南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (7): 1272-1283.doi: 10.12122/j.issn.1673-4254.2024.07.07
收稿日期:2024-02-15
出版日期:2024-07-20
发布日期:2024-07-25
通讯作者:
吴宁
E-mail:Chengyaocy01@163.com;wuning@gmc.edu.cn
作者简介:程 瑶,在读硕士研究生,E-mail: Chengyaocy01@163.com
基金资助:
Yao CHENG( ), Yuanying WANG, Feiyang YAO, Pan HU, Mingxian CHEN, Ning WU(
), Yuanying WANG, Feiyang YAO, Pan HU, Mingxian CHEN, Ning WU( )
)
Received:2024-02-15
Online:2024-07-20
Published:2024-07-25
Contact:
Ning WU
E-mail:Chengyaocy01@163.com;wuning@gmc.edu.cn
Supported by:摘要:
目的 探究2型登革病毒 (DENV-2)感染人脐静脉内皮细胞(HUVECs)对细胞自噬的影响及黄芩苷(BA)抗DENV-2感染的具体作用机制。 方法 HUVECs体外培养,设置对照组:HUVECs正常培养;DENV-2感染组:HUVECs+DENV-2;黄芩苷组:HUVECs+DENV-2+BA。用DENV-2感染HUVECs通过透射电镜观察自噬;Western blotting检测细胞自噬相关蛋白LC3、P62的表达,采用溶酶体红色荧光探针染色法观察DENV-2感染后HUVECs内溶酶体的pH变化;蛋白质组学筛查DENV-2感染HUVECs后差异蛋白的表达情况;使用CCK-8法测定黄芩苷对HUVECs活性的影响; RT-qPCR检测细胞内病毒RNA的复制情况;检测病毒NS1蛋白的表达情况;通过透射电镜观察细胞自噬;采用Lyso-Tracker Red染色法观察黄芩苷对DENV-2感染后HUVECs内溶酶体酸化的影响;检测细胞自噬相关蛋白ATG5、Beclin-1、LC3、P62的表达,自噬小体和溶酶体融合的关键蛋白STX17、SNAP29、VAMP8表达情况及PI3K/AKT信号通路相关蛋白的表达。 结果 DENV-2感染可诱导细胞自噬小体的形成;DENV-2感染后LC3 Ⅱ/LC3 I表达逐渐增高,48 h达到峰值(P<0.05);p62表达水平在感染前中期无明显变化,48 h后降低(P<0.05);Lysotracker red染色结果显示DENV-2感染组红色亮点较多,且染色明亮。CCK-8结果显示50 μg/mL被视为黄芩苷对HUVECs细胞的最大无毒剂量。RT-qPCR结果显示随着黄芩苷浓度增加药物对病毒的抑制作用增强。黄芩苷可降低DENV-2 NS1蛋白的表达(P<0.001)。与DENV-2组相比,经过黄芩苷(50 μg/mL)处理后,自噬减少,加入黄芩苷后抑制溶酶体酸化。黄芩苷组48 h LC3 II/LC3 I比值降低(P<0.05),48 h时p62表达增加(P<0.05)。经过黄芩苷处理后DENV-2诱导的自噬相关蛋白Beclin-1、ATG 5、STX17、SNAP29和VAMP8的表达下调(P<0.01)。蛋白组学结果显示PI3K-AKT通路在DENV的发病机制中可能发挥重要作用。加入PI3K抑制剂(LY294002)后,DENV-2的RNA表达水平降低,各组p-PI3K/PI3K、p-AKT/AKT、p-mTOR/mTOR蛋白相对表达水平的蛋白表达水平降低(P<0.01)。用黄芩苷处理后,DENV-2感染组PI3K、AKT的mRNA表达水平均显著降低(P<0.05),而mTOR mRNA表达无明显变化。DENV-2感染组上调的p-PI3K、p-AKT的蛋白表达水平均降低(P<0.05),而p-mTOR表达无明显变化。DENV-2诱导的LC3、P62蛋白表达降低(P<0.05);在黄芩苷治疗组中也抑制了病毒诱导的自噬。 结论 DENV-2感染可促进HUVECs细胞自噬的发生,黄芩苷可抑制DENV-2的RNA和NS1蛋白的表达,可能通过抑制自噬发生及自噬体和溶酶体融合,降低DENV-2诱导的自噬,其作用机制可能是通过PI3K/AKT信号通路调控。
程瑶, 王远迎, 姚飞扬, 胡盼, 陈铭勰, 吴宁. 黄芩苷通过调控PI3K/AKT信号通路抑制登革病毒感染诱导的人静脉内皮细胞的自噬[J]. 南方医科大学学报, 2024, 44(7): 1272-1283.
Yao CHENG, Yuanying WANG, Feiyang YAO, Pan HU, Mingxian CHEN, Ning WU. Baicalin suppresses type 2 dengue virus-induced autophagy of human umbilical vein endothelial cells by inhibiting the PI3K/AKT pathway[J]. Journal of Southern Medical University, 2024, 44(7): 1272-1283.
