南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (8): 1672-1681.doi: 10.12122/j.issn.1673-4254.2025.08.12
• • 上一篇
张潇1( ), 满景洲1, 张勇2, 郑云剑2, 王和平1, 袁怡君1(
), 满景洲1, 张勇2, 郑云剑2, 王和平1, 袁怡君1( ), 谢曦1(
), 谢曦1( )
)
收稿日期:2025-01-21
出版日期:2025-08-20
发布日期:2025-09-05
通讯作者:
袁怡君,谢曦
E-mail:740597089@qq.com;yijun.yuan@hainanu.edu.cn;xiexi@hainanu.edu.cn
作者简介:张 潇,在读硕士研究生,E-mail: 740597089@qq.com
基金资助:
Xiao ZHANG1( ), Jingzhou MAN1, Yong ZHANG2, YunJian ZHENG2, Heping WANG1, Yijun YUAN1(
), Jingzhou MAN1, Yong ZHANG2, YunJian ZHENG2, Heping WANG1, Yijun YUAN1( ), Xi XIE1(
), Xi XIE1( )
)
Received:2025-01-21
Online:2025-08-20
Published:2025-09-05
Contact:
Yijun YUAN, Xi XIE
E-mail:740597089@qq.com;yijun.yuan@hainanu.edu.cn;xiexi@hainanu.edu.cn
摘要:
目的 通过博来霉素(BLM)诱导的小鼠肺纤维化模型和TGF-β1诱导的人胚肺成纤维细胞(HFL1)模型探究雨生红球藻(HP)对肺纤维化的影响。 方法 将30只C57BL/6雄性小鼠随机分为5组:对照组(Control)、模型组(BLM)、HP低剂量组(BLM +HP 3 mg/kg)、HP高剂量组(BLM+HP 21 mg/kg)、阳性药物吡非尼酮(PFD)组(BLM + PFD 300 mg/kg),n=6。21 d后检测小鼠呼吸功能、肺组织病理学变化以及纤维化标志物的表达。在5 ng/mL TGF-β1诱导的HFL1模型,分别给予低、中、高剂量的 HP(120、180、240 μg/mL),以PFD(1.85 μg/mL)作为阳性对照药物,培养48 h后,检测纤维化标志物的表达水平。正常组、模型组、HP高剂量组进行mRNA测序和分析。 结果 HP在呼吸功能、肺组织形态结构、炎性浸润、胶原纤维沉积和纤维化蛋白质的表达方面均能减轻BLM引起的肺功能损伤和组织形态结构的纤维化,且高剂量HP表现出与PFD相似的效果。体外实验也显示,与模型组相比,高剂量HP可降低α-SMA(P<0.01)和FN(P<0.05)的蛋白质表达,以及α-SMA(P<0.05)和FN(P<0.01)mRNA表达,进一步转录组分析显示多个可能参与HP调控肺纤维化的关键基因和通路。 结论 HP粉末在体内外均表现出改善肺纤维化的作用,可能通过多种活性物质发挥协同作用,这为肺纤维化疾病的预防和治疗提供了研究基础。
张潇, 满景洲, 张勇, 郑云剑, 王和平, 袁怡君, 谢曦. 雨生红球藻抑制肺成纤维细胞向肌成纤维细胞转化改善博来霉素诱导的小鼠肺纤维化[J]. 南方医科大学学报, 2025, 45(8): 1672-1681.
Xiao ZHANG, Jingzhou MAN, Yong ZHANG, YunJian ZHENG, Heping WANG, Yijun YUAN, Xi XIE. Haematococcus pluvialis alleviates bleomycin-induced pulmonary fibrosis in mice by inhibiting transformation of lung fibroblasts into myofibroblast[J]. Journal of Southern Medical University, 2025, 45(8): 1672-1681.
| Primer | Forward primer sequence (5'-3') | Reverse primer sequence (5'-3') |
|---|---|---|
| Mouse Col1 | CTTTGCTTCCCAGATGTCCT | CGGTGTCCCTTCATTCCAG |
