Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (5): 859-866.doi: 10.12122/j.issn.1673-4254.2024.05.07
• Clinical Research • Previous Articles Next Articles
Wei ZHOU1( ), Jun NIE1, Jia HU2, Yizhi JIANG3, Dafa ZHANG1(
), Jun NIE1, Jia HU2, Yizhi JIANG3, Dafa ZHANG1( )
)
Received:2023-10-26
Online:2024-05-20
Published:2024-06-06
Contact:
Dafa ZHANG
E-mail:welson1987@163.com;zhangdafa2256@126.com
Supported by:Wei ZHOU, Jun NIE, Jia HU, Yizhi JIANG, Dafa ZHANG. Differential expressions of endoplasmic reticulum stress-associated genes in aortic dissection and their correlation with immune cell infiltration[J]. Journal of Southern Medical University, 2024, 44(5): 859-866.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.05.07
| Primers | Sequence (5'-3') |
|---|---|
| β-Actin | F:AGCGAGCATCCCCCAAAGTT; R:GGGCACGAAGGCTCATCATT |
| AGER | F: GTGTCCTTCCCAACGGCTC; R: ATTGCCTGGCACCGGAAAA |
Tab.1 Primer sequences for qRT-PCR
| Primers | Sequence (5'-3') |
|---|---|
| β-Actin | F:AGCGAGCATCCCCCAAAGTT; R:GGGCACGAAGGCTCATCATT |
| AGER | F: GTGTCCTTCCCAACGGCTC; R: ATTGCCTGGCACCGGAAAA |
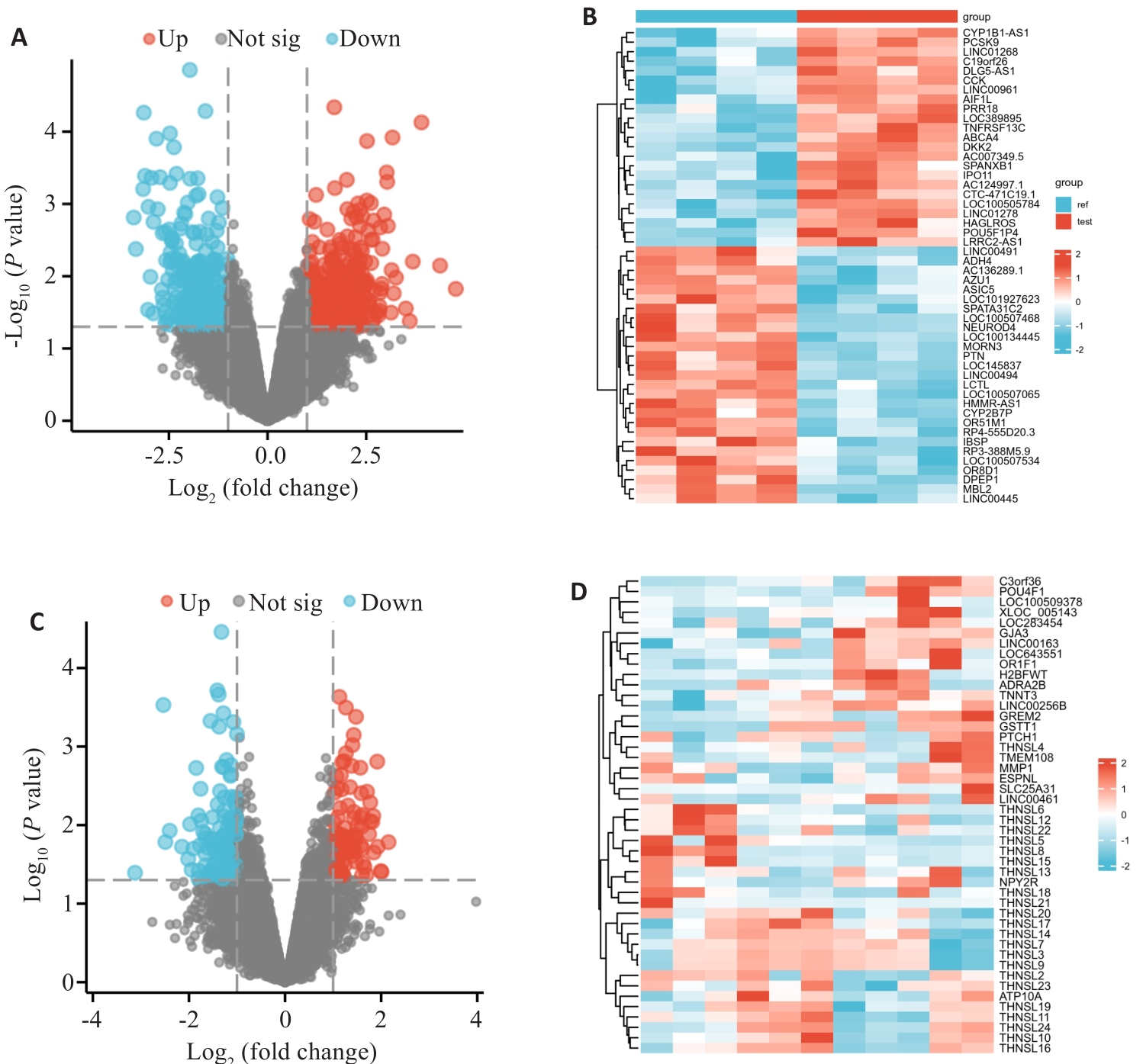
Fig.1 Differentially expressed genes (DEGs) in AD. A: Volcano plot of the DEGs in GSE190635. Red points represent up-regulated genes, and blue points represent down-regulated genes; Black points represent genes without significant difference (NS). B: Heatmap of DEGs in GSE190635. C: Volcano plot for the DEGs in GSE98770. D: Heatmap of the DEGs in GSE98770.