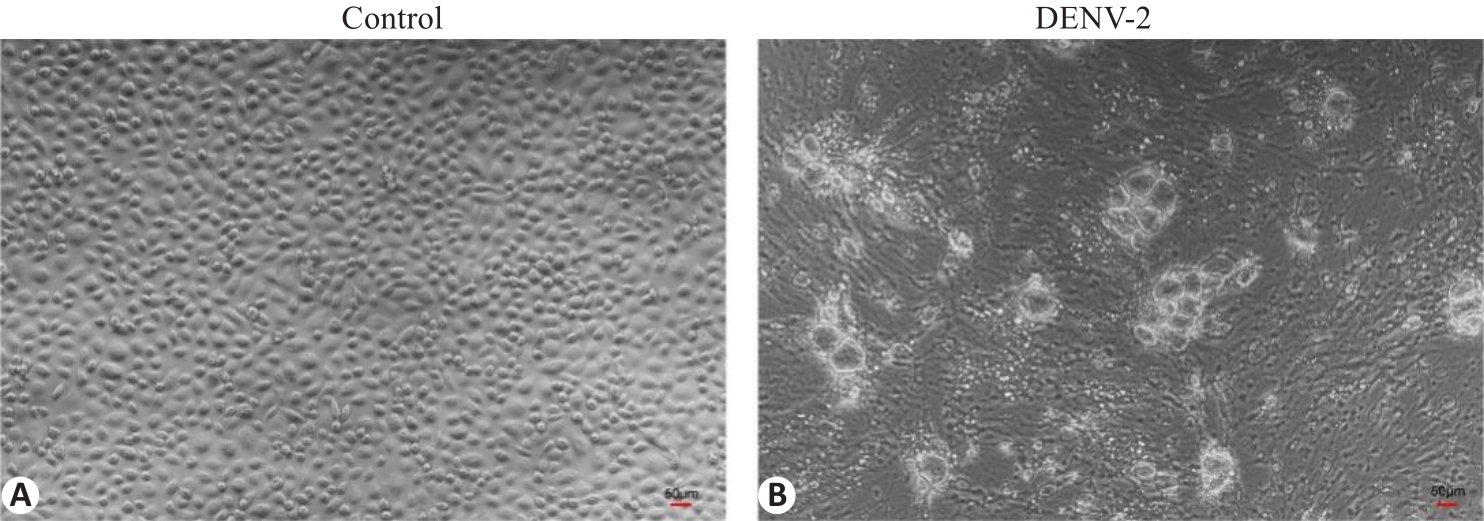
图1 DENV-2感染C6/36细胞病变图
Fig.1 Pathological changes of C6/36 cells infected by DENV-2 (Original magnification: ×100). A: Blank control cells. B: DENV-2-infected cells.
| Dilution of virus solution | Number of CPE occurrences | No CPE count | Total number | % of CPE holes present | |
|---|---|---|---|---|---|
| Number of CPE holes | No CPE count | ||||
| 10-3 | 8 | 0 | 34 | 0 | 100 |
| 10-4 | 8 | 0 | 26 | 0 | 100 |
| 10-5 | 8 | 0 | 18 | 0 | 100 |
| 10-6 | 8 | 0 | 10 | 2 | 100 |
| 10-7 | 2 | 6 | 2 | 6 | 25 |
| 10-8 | 0 | 8 | 0 | 14 | 0 |
| 10-9 | 0 | 8 | 0 | 22 | 0 |
| 10-10 | 0 | 8 | 0 | 30 | 0 |
表1 DENV-2病毒滴度结果统计表
Tab.1 Virulence of DENV-2 virus at different titers in C6/36 cells
| Dilution of virus solution | Number of CPE occurrences | No CPE count | Total number | % of CPE holes present | |
|---|---|---|---|---|---|
| Number of CPE holes | No CPE count | ||||
| 10-3 | 8 | 0 | 34 | 0 | 100 |
| 10-4 | 8 | 0 | 26 | 0 | 100 |
| 10-5 | 8 | 0 | 18 | 0 | 100 |
| 10-6 | 8 | 0 | 10 | 2 | 100 |
| 10-7 | 2 | 6 | 2 | 6 | 25 |
| 10-8 | 0 | 8 | 0 | 14 | 0 |
| 10-9 | 0 | 8 | 0 | 22 | 0 |
| 10-10 | 0 | 8 | 0 | 30 | 0 |
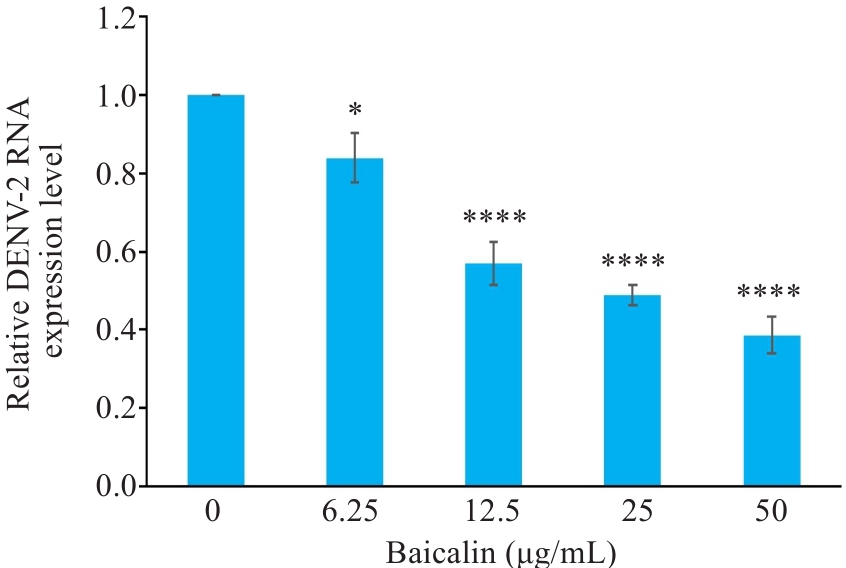
图6 黄芩苷降低DENV-2感染HUVECs的DENV-2 RNA相对表达量
Fig.6 Baicalin reduces relative expression of DENV-2 RNA in infected HUVECs. *P<0.05, ****P<0.001 vs 0 μg/mL group (n=3).