| Mouse Col3 | GGCAGTGATGGGCAACCT | TCCCTTCGCACCGTTCTT |
| Mouse α-SMA | GGGAGTAATGGTTGGAATG | CTCAAACATAATCTGGGTCA |
| Mouse FN1 | CATTGTTACCAACTGGGACG | CCAGAGGCATACAGGGAC |
| Mouse Col5 | GGACTCGGCGGAACATTG | GGGAGTTGAGGGAACCAAA |
| Mouse GAPDH | TGTTTCCTCGTCCCGTAG | CAATCTCCACTTTGCCACT |
| Human Col1 | AAGGTGTTGTGCGATGACG | TGGTCGGTGGGTGACTCTG |
| Human Col3 | GAGCTGGCTACTTCTCGC | TCTATCCGCATAGGACTGAC |
| Human α-SMA | GGGGTGATGGTGGGAATG | GCAGGGTGGGATGCTCTT |
| Human FN1 | ATGGAGGAAGCCGAGGTT | AGCGGTTTGCGATGGTAC |
| Human Col5 | TGGCAAGTGGCACAGAATT | TCACCCTCAAACACCTCCTC |
| Human β-actin | GGAAATCGTGCGTGACATT | CAGGCAGCTCGTAGCTCTT |
表1 引物序列
Tab.1 Primer sequences for qRT-PCR
| Primer | Forward primer sequence (5'-3') | Reverse primer sequence (5'-3') |
|---|---|---|
| Mouse Col1 | CTTTGCTTCCCAGATGTCCT | CGGTGTCCCTTCATTCCAG |
| Mouse Col3 | GGCAGTGATGGGCAACCT | TCCCTTCGCACCGTTCTT |
| Mouse α-SMA | GGGAGTAATGGTTGGAATG | CTCAAACATAATCTGGGTCA |
| Mouse FN1 | CATTGTTACCAACTGGGACG | CCAGAGGCATACAGGGAC |
| Mouse Col5 | GGACTCGGCGGAACATTG | GGGAGTTGAGGGAACCAAA |
| Mouse GAPDH | TGTTTCCTCGTCCCGTAG | CAATCTCCACTTTGCCACT |
| Human Col1 | AAGGTGTTGTGCGATGACG | TGGTCGGTGGGTGACTCTG |
| Human Col3 | GAGCTGGCTACTTCTCGC | TCTATCCGCATAGGACTGAC |
| Human α-SMA | GGGGTGATGGTGGGAATG | GCAGGGTGGGATGCTCTT |
| Human FN1 | ATGGAGGAAGCCGAGGTT | AGCGGTTTGCGATGGTAC |
| Human Col5 | TGGCAAGTGGCACAGAATT | TCACCCTCAAACACCTCCTC |
| Human β-actin | GGAAATCGTGCGTGACATT | CAGGCAGCTCGTAGCTCTT |
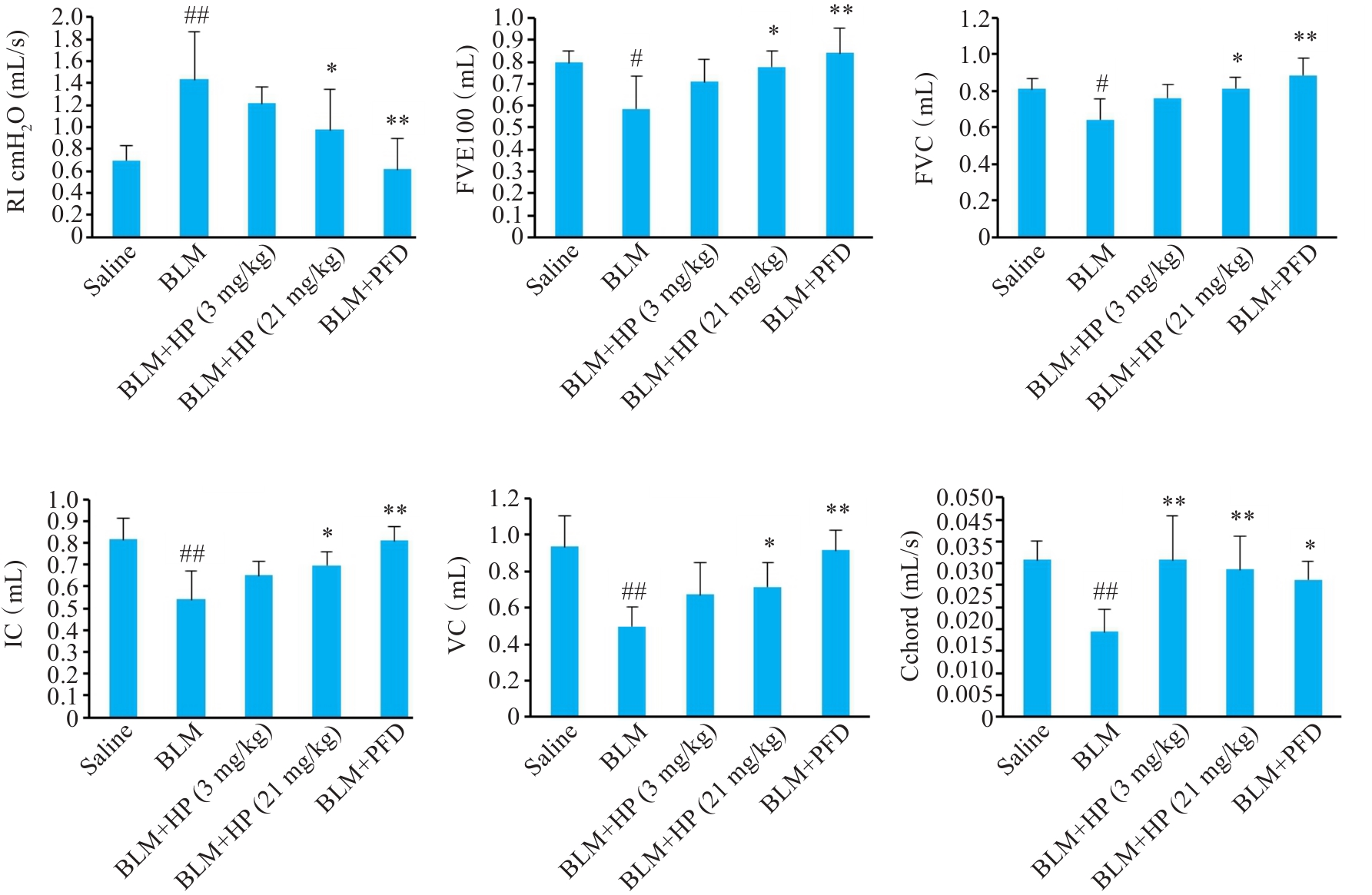
图1 HP对博来霉素诱导的小鼠肺纤维化模型肺呼吸功能影响
Fig.1 Effect of HP on pulmonary respiratory function in bleomycin-induced mouse pulmonary fibrosis model. Saline: Control group; BLM: Bleomycin-induced model group; BLM+HP (3 mg/kg): Low-dose HP group; BLM+HP (21 mg/kg): High-dose HP group; BLM+PFD: Positive drug pirfenidone control group. #P<0.05, ##P<0.01 vs Saline group; *P<0.05, **P<0.01 vs BLM group.