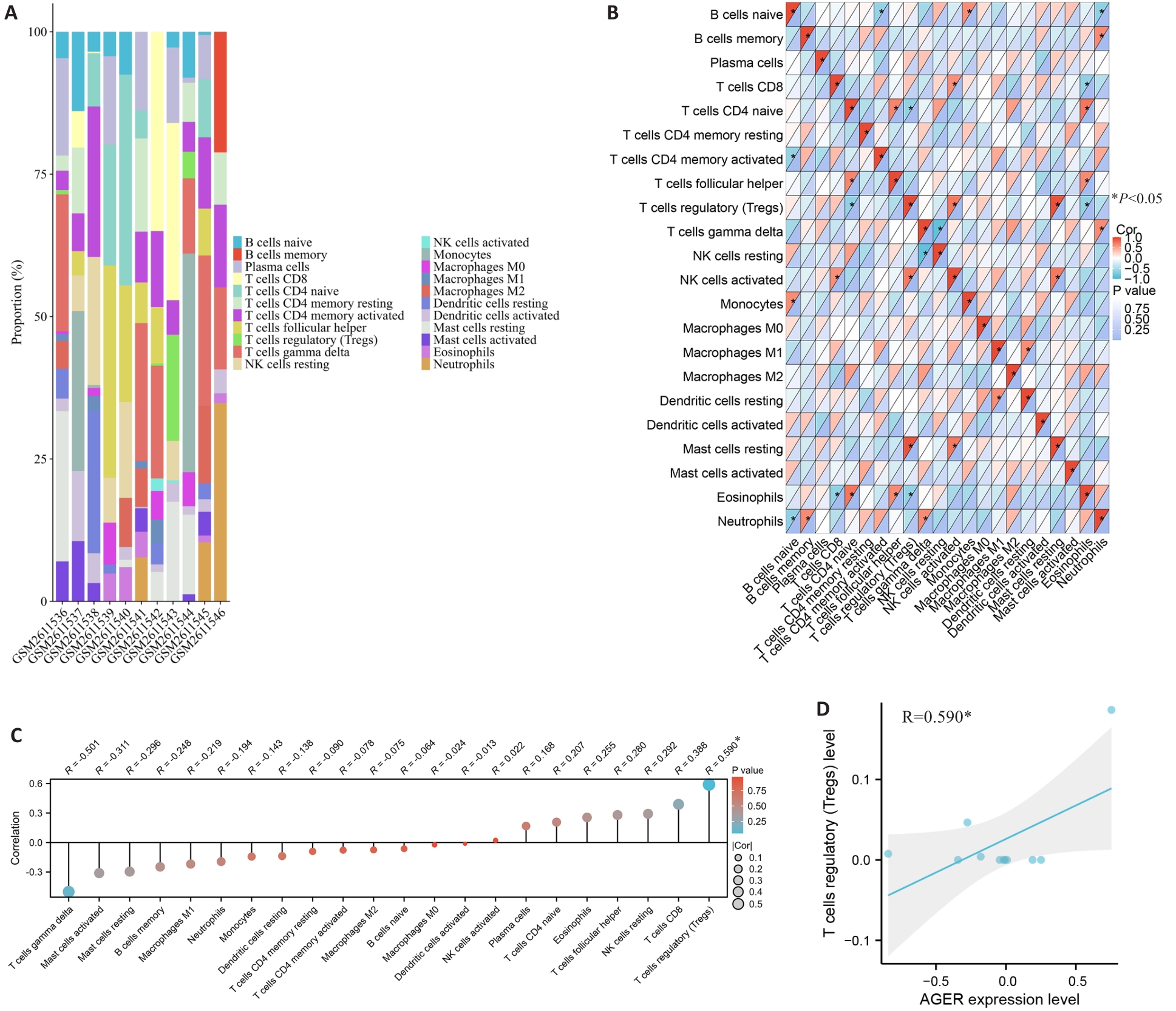
Fig.4 Immune cell infiltration analysis in the aorta of AD patients. A: CIBERSORT algorithm-based topography of the 22 immune cells in AD samples in GSE98770 dataset. B: Correlation analysis of the 22 immune cells. C: Correlation analysis of AGER with the 22 immune cells. D: Correlation analysis between Treg cell percentage and AGER expression level. Pearson method, *P<0.05.
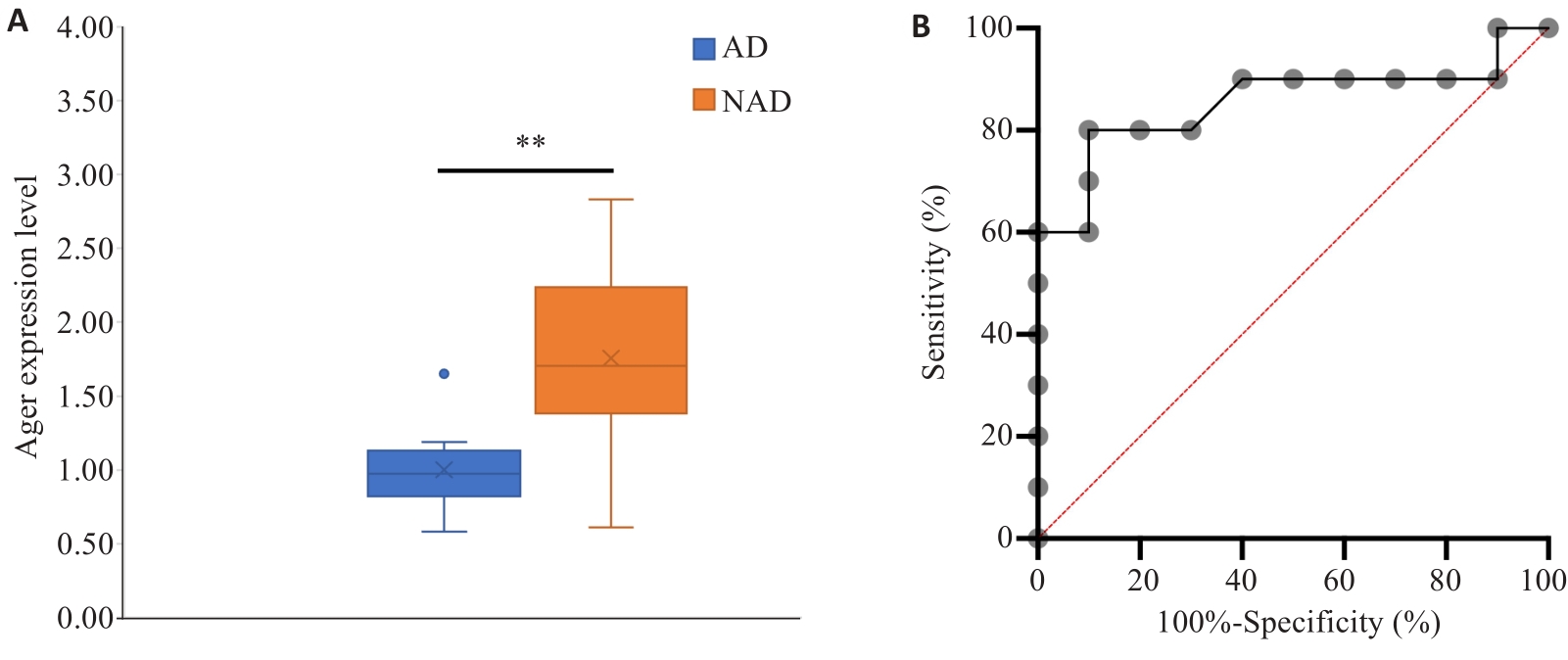
Fig.5 Validation of AGER mRNA expression in clinical aorta specimens. A: qRT-PCR detection of AGER expression in AD and NAD groups. B: ROC curve analysis. **P<0.01.