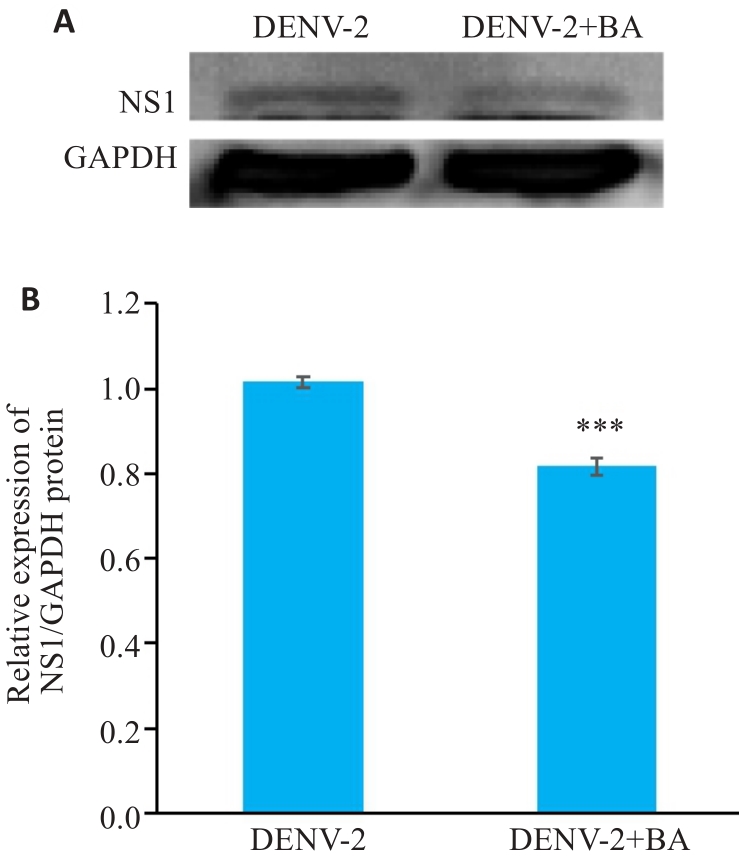
图7 黄芩苷抑制DENV-2 NS1蛋白表达水平
Fig.7 Western blots (A) and quantitative analysis (B) of the protein expressions of DENV-2 NS1 protein in HUVECs infected by DENV-2. ***P<0.001 vs DENV-2 group (n=3).
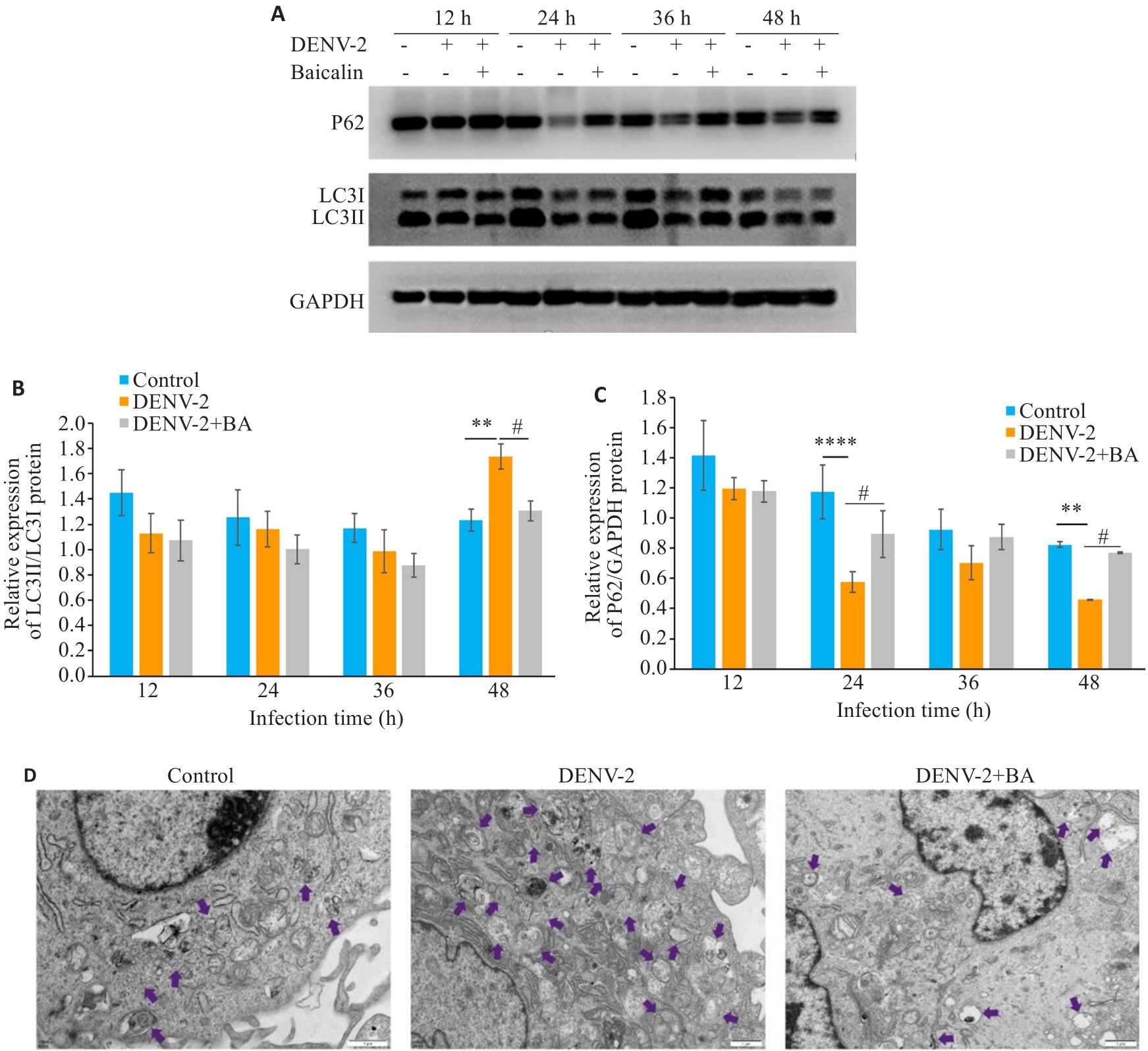
图8 黄芩苷抑制DENV-2感染HUVEs诱导的自噬
Fig.8 Baicalin inhibits autophagy induced by DENV-2 infection in HUVECs. A-C: Western blotting of protein expressions of LC3 and P62 in HUVECs infected by DENV-2. D: Transmission electron microscopy showing autophagy of HUVECs infected with DENV-2 after baicalin treatment (×15000). **P<0.01, ****P<0.001 vs Contorl group. #P<0.05 vs DENV-2 group (n=3).