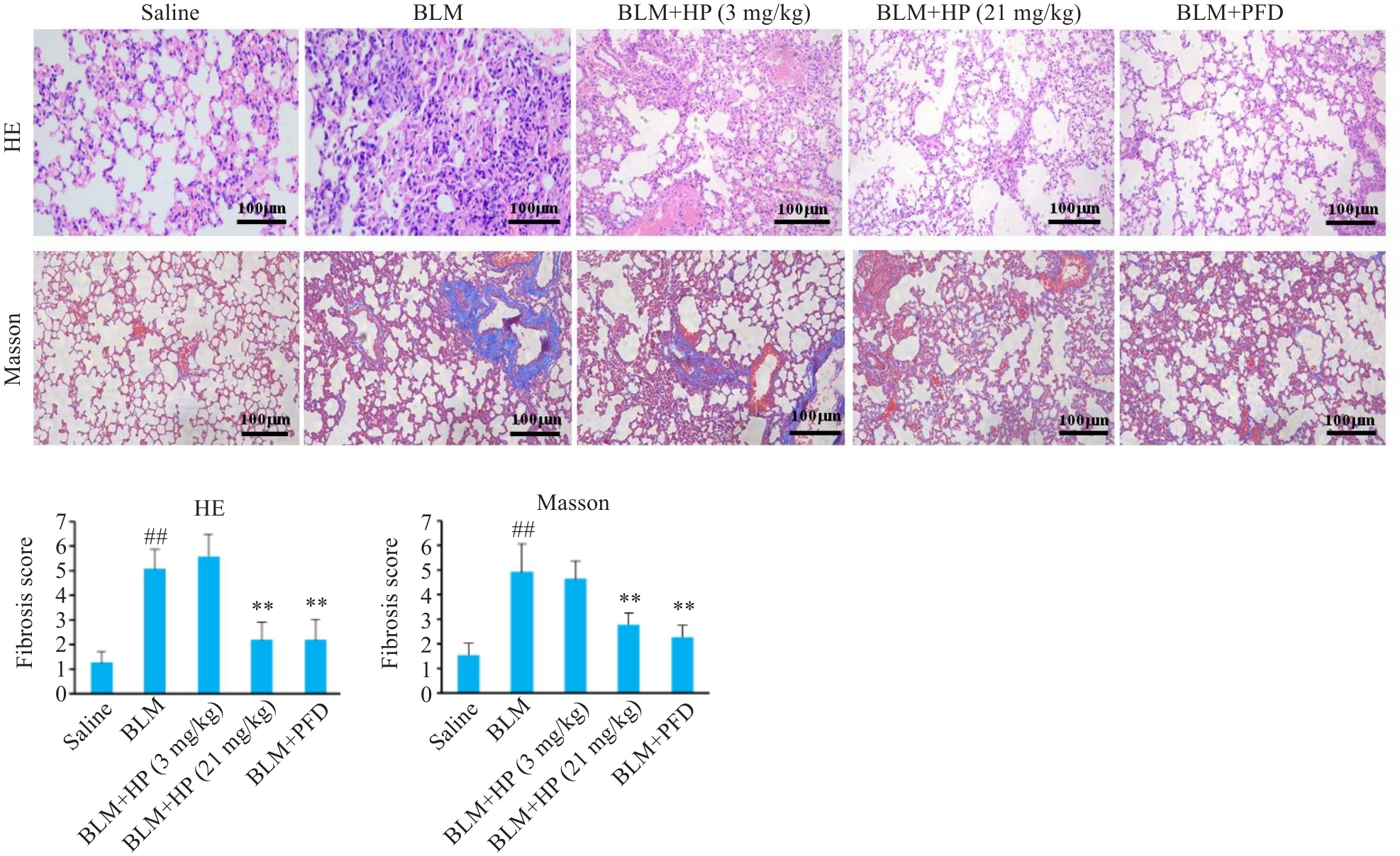
图2 HP对博来霉素诱导的小鼠肺纤维化模型肺组织形态和胶原沉积的影响
Fig.2 Effects of HP on lung tissue histology and collagen deposition in mice with bleomycin-induced pulmonary fibrosis. ##P<0.01 vs Saline group; **P<0.01 vs BLM group.
