800-806. |
| [15] | YU Jingwen, YANG Meijie, JIANG Li, XIAO Zhibo, LI Shuang, CHEN Jinyun. Preoperative MR T2WI signal characteristics of adenomyosis are closely related with the outcome of high-intensity focused ultrasound ablation: a propensity score-matched cohort study [J]. Journal of Southern Medical University, 2023, 43(4): 597-603. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||