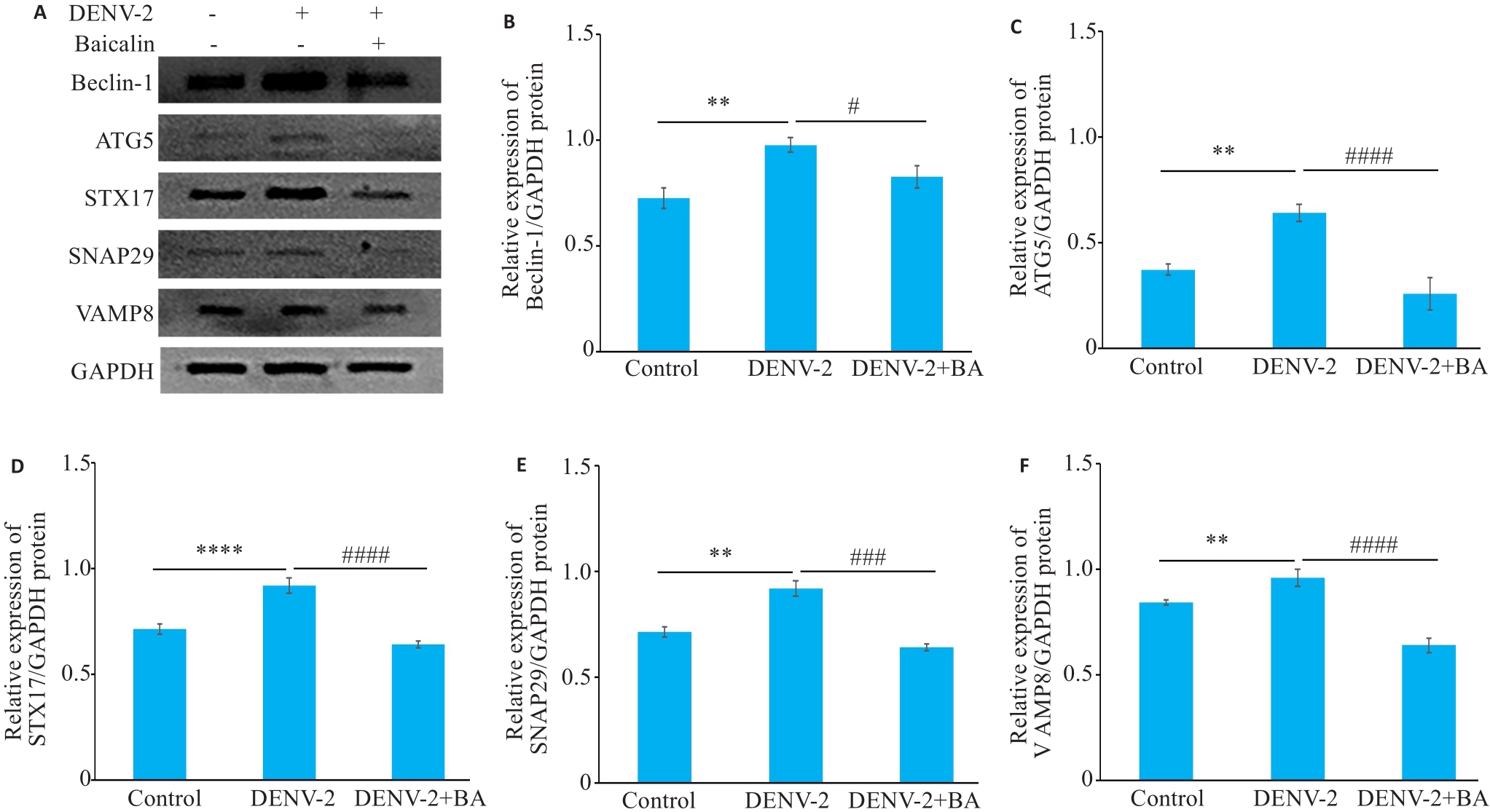
图10 黄芩苷抑制DENV-2感染HUVECs后自噬相关蛋白水平
Fig.10 Baicalin inhibits expressions of autophagy-related proteins in HUVECs infected with DENV-2. A: Western blotting of Beclin-1, ATG 5, STX17, SNAP29 and VAMP8 proteins. B-F: Relative expression levels of Beclin-1, ATG 5, STX17, SNAP29 and VAMP8 proteins. **P<0.01, ****P<0.001. #P<0.05, ###P<0.01, ####P<0.001 (n=3).