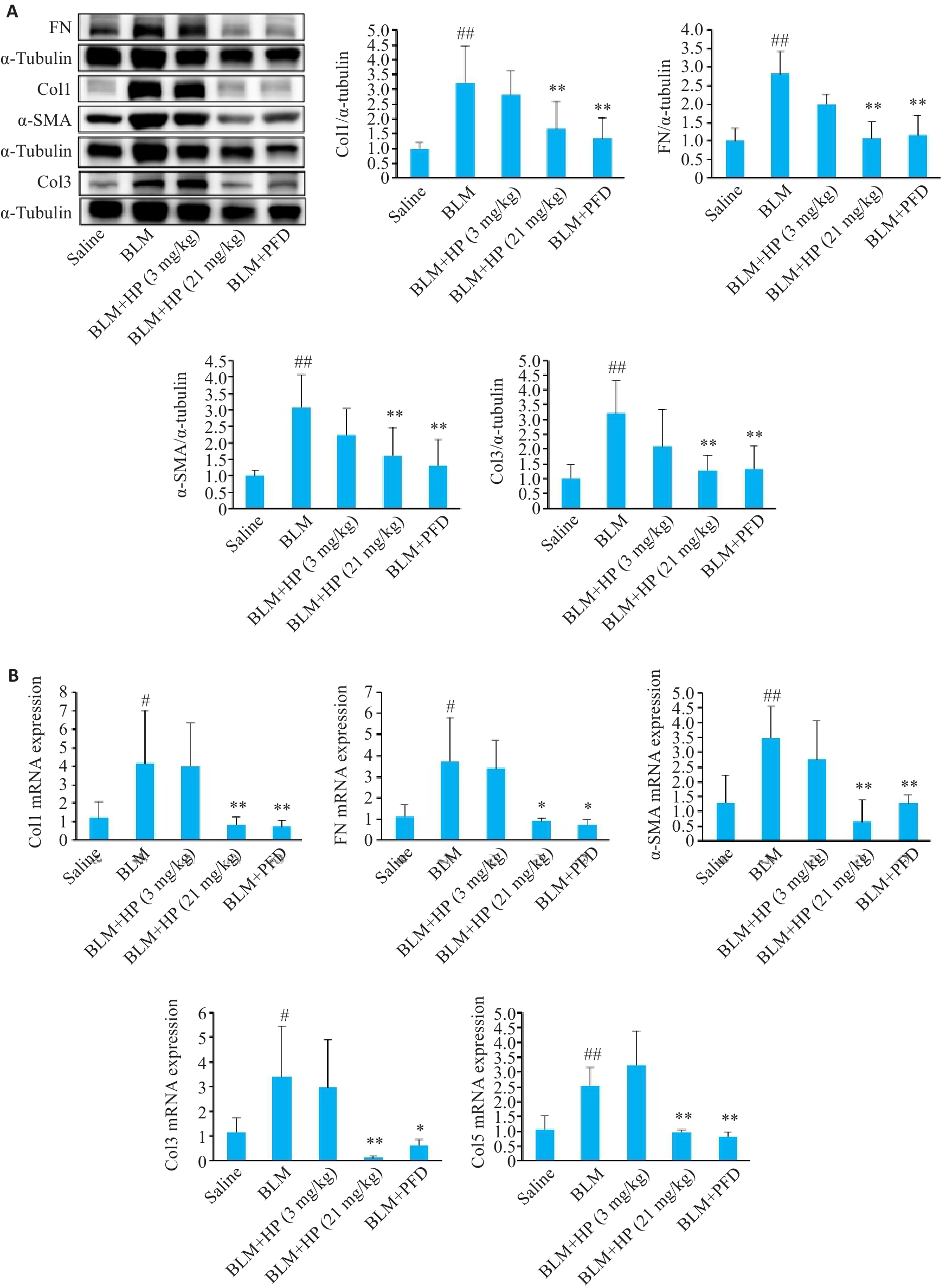
图3 Western blotting(A)和qRT-PCR(B)检测HP对博来霉素诱导的小鼠肺纤维化模型肺组织纤维化蛋白表达水平的影响
Fig.3 Western blotting (A) and qRT-PCR (B) for assessing the effect of HP on expressions of fibrotic proteins in the lung tissue of mice with bleomycin-induced pulmonary fibrosis. #P<0.05, ##P<0.01 vs saline group; *P<0.05, **P<0.01 vs BLM group.
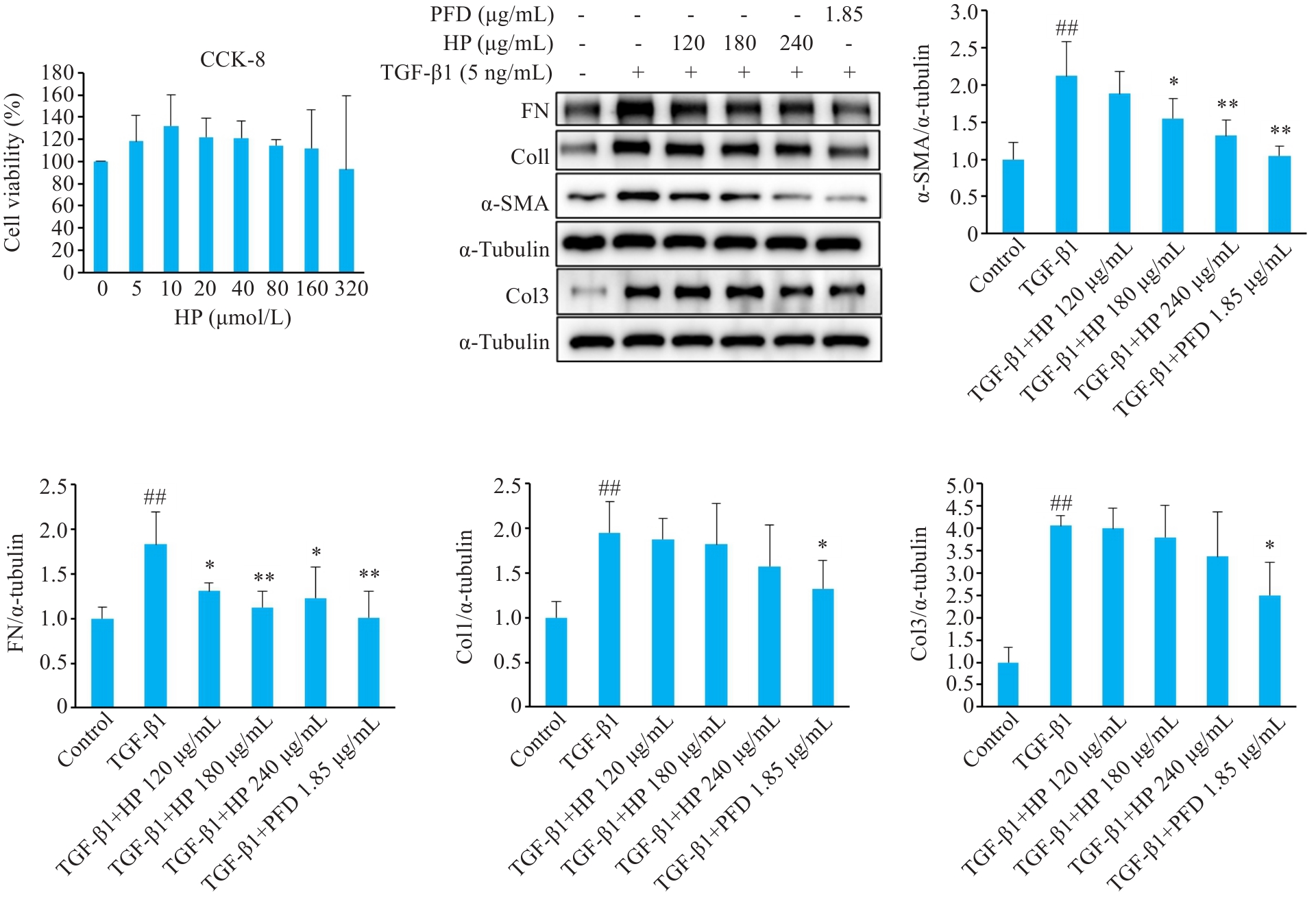
图4 不同浓度的HP对TGF-β1诱导的HFL1细胞模型纤维化蛋白表达水平的影响
Fig.4 Effect of different concentrations of HP on expression levels of fibrotic proteins in TGF-β1-induced HFL1 cell model. CCK-8 was used for detecting changes in proliferation of HFL1 cells treated with different concentrations of HP. Western blotting was used to detect the expression levels of fibrotic proteins of the treated cells. Except for the control group, all the other groups were induced with 5 ng/mL TGF-β1. ##P<0.01 vs Control group; *P<0.05, **P<0.01 vs TGF-β1 group.