| TF | Target | Type | PMID |
|---|---|---|---|
| NFKB1 | AGER | Activation | 18622638;19616578 |
| PPARG | AGER | Repression | 18855759 |
| RELA | AGER | Activation | 18622638;19616578 |
Tab.2 Transcriptional factors (TF) of AGER
| TF | Target | Type | PMID |
|---|---|---|---|
| NFKB1 | AGER | Activation | 18622638;19616578 |
| PPARG | AGER | Repression | 18855759 |
| RELA | AGER | Activation | 18622638;19616578 |
| 1 | Mussa FF, Horton JD, Moridzadeh R, et al. Acute aortic dissection and intramural hematoma: a systematic review[J]. JAMA, 2016, 316(7): 754-63. DOI: 10.1001/jama.2016.10026 |
| 2 | Qin H, Wu H, Chen Y, et al. Early detection of postoperative acute kidney injury in acute stanford type A aortic dissection with Doppler renal resistive index[J]. J Ultrasound Med, 2017, 36(10): 2105-11. DOI: 10.1002/jum.14236 |
| 3 | Chiu P, Miller DC. Evolution of surgical therapy for Stanford acute type A aortic dissection[J]. Ann Cardiothorac Surg, 2016, 5(4): 275-95. DOI: 10.21037/acs.2016.05.05 |
| 4 | Alfson DB, Ham SW. Type B aortic dissections: current guidelines for treatment[J]. Cardiol Clin, 2017, 35(3): 387-410. DOI: 10.1016/j.ccl.2017.03.007 |
| 5 | Del Porto F, di Gioia C, Tritapepe L, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection[J]. Cardiology, 2014, 127(2): 123-9. DOI: 10.1159/000355253 |
| 6 | del Porto F, Proietta M, Tritapepe L, et al. Inflammation and immune response in acute aortic dissection[J]. Ann Med, 2010, 42(8): 622-9. DOI: 10.3109/07853890.2010.518156 |
| 7 | Balch WE, Morimoto RI, Dillin A, et al. Adapting proteostasis for disease intervention [J]. Science, 2008, 319: 916-9. DOI: 10.1126/science.1141448 |
| 8 | Hurtley SM, Bole DG, Hoover-Litty H, et al. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP)[J]. J Cell Biol, 1989, 108(6): 2117-26. DOI: 10.1083/jcb.108.6.2117 |
| 9 | Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum [J]. Science, 2011, 334: 1086-90. DOI: 10.1126/science.1209235 |
| 10 | Travers KJ, Patil CK, Wodicka L, et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation[J]. Cell, 2000, 101(3): 249-58. DOI: 10.1016/s0092-8674(00)80835-1 |
| 11 | Wang L, Song RD, Ma MH, et al. Inhibition of autophagy can promote the apoptosis of bladder cancer cells induced by SC66 through the endoplasmic reticulum stress pathway[J]. Chem Biol Interact, 2023, 384: 110725. DOI: 10.1016/j.cbi.2023.110725 |
| 12 | Choi SW, Cho W, Oh H, et al. Madecassoside ameliorates hepatic steatosis in high-fat diet-fed mice through AMPK/autophagy-mediated suppression of ER stress[J]. Biochem Pharmacol, 2023, 217: 115815. DOI: 10.1016/j.bcp.2023.115815 |
| 13 | Zhu X, Chen X, Shen X, et al. PP4R1 accelerates the malignant progression of NSCLC via up-regulating HSPA6 expression and HSPA6-mediated ER stress[J]. Biochim Biophys Acta Mol Cell Res, 2024, 1871(1): 119588. DOI: 10.1016/j.bbamcr.2023.119588 |
| 14 | Zhu ZW, Pu J, Li YN, et al. RBM25 regulates hypoxic cardiomyocyte apoptosis through CHOP-associated endoplasmic reticulum stress[J]. Cell Stress Chaperones, 2023, 28(6): 861-76. DOI: 10.1007/s12192-023-01380-7 |