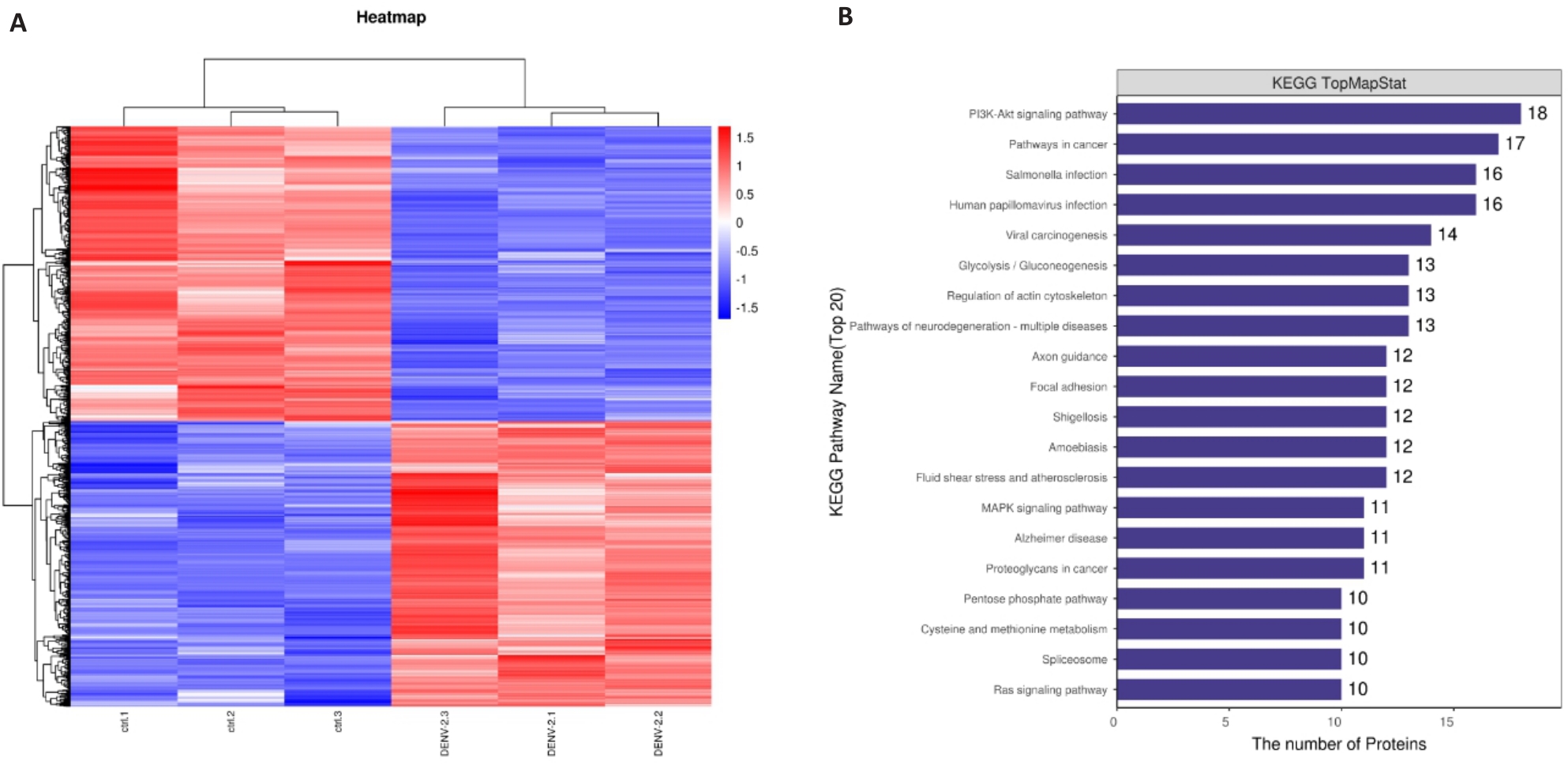
图11 DENV-2感染后HUEVCs蛋白质组学分析
Fig.11 Proteomic analysis of HUEVCs with DENV-2 infection. A: Heat map of differential proteins in healthy adults and patients with bronchial asthma. B: KEGG signaling pathways enrichment analysis.
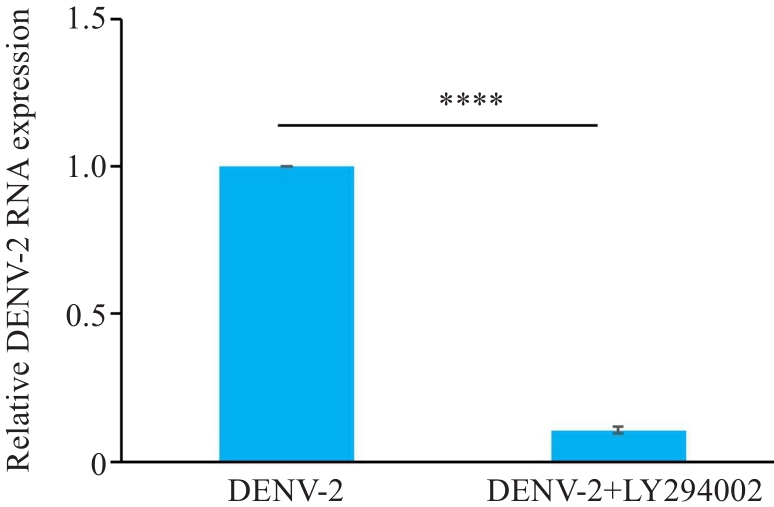
图12 LY294002降低DENV-2感染HUVECs的DENV-2 RNA相对表达量
Fig.12 LY294002 reduces relative expression of DENV-2 RNA in HUVECs infected with DENV-2. ****P<0.001 (n=3).