图5 免疫荧光(A)和qRT-PCR(B)检测高剂量HP(240 μg/mL)对TGF-β1诱导的HFL1细胞模型纤维化蛋白表达水平的影响
Fig.5 Immunofluorescence (A) and qRT-PCR (B) for detecting the effects of high-dose HP (240 μg/mL) on expression levels of fibrotic proteins in TGF-β1-induced HFL1 cells. Model: TGF-β1-induced cells; HP: Cells treated with 240 μg/mL HP. #P<0.05, ##P<0.01 vs Control group. *P<0.05, **P<0.01 vs Model group.
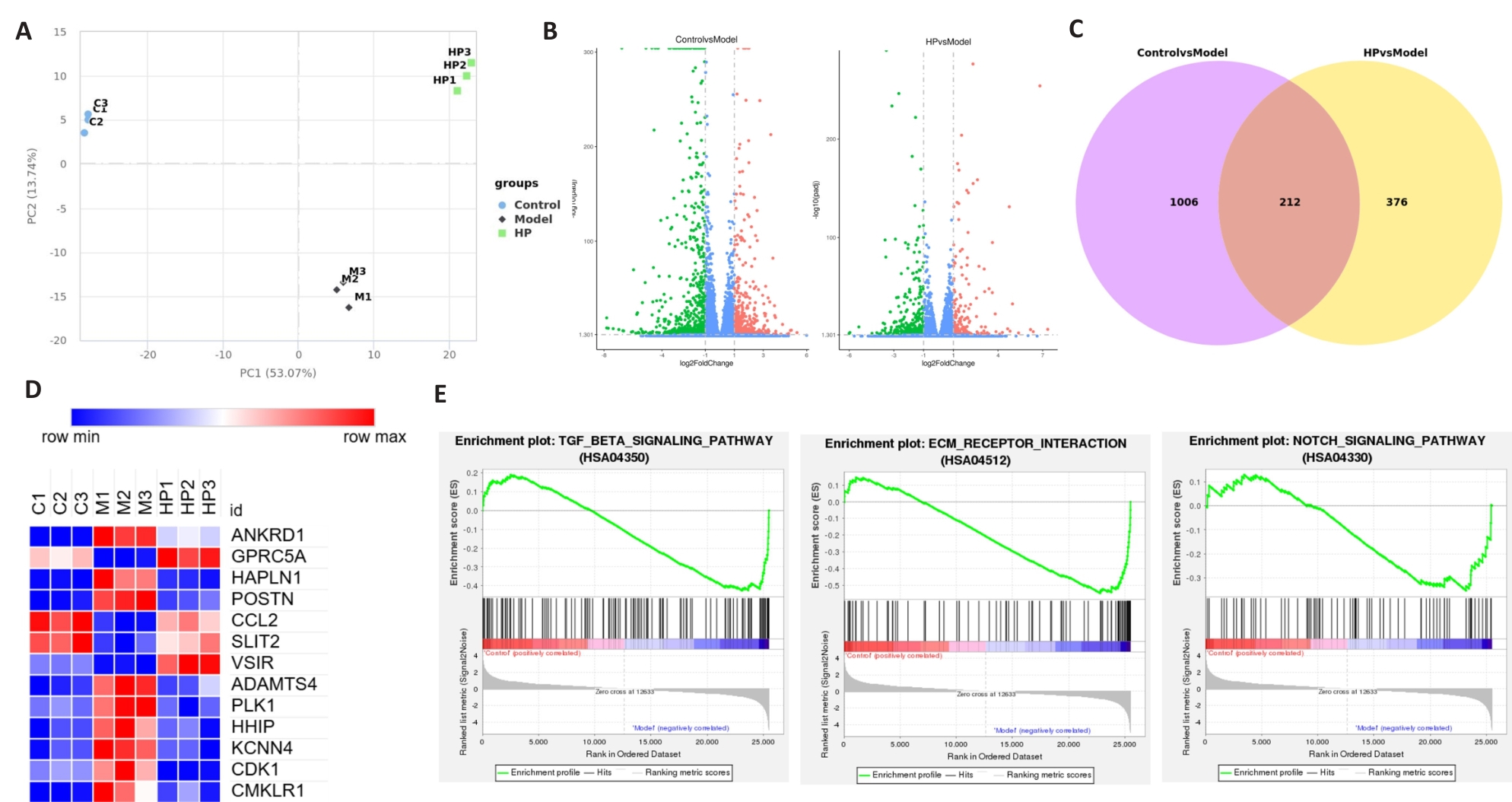
图6 转录组分析显示高剂量HP(240 μg/mL)对TGF-β1诱导的HFL1细胞模型肺纤维相关基因表达水平和主要通路的影响
Fig.6 Transcriptome analysis of the effects of high-dose HP (240 μg/mL) on pulmonary fibrosis-associated gene expression levels and major pathways in TGF-β1-induced HFL1 cell models. C1, C2, and C3: Three replicates of control cells; M1, M2, and M3: Three replicates of TGF-β1-induced cells; HP1, HP2, and HP3: Three replicates of cells treated with 240 μg/mL HP. A: PCA analysis. B: DEGs analysis. C: Venny diagram. D: Gene expression. E: Enrichment plot.