| 15 | Zhao Y, Liu Y, Deng J, et al. Ginsenoside F4 alleviates skeletal muscle insulin resistance by regulating PTP1B in type II diabetes mellitus[J]. J Agric Food Chem, 2023, 71(39): 14263-75. DOI: 10.1021/acs.jafc.3c01262 |
| 16 | Zhu Q, Guo R, Liu C, et al. Endoplasmic reticulum stress-mediated apoptosis contributing to high glucose-induced vascular smooth muscle cell calcification[J]. J Vasc Res, 2015, 52(5): 291-8. DOI: 10.1159/000442980 |
| 17 | Deng BY, Liao F, Liu YH, et al. Comprehensive analysis of endoplasmic reticulum stress-associated genes signature of ulcerative colitis[J]. Front Immunol, 2023, 14: 1158648. DOI: 10.3389/fimmu.2023.1158648 |
| 18 | Franz M, Rodriguez H, Lopes C, et al. GeneMANIA update 2018[J]. Nucleic Acids Res, 2018, 46(W1): W60-4. DOI: 10.1093/nar/gky311 |
| 19 | Chen BB, Khodadoust MS, Liu CL, et al. Profiling tumor infiltrating immune cells with CIBERSORT[J]. Methods Mol Biol, 2018, 1711: 243-59. DOI: 10.1007/978-1-4939-7493-1_12 |
| 20 | Han H, Cho JW, Lee S, et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions[J]. Nucleic Acids Res, 2018, 46(D1): D380-6. DOI: 10.1093/nar/gkx1013 |
| 21 | Zhou GY, Soufan O, Ewald J, et al. NetworkAnalyst 3.0: a visual analytics platform for comprehensive gene expression profiling and meta-analysis[J]. Nucleic Acids Res, 2019, 47(W1): W234-41. DOI: 10.1093/nar/gkz240 |
| 22 | Chen HL, Luo SX, Chen HM, et al. ATF3 regulates SPHK1 in cardiomyocyte injury via endoplasmic reticulum stress[J]. Immun Inflamm Dis, 2023, 11(9): e998. DOI: 10.1002/iid3.998 |
| 23 | Zhou FY, Gao HY, Shang LR, et al. Oridonin promotes endoplasmic reticulum stress via TP53-repressed TCF4 transactivation in colorectal cancer[J]. J Exp Clin Cancer Res, 2023, 42(1): 150. DOI: 10.1186/s13046-023-02702-4 |
| 24 | Corica D, Pepe G, Currò M, et al. Methods to investigate advanced glycation end-product and their application in clinical practice[J]. Methods, 2022, 203: 90-102. DOI: 10.1016/j.ymeth.2021.12.008 |
| 25 | Mishra M, Prasad K. AGE-RAGE stress, stressors, and antistressors in health and disease[J]. Int J Angiol, 2018, 27(1): 1-12. DOI: 10.1055/s-0037-1613678 |
| 26 | Reddy VP, Aryal P, Darkwah EK. Advanced glycation end products in health and disease[J]. Microorganisms, 2022, 10(9): 1848. DOI: 10.3390/microorganisms10091848 |
| 27 | Fishman SL, Sonmez H, Basman C, et al. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review[J]. Mol Med, 2018, 24(1): 59-66. DOI: 10.1186/s10020-018-0060-3 |
| 28 | Burr SD, Harmon MB, Jr JAS. The impact of diabetic conditions and AGE/RAGE signaling on cardiac fibroblast migration[J]. Front Cell Dev Biol, 2020, 8: 112-23. DOI: 10.3389/fcell.2020.00112 |
| 29 | Grond-Ginsbach C, Pjontek R, Aksay SS, et al. Spontaneous arterial dissection: phenotype and molecular pathogenesis[J]. Cell Mol Life Sci, 2010, 67(11): 1799-815. DOI: 10.1007/s00018-010-0276-z |
| 30 | Raffort J, Lareyre F, Clément M, et al. Monocytes and macrophages in abdominal aortic aneurysm[J]. Nat Rev Cardiol, 2017, 14: 457-71. DOI: 10.1038/nrcardio.2017.52 |