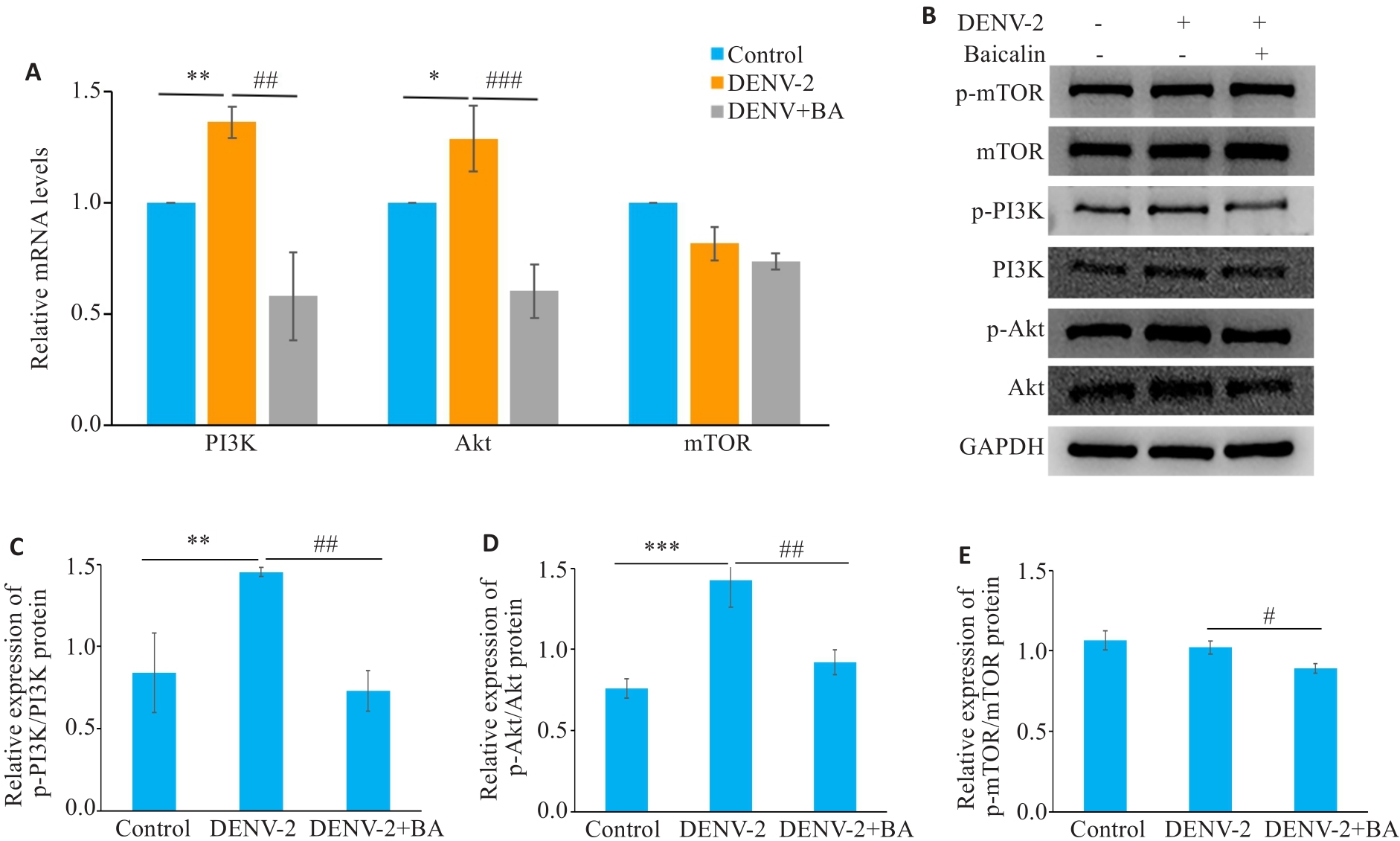
图13 黄芩苷抑制DENV-2诱导的PI3K/AKT信号通路的表达量
Fig.13 Baicalin inhibits the expression level of PI3K/AKT signaling pathway in DENV-2-infected HUVECs. A: Baicalin reduces the relative expressions of PI3K, AKT and mTOR mRNA in DENV-2-infected HUVECs. B: Baicalin inhibits the expression levels of PI3K/AKT signaling pathway-related proteins in DENV-2-infected HUVECs. C: Ratio of p-PI3K/PI3K protein expression. D: Ratio of p-AKT/AKT protein expression. E: Relative expressions of p-mTOR/mTOR protein. *P<0.05, **P<0.01, ***P<0.001; #P<0.05, ##P<0.01, ###P<0.001 (n=3).