| Gene | Full name | Expression in the model | Function |
|---|---|---|---|
| ANKRD1 | Ankyrin repeat domain-containing protein 1 | Upregulation | Pro-fibrosis[ |
| GPRC5A | G protein-coupled receptor class C group 5 member A | Downregulation | Anti-fibrosis[ |
| HAPLN1 | Hyaluronan and proteoglycan link protein 1 | Upregulation | Pro-fibrosis [ |
| POSTN | Periostin | Upregulation | Pro-fibrosis [ |
| CCL2 | C-C motif chemokine 2 | Downregulation | Anti-fibrosis [ |
| SLIT2 | Slit homolog 2 protein | Downregulation | Anti-fibrosis [ |
| VSIR | V-type immunoglobulin domain-containing suppressor of T-cell activation | Downregulation | Anti-fibrosis [ |
| ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs | Upregulation | Pro-fibrosis [ |
| PLK1 | Serine/threonine-protein kinase | Upregulation | Pro-fibrosis [ |
| HHIP | Hedgehog-interacting protein | Upregulation | Pro-fibrosis [ |
| KCNN4 | Intermediate conductance calcium-activated potassium channel protein 4 | Upregulation | Pro-fibrosis [ |
| CDK1 | Cyclin-dependent kinase 1 | Upregulation | Pro-fibrosis [ |
| CMKLR1 | Chemokine-like receptor 1 | Upregulation | Pro-fibrosis [ |
表2 与纤维化相关的差异表达基因
Tab.2 Differentially expressed genes related to fibrosis
| Gene | Full name | Expression in the model | Function |
|---|---|---|---|
| ANKRD1 | Ankyrin repeat domain-containing protein 1 | Upregulation | Pro-fibrosis[ |
| GPRC5A | G protein-coupled receptor class C group 5 member A | Downregulation | Anti-fibrosis[ |
| HAPLN1 | Hyaluronan and proteoglycan link protein 1 | Upregulation | Pro-fibrosis [ |
| POSTN | Periostin | Upregulation | Pro-fibrosis [ |
| CCL2 | C-C motif chemokine 2 | Downregulation | Anti-fibrosis [ |
| SLIT2 | Slit homolog 2 protein | Downregulation | Anti-fibrosis [ |
| VSIR | V-type immunoglobulin domain-containing suppressor of T-cell activation | Downregulation | Anti-fibrosis [ |
| ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs | Upregulation | Pro-fibrosis [ |
| PLK1 | Serine/threonine-protein kinase | Upregulation | Pro-fibrosis [ |
| HHIP | Hedgehog-interacting protein | Upregulation | Pro-fibrosis [ |
| KCNN4 | Intermediate conductance calcium-activated potassium channel protein 4 | Upregulation | Pro-fibrosis [ |
| CDK1 | Cyclin-dependent kinase 1 | Upregulation | Pro-fibrosis [ |
| CMKLR1 | Chemokine-like receptor 1 | Upregulation | Pro-fibrosis [ |
| [1] | Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets[J]. Matrix Biol, 2018, 71: 112-27. doi:10.1016/j.matbio.2018.03.021 |
| [2] | Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis[J]. Lancet, 2017, 389(10082): 1941-52. doi:10.1016/s0140-6736(17)30866-8 |
| [3] | Cruwys S, Hein P, Humphries B, et al. Drug discovery and development in idiopathic pulmonary fibrosis: challenges and opportunities[J]. Drug Discov Today, 2020, 25(12): 2277-83. doi:10.1016/j.drudis.2020.09.019 |
| [4] | 丛晓梅, 臧晓南, 王振东, 等. 雨生红球藻虾青素合成关键酶的原核表达及纯化[J]. 海洋湖沼通报, 2022, 44(6): 49-56. doi:10.13984/j.cnki.cn37-1141.2022.06.007 |
| [5] | Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin[J]. Trends Biotechnol, 2000, 18(4): 160-7. doi:10.1016/s0167-7799(00)01433-5 |
| [6] | 李文学, 白慧颖, 赵宏晶, 等. 雨生红球藻虾青素积累条件优化研究[J]. 食品研究与开发, 2021, 42(16): 152-6. doi:10.12161/j.issn.1005-6521.2021.16.022 |
| [7] | Li K, Wang W, Xiao W. Astaxanthin: a promising therapeutic agent for organ fibrosis[J]. Pharmacol Res, 2023, 188: 106657. doi:10.1016/j.phrs.2023.106657 |
| [8] | Cheng J, Eroglu A. The promising effects of astaxanthin on lung diseases[J]. Adv Nutr, 2021, 12(3): 850-64. doi:10.1093/advances/nmaa143 |