| 31 | Golledge J, Karan M, Moran CS, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions[J]. Eur Heart J, 2008, 29(5): 665-72. DOI: 10.1093/eurheartj/ehm557 |
| 32 | Rao ZQ, Zheng YD, Xu L, et al. Endoplasmic reticulum stress and pathogenesis of vascular calcification[J]. Front Cardiovasc Med, 2022, 9: 918056. DOI: 10.3389/fcvm.2022.918056 |
| 33 | Kurihara T, Shimizu-Hirota R, Shimoda M, et al. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection[J]. Circulation, 2012, 126(25): 3070-80. DOI: 10.1161/circulationaha.112.097097 |
| 34 | Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6[J]. Nat Rev Cardiol, 2021, 18: 58-68. DOI: 10.1038/s41569-020-0431-7 |
| 35 | An Z, Qiao F, Lu QJ, et al. Interleukin-6 downregulated vascular smooth muscle cell contractile proteins via ATG4B-mediated autophagy in thoracic aortic dissection[J]. Heart Vessels, 2017, 32(12): 1523-35. DOI: 10.1007/s00380-017-1054-8 |
| 36 | Xu HJ, Li Y, Wang H, et al. Systemic immune-inflammation index predicted short-term outcomes in ATAD patients undergoing surgery[J]. J Card Surg, 2022, 37(4): 969-75. DOI: 10.1111/jocs.16300 |
| 37 | Ait-Oufella H, Wang Y, Herbin O, et al. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice[J]. Arterioscler Thromb Vasc Biol, 2013, 33(10): 2374-9. DOI: 10.1161/atvbaha.113.301280 |
| 38 | Hu XX, Schwarz JK, Lewis JS Jr, et al. A microRNA expression signature for cervical cancer prognosis[J]. Cancer Res, 2010, 70(4): 1441-8. DOI: 10.1158/0008-5472.can-09-3289 |
| 39 | Xu ZJ, Lang DH, Wang D, et al. LncRNA FGD5-AS1 promotes abdominal aortic aneurysm growth through the activation of MMP3 in vascular smooth muscle cells[J]. Int Heart J, 2023, 64(3): 470-82. DOI: 10.1536/ihj.22-106 |
| 40 | Wang Y, Zhang cheng-xin, Ge sheng-lin, et al. CTBP1-AS2 inhibits proliferation and inducesautophagy in ox-LDL-stimulated vascular smooth musclecells by regulating miR-195-5p/ATG14[J]. Int J Mol Med, 2020, 46(2): 839-48. DOI: 10.3892/ijmm.2020.4624 |
| [1] | Zhiwei ZUO, Qingliang MENG, Jiakang CUI, Kelei GUO, Hua BIAN. An artificial neural network diagnostic model for scleroderma and immune cell infiltration analysis based on mitochondria-associated genes [J]. Journal of Southern Medical University, 2024, 44(5): 920-929. |
| [2] | Bei PEI, Yi ZHANG, Siyuan WEI, Yu MEI, Biao SONG, Gang DONG, Ziang WEN, Xuejun LI. Identification of potential pathogenic genes of intestinal metaplasia based on transcriptomic sequencing and bioinformatics analysis [J]. Journal of Southern Medical University, 2024, 44(5): 941-949. |
| [3] | Qinzhi WANG, Bing SONG, Shirui HAO, Zhiyuan XIAO, Lianhui JIN, Tong ZHENG, Fang CHAI. Bioinformatic analysis of CCND2 expression in papillary thyroid carcinoma and its impact on immune infiltration [J]. Journal of Southern Medical University, 2024, 44(5): 981-988. |
| [4] | LIANG Yihao, LAI Yingjun, YUAN Yanwen, YUAN Wei, ZHANG Xibo, ZHANG Bashan, LU Zhifeng. Screening of differentially expressed genes in gastric cancer based on GEO database and function and pathway enrichment analysis [J]. Journal of Southern Medical University, 2024, 44(3): 605-616. |