| 1 | Rodriguez DM, Major CG, Sánchez-González L, et al. Dengue vaccine acceptability before and after the availability of COVID-19 vaccines in Puerto Rico[J]. Vaccine, 2023, 41(24): 3627-35. |
| 2 | Sarker A, Dhama N, Gupta RD. Dengue virus neutralizing antibody: a review of targets, cross-reactivity, and antibody-dependent enhancement[J]. Front Immunol, 2023, 14: 1200195. |
| 3 | Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology[J]. Can J Microbiol, 2021, 67(10): 687-702. |
| 4 | El Sahili A, Lescar J. Dengue virus non-structural protein 5[J]. Viruses, 2017, 9(4): 91. |
| 5 | Medeiros AS, Costa DMP, Branco MSD, et al. Dengue virus in Aedes aegypti and Aedes albopictus in urban areas in the state of Rio Grande do Norte, Brazil: importance of virological and entomological surveillance[J]. PLoS One, 2018, 13(3): e0194108. |
| 6 | Yu L, Wen YF, Xiang MR, et al. The limitation of rapid tests for DENV2 infection in host with unique immune status: low NS1 antigenemia and deficient antibody responses[J]. Virol Sin, 2020, 35(4): 478-80. |
| 7 | Li N, Li HD, Chen ZJ, et al. Estimating dengue transmission intensity in China using catalytic models based on serological data[J]. Trop Med Infect Dis, 2023, 8(2): 116. |
| 8 | Brady OJ, Hay SI. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus[J]. Annu Rev Entomol, 2020, 65: 191-208. |
| 9 | Lun XC, Yang R, Lin LH, et al. Effects of the source of information and knowledge of dengue fever on the mosquito control behavior of residents of border areas of Yunnan, China[J]. Parasit Vectors, 2023, 16(1): 311. |
| 10 | Moghaddam E, Teoh BT, Sam SS, et al. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus[J]. Sci Rep, 2014, 4: 5452. |
| 11 | Bao M, Ma Y, Liang M, et al. Research progress on pharmacological effects and new dosage forms of baicalin[J]. Vet Med Sci. 2022, 8(6):2773-84. |
| 12 | Zandi K, Teoh BT, Sam SS, et al. Novel antiviral activity of baicalein against dengue virus[J]. BMC Complement Altern Med, 2012, 12: 214. |
| 13 | Dhiman M, Sharma L, Dadhich A, et al. Traditional knowledge to contemporary medication in the treatment of infectious disease dengue: a review[J]. Front Pharmacol, 2022, 13: 750494. |
| 14 | Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life[J]. Cells, 2018, 7(12): E278. |
| 15 | Lee YR, Lei HY, Liu MT, et al. Autophagic machinery activated by dengue virus enhances virus replication[J]. Virology, 2008, 374(2): 240-8. |
| 16 | Panyasrivanit M, Khakpoor A, Wikan N, et al. Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes[J]. J Gen Virol, 2009, 90(Pt 2): 448-56. |
| 17 | Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism[J]. Cell Host Microbe, 2010, 8(5): 422-32. |
| 18 | McLean JE, Wudzinska A, Datan E, et al. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication[J]. J Biol Chem, 2011, 286(25): 22147-59. |
| 19 | Zhang QW, Sun JX, Wang YL, et al. Antimycobacterial and anti-inflammatory mechanisms of baicalin via induced autophagy in macrophages infected with Mycobacterium tuberculosis [J]. Front Microbiol, 2017, 8: 2142. |
| 20 | Kang CY, Wang LB, Kang MY, et al. Baicalin alleviates 6-hydroxydopamine-induced neurotoxicity in PC12 cells by down-regulation of microRNA-192-5p[J]. Brain Res, 2019, 1708: 84-92. |
| 21 | WHO [(accessed on 17 March 2023)]. Available online: . |
| 22 | Yi BW, Chew BXZ, Chen HX, et al. Antiviral activity of catechin against dengue virus infection[J]. Viruses, 2023, 15(6): 1377. |
| 23 | Bhatt P, Sabeena SP, Varma M, et al. Current understanding of the pathogenesis of dengue virus infection[J]. Curr Microbiol, 2021, 78(1): 17-32. |
| 24 | Uno N, Ross TM. Dengue virus and the host innate immune response[J]. Emerg Microbes Infect, 2018, 7(1): 167. |
| 25 | Wu SY, Chen YL, Lee YR, et al. The autophagosomes containing dengue virus proteins and full-length genomic RNA are infectious[J]. Viruses, 2021, 13(10): 2034. |