| [9] | 王裕玉, 宋庆辉, 贾 晶, 等. 雨生红球藻的营养价值及其在鱼类饲料中的应用[J]. 饲料研究, 2023, 46(17): 167-72. doi:10.13557/j.cnki.issn1002-2813.2023.17.033 |
| [10] | Zhao J, Meng M, Zhang J, et al. Astaxanthin ameliorates renal interstitial fibrosis and peritubular capillary rarefaction in unilateral ureteral obstruction[J]. Mol Med Rep, 2019, 19(4): 3168-78. doi:10.3892/mmr.2019.9970 |
| [11] | Ma HT, Chen SH, Xiong HY, et al. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice[J]. Food Funct, 2020, 11(5): 4659-71. doi:10.1039/c9fo02429h |
| [12] | Sarker M, Chowdhury N, Bristy AT, et al. Astaxanthin protects fludrocortisone acetate-induced cardiac injury by attenuating oxidative stress, fibrosis, and inflammation through TGF-β/Smad signaling pathway[J]. Biomed Pharmacother, 2024, 181: 117703. doi:10.1016/j.biopha.2024.117703 |
| [13] | 权博文, 吴 桐, 刘 庆, 等. 蒙古扁桃种仁不同极性部位对博来霉素致大鼠肺纤维化的保护作用[J]. 食品工业科技, 2020, 41(22): 305-9. doi:10.13386/j.issn1002-0306.2020050143 |
| [14] | Zhang J, Xu P, Wang Y, et al. Astaxanthin prevents pulmonary fibrosis by promoting myofibroblast apoptosis dependent on Drp1-mediated mitochondrial fission[J]. J Cell Mol Med, 2015, 19(9): 2215-31. doi:10.1111/jcmm.12609 |
| [15] | Wang MR, Zhang JJ, Song XD, et al. Astaxanthin ameliorates lung fibrosis in vivo and in vitro by preventing transdifferentiation, inhibiting proliferation, and promoting apoptosis of activated cells[J]. Food Chem Toxicol, 2013, 56: 450-8. doi:10.1016/j.fct.2013.03.004 |
| [16] | Song X, Wang B, Lin S, et al. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway[J]. J Cell Mol Med, 2014, 18(11): 2198-212. doi:10.1111/jcmm.12347 |
| [17] | Mazzeo L, Ghosh S, Di Cicco E, et al. ANKRD1 is a mesenchymal-specific driver of cancer-associated fibroblast activation bridging androgen receptor loss to AP-1 activation[J]. Nat Commun, 2024, 15(1): 1038. doi:10.1038/s41467-024-45308-w |
| [18] | Huang ZL, Wang SQ, Liu YT, et al. GPRC5A reduction contributes to pollutant benzo [a] Pyrene injury via aggravating murine fibrosis, leading to poor prognosis of IIP patients[J]. Sci Total Environ, 2020, 739: 139923. doi:10.1016/j.scitotenv.2020.139923 |
| [19] | Evanko SP, Gooden MD, Kang I, et al. A role for HAPLN1 during phenotypic modulation of human lung fibroblasts in vitro [J]. J Histochem Cytochem, 2020, 68(11): 797-811. doi:10.1369/0022155420966663 |
| [20] | Wu S, Liu M, Zhang M, et al. The gene expression of CALD1 CDH2 and POSTN in fibroblast are related to idiopathic pulmonary fibrosis[J]. Front Immunol, 2024, 15: 1275064. doi:10.3389/fimmu.2024.1275064 |
| [21] | Kalderén C, Stadler C, Forsgren M, et al. CCL2 mediates anti-fibrotic effects in human fibroblasts independently of CCR2[J]. Int Immunopharmacol, 2014, 20(1): 66-73. doi:10.1016/j.intimp.2014.02.020 |
| [22] | He CY, Gu L, Li AM, et al. Recombinant Slit2 attenuates tracheal fibroblast activation in benign central airway obstruction by inhibiting the TGF-β1/Smad3 signaling pathway[J]. Mol Cell Probes, 2024, 73: 101947. doi:10.1016/j.mcp.2023.101947 |
| [23] | Kim MG, Yun D, Kang CL, et al. Kidney VISTA prevents IFN-γ/IL-9 axis-mediated tubulointerstitial fibrosis after acute glomerular injury[J]. J Clin Investig, 2022, 132(1): e151189. doi:10.1172/JCI151189 |
| [24] | Boyd DF, Allen EK, Randolph AG, et al. Exuberant fibroblast activity compromises lung function via ADAMTS4[J]. Nature, 2020, 587(7834): 466-71. doi:10.1038/s41586-020-2877-5 |
| [25] | Maria V, Murugan E P, Athiramol S, et al. Inhibition of the extracellular enzyme ADAMTS4 prevents cardiac fibrosis and dysfunction[J]. Cardiovascular Res, 2023, 119(10): 1915-27. |