| [5] | ZHANG Xiaoyan, WANG Xie, WANG Jie, SHAO Nan, CAI Biao, XIE Daojun. Huangpu Tongqiao Capsule improves cognitive impairment in rats with Wilson disease by inhibiting endoplasmic reticulum stress-mediated apoptosis pathway [J]. Journal of Southern Medical University, 2024, 44(3): 447-454. |
| [6] | CHENG Jiacong, LI Zhihui, LIU Yao, LI Cheng, HUANG Xin, TIAN Yinxin, SHEN Fubing. Bioinformatics analysis and validation of the interaction between PML protein and TAB1 protein [J]. Journal of Southern Medical University, 2024, 44(1): 179-186. |
| [7] | WANG Qiusheng, ZHANG Zhen, WANG Lian, WANG Yu, YAO Xinyu, WANG Yueyue, ZHANG Xiaofeng, GE Sitang, ZUO Lugen. High expression of death-associated protein 5 promotes glucose metabolism in gastric cancer cells and correlates with poor survival outcomes [J]. Journal of Southern Medical University, 2023, 43(7): 1063-1070. |
| [8] | ZHANG Weijian, ZOU Zhuoyue, ZHU Yongna, WANG Min, MA Caiyun, WU Junjie, SHI Xin, LIU Xi. Expression of interleukin-34 in tongue squamous cell carcinoma and its clinical implications [J]. Journal of Southern Medical University, 2023, 43(12): 2111-2117. |
| [9] | WANG Zining, XIAO Cangsong, LI Shuanglei, CHI Haitao, WANG Junhui, CHEN Lei, ZHAO Qiang, YANG Ming. Learning curve and clinical efficacy of aortic surgery through upper hemisternotomy [J]. Journal of Southern Medical University, 2023, 43(11): 1919-1925. |
| [10] | XIN Baoshan, LIU Gang, ZHANG Chengxiong, WANG Bing, SHI Lianghui. LncRNA LINC00342 regulates gastric cancer cell proliferation, migration and invasion by targeting miR-596 [J]. Journal of Southern Medical University, 2023, 43(10): 1761-1770. |
| [11] | LIU Zhiyu, FANG Fengzhu, LI Jing, ZHAO Guangyue, ZANG Quanjin, ZHANG Feng, DIE Jun. RHPN2 is highly expressed in osteosarcoma cells to promote cell proliferation and migration and inhibit apoptosis [J]. Journal of Southern Medical University, 2022, 42(9): 1367-1373. |
| [12] | ZHANG Wenjing, HU Zhaoting. Jiangtang Sanhuang tablet inhibits endoplasmic reticulum stress and autophagy in diabetic mouse islet cells [J]. Journal of Southern Medical University, 2022, 42(9): 1317-1323. |
| [13] | HONG Haining, ZHU Haonan, LI Chao, ZANG Chao, SANG Haiwei, CHEN Liwei, WANG Ansheng. FNDC1 is highly expressed in lung adenocarcinoma and closely related with poor prognosis [J]. Journal of Southern Medical University, 2022, 42(8): 1182-1190. |
| [14] | HUANG Ping, ZHU Jing, LI Hhua, WANG Yanzhao, TANG Yiming, LIU Qiang. Bioinformatic analysis of differentially expressed proteins in the dorsal raphe nucleus of rats after continuous treatment with olanzapine [J]. Journal of Southern Medical University, 2022, 42(8): 1221-1229. |
| [15] | LUO Lan, TONG Jiaqi, LI Lu, JIN Mu. Xenon post-conditioning protects against spinal cord ischemia-reperfusion injury in rats by downregulating mTOR pathway and inhibiting endoplasmic reticulum stress-induced neuronal apoptosis [J]. Journal of Southern Medical University, 2022, 42(8): 1256-1262. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||