| 26 | Oo A, Teoh BT, Sam SS, et al. Baicalein and baicalin as Zika virus inhibitors[J]. Arch Virol, 2019, 164(2): 585-93. |
| 27 | Tramontini Gomes de Sousa Cardozo F, Baimukanova G, Lanteri MC, et al. Serum from dengue virus-infected patients with and without plasma leakage differentially affects endothelial cells barrier function in vitro [J]. PLoS One, 2017, 12(6): e0178820. |
| 28 | Anasir MI, Poh CL. Discovery of B-cell epitopes for development of dengue vaccines and antibody therapeutics[J]. Med Microbiol Immunol, 2022, 211(1): 1-18. |
| 29 | Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication[J]. Virology, 1996, 220(1): 232-40. |
| 30 | Zhu HY, Han L, Shi XL, et al. Baicalin inhibits autophagy induced by influenza A virus H3N2[J]. Antiviral Res, 2015, 113: 62-70. |
| 31 | Oo A, Rausalu K, Merits A, et al. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection[J]. Antiviral Res, 2018, 150: 101-11. |
| 32 | 金 跃, 李 祥, 王晓丽, 等. 黄芩苷体外抑制柯萨奇病毒B组3型感染的机制研究[J]. 南京医科大学学报: 自然科学版, 2019, 39(2): 196-200. |
| 33 | Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms[J]. Autophagy, 2018, 14(2): 207-15. |
| 34 | Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persister cells[J]. Nat Rev Microbiol, 2017, 15: 453-64. |
| 35 | Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion[J]. Autophagy, 2021, 17(10): 2680-8. |
| 36 | Xie YB, Shi XF, Sheng K, et al. PI3K/akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review)[J]. Mol Med Rep, 2019, 19(2): 783-91. |
| 37 | Wang BY, Zhong Y, Li QJ, et al. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy[J]. Aging, 2018, 10(10): 2772-82. |
| 38 | Lee CJ, Liao CL, Lin YL. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection[J]. J Virol, 2005, 79(13): 8388-99. |
| 39 | Kang CY, Wang LB, Kang MY, et al. Baicalin alleviates 6-hydroxydopamine-induced neurotoxicity in PC12 cells by down-regulation of microRNA-192-5p[J]. Brain Res, 2019, 1708: 84-92. |
| [1] | 李玮怡, 江露, 张宗星, 陈丹, 包卓玛, 黄丽, 袁林. 强骨康疏方通过抑制HIF-1α/BNIP3自噬信号通路减少类风湿性关节炎大鼠的破骨细胞分化[J]. 南方医科大学学报, 2025, 45(7): 1389-1396. |
| [2] | 吴璇, 方家敏, 韩玮玮, 陈琳, 孙菁, 金齐力. 高表达PRELID1促进胃癌细胞上皮间质转化并与不良预后相关[J]. 南方医科大学学报, 2025, 45(7): 1535-1542. |
| [3] | 王心恒, 邵小涵, 李童童, 张璐, 杨勤军, 叶卫东, 童佳兵, 李泽庚, 方向明. 平喘宁方通过调控HMGB1/Beclin-1轴介导的自噬改善患寒哮证大鼠的气道炎症[J]. 南方医科大学学报, 2025, 45(6): 1153-1162. |
| [4] | 陈悦, 肖林雨, 任侣, 宋雪, 李静, 胡建国. 水晶兰苷通过抑制PI3K/AKT信号通路减少神经元凋亡改善脊髓损伤后小鼠的运动功能[J]. 南方医科大学学报, 2025, 45(4): 774-784. |
| [5] | 储菲, 陈孝华, 宋博文, 杨晶晶, 左芦根. 苏荠宁黄酮通过抑制PI3K/AKT信号通路拮抗肠上皮细胞凋亡改善小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(4): 819-828. |
| [6] | 董妍妍, 张可敬, 储俊, 储全根. 抵当汤含药血清通过PI3K/Akt/mTOR信号通路增强高糖诱导的大鼠肾小球内皮细胞自噬[J]. 南方医科大学学报, 2025, 45(3): 461-469. |
| [7] | 廖茗, 钟文华, 张冉, 梁娟, 徐文陶睿, 万文珺, 吴超, 李曙. 源自蛇毒的蛋白C激活剂通过调控HIF-1α抑制BNIP3活性氧生成保护人脐静脉内皮细胞免受缺氧-复氧损伤[J]. 南方医科大学学报, 2025, 45(3): 614-621. |
| [8] | 黄菊, 殷丽霞, 牛民主, 耿志军, 左芦根, 李静, 胡建国. 紫花前胡苷通过抑制肠上皮细胞焦亡改善2,4,6-三硝基苯磺酸诱导的小鼠实验性结肠炎[J]. 南方医科大学学报, 2025, 45(2): 261-268. |
| [9] | 徐皓男, 张放, 黄钰莹, 姚其盛, 管悦琴, 陈浩. 百蕊草通过调节肠道菌群和调控EGFR/PI3K/Akt信号通路改善小鼠抗生素相关性腹泻[J]. 南方医科大学学报, 2025, 45(2): 285-295. |
| [10] | 裴月娇, 刘慧敏, 昕宇, 刘波. miR-124通过调控PI3K/AKT信号通路改善睡眠剥夺大鼠认知功能[J]. 南方医科大学学报, 2025, 45(2): 340-346. |
| [11] | 郭克磊, 李颖利, 宣晨光, 侯紫君, 叶松山, 李林运, 陈丽平, 韩立, 卞华. 益气养阴化浊通络方通过调控miR-21a-5p/FoxO1/PINK1介导的线粒体自噬减轻糖尿病肾病小鼠的足细胞损伤[J]. 南方医科大学学报, 2025, 45(1): 27-34. |
| [12] | 展俊平, 黄硕, 孟庆良, 范围, 谷慧敏, 崔家康, 王慧莲. 缺氧微环境下补阳还五汤通过抑制BNIP3-PI3K/Akt通路抑制类风湿关节炎滑膜成纤维细胞的线粒体自噬[J]. 南方医科大学学报, 2025, 45(1): 35-42. |
| [13] | 陈志亮, 杨永刚, 黄霞, 成彦, 瞿媛, 衡琪琪, 符羽佳, 李可薇, 顾宁. 外泌体miRNA差异表达可作为诊断慢性心力衰竭合并高尿酸血症患者新型分子标志物及靶基因功能分析[J]. 南方医科大学学报, 2025, 45(1): 43-51. |
| [14] | 张玉如, 万磊, 方昊翔, 李方泽, 王丽文, 李柯霏, 闫佩文, 姜辉. miR-155-5p介导PIK3R1负调控PI3K/AKT信号通路促进原发性干燥综合征人唾液腺上皮细胞增殖[J]. 南方医科大学学报, 2025, 45(1): 65-71. |
| [15] | 左涵珺, 段兆达, 王朝, 郭涛, 石金沙, 石浩龙, 李娟娟. 天麻素经PI3K/AKT通路改善新生大鼠缺氧缺血性脑损伤后小胶质细胞介导的炎症反应[J]. 南方医科大学学报, 2024, 44(9): 1712-1719. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||