| [26] | Chen Y, Chen X, Ji YR, et al. PLK1 regulates hepatic stellate cell activation and liver fibrosis through Wnt/β-catenin signalling pathway[J]. J Cell Mol Med, 2020, 24(13): 7405-16. doi:10.1111/jcmm.15356 |
| [27] | Du Y, Shang Y, Qian Y, et al. Plk1 promotes renal tubulointerstitial fibrosis by targeting autophagy/lysosome axis[J]. Cell Death Dis, 2023, 14(8): 571. doi:10.1038/s41419-023-06093-4 |
| [28] | Zhao XP, Chang SY, Liao MC, et al. Hedgehog interacting protein promotes fibrosis and apoptosis in glomerular endothelial cells in murine diabetes[J]. Sci Rep, 2018, 8(1): 5958. doi:10.1038/s41598-018-24220-6 |
| [29] | Mann C, Kaistha BP, Kacik M, et al. Downregulation of miR-503 in activated kidney fibroblasts disinhibits KCNN4 in an in vitro model of kidney fibrosis[J]. Kidney Blood Press Res, 2019, 44(1): 113-22. doi:10.1159/000498875 |
| [30] | Yang L, Han B, Zhang M, et al. Activation of BK channels prevents hepatic stellate cell activation and liver fibrosis through the suppression of TGFβ1/SMAD3 and JAK/STAT3 profibrotic signaling pathways[J]. Front Pharmacol, 2020, 11: 165. doi:10.3389/fphar.2020.00165 |
| [31] | Sai C, Yunhan J, Zhao J, et al. Cyclin dependent kinase 1 (CDK1) activates cardiac fibroblasts via directly phosphorylating paxillin at Ser244[J]. Int Heart J, 2019, 60(2): 374-83. doi:10.1536/ihj.18-073 |
| [32] | Yamamoto T, Matsushima S, Okabe K, et al. Cyclin dependent kinase 1 (CDK1) positively regulates cardiac hypertrophy and fibrosis via TGF-beta pathway[J]. Eur Heart J, 2020, 41(): ehaa946.3743. doi:10.1093/ehjci/ehaa946.3743 |
| [33] | Cai R, Cao R, Liu Y, et al. Chemerin promotes proliferation of cardiac fibroblasts via CMKLR1/PI3k/Akt/NF‑κB signaling pathway[DB/OL]. 2024-04-01. . doi:10.21203/rs.3.rs-4142000/v1 |
| [34] | Mannes PZ, Adams TS, Farsijani S, et al. Noninvasive assessment of the lung inflammation-fibrosis axis by targeted imaging of CMKLR1[J]. Sci Adv, 2024, 10(25): 9817. doi:10.1126/sciadv.adm9817 |
| [35] | 唐海南, 柏 月, 许金震, 等. 雨生红球藻粉对斑节对虾肝胰腺免疫性能及甲醛胁迫下相关基因表达的影响[J]. 厦门大学学报(自然科学版), 2024, 64(8): 1-11. |
| [36] | Zhang T, He X, Caldwell L, et al. NUAK1 promotes organ fibrosis via YAP and TGF-β/SMAD signaling[J]. Sci Transl Med, 2022, 14(637): eaaz4028. doi:10.1126/scitranslmed.aaz4028 |
| [37] | Yan Z, Kui Z, Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis[J]. Autoimmun Rev, 2014, 13(10): 1020-5. doi:10.1016/j.autrev.2014.08.028 |
| [38] | Suri GS, Kaur G, Jha CK, et al. Understanding idiopathic pulmonary fibrosis-Clinical features, molecular mechanism and therapies[J]. Exp Gerontol, 2021, 153: 111473. doi:10.1016/j.exger.2021.111473 |
| [39] | 李斯宇, 庞立健, 吕晓东, 等. 特发性肺纤维化复杂网络发病机制与络病理论[J]. 中华中医药杂志, 2022, 37(2): 640-5. |
| [1] | 李国永, 黎仁玲, 刘艺婷, 柯宏霞, 李菁, 王新华. 牛蒡子治疗小鼠病毒性肺炎后肺纤维化的机制:基于代谢组学、网络药理学和实验验证方法[J]. 南方医科大学学报, 2025, 45(6): 1185-1199. |
| [2] | 代振佳, 高群伟, 应梦娇, 王澳, 洪娟, 王春景, 郭俣, 刘长青, 刘高峰. C6TSEDRVAJZ小分子化合物组合诱导人胎盘成纤维细胞分化为上皮样细胞[J]. 南方医科大学学报, 2025, 45(2): 322-330. |
| [3] | 曹周芳, 汪元, 王梦娜, 孙玥, 刘菲菲. LINC00837/miR-671-5p/SERPINE2功能轴促进类风湿关节炎成纤维细胞样滑膜细胞的恶性病理学过程[J]. 南方医科大学学报, 2025, 45(2): 371-378. |
| [4] | 展俊平, 黄硕, 孟庆良, 范围, 谷慧敏, 崔家康, 王慧莲. 缺氧微环境下补阳还五汤通过抑制BNIP3-PI3K/Akt通路抑制类风湿关节炎滑膜成纤维细胞的线粒体自噬[J]. 南方医科大学学报, 2025, 45(1): 35-42. |
| [5] | 骆金光, 陶怀祥, 闻志远, 陈龙, 胡昊, 关翰. 肿瘤相关成纤维细胞上调hsa-miR-18b-5p靶向FBXL3促进前列腺癌的增殖及转移[J]. 南方医科大学学报, 2024, 44(7): 1284-1296. |
| [6] | 何 程, 陈 炜, 张念志, 栾 军, 王三凤, 张 尤. 参七虫草方通过ASS1/src/STAT3信号通路改善肺纤维化大鼠的炎症反应[J]. 南方医科大学学报, 2024, 44(4): 644-651. |
| [7] | 王梓凝, 杨 明, 李双磊, 迟海涛, 王军惠, 肖苍松. 心肌梗死后心肌纤维化小鼠心肌线粒体功能和能量代谢重塑相关性的转录组学分析[J]. 南方医科大学学报, 2024, 44(4): 666-674. |
| [8] | 邵 珊, 白薇超, 邹鹏程, 罗敏娜, 赵新汉, 雷建军. 二甲双胍阻断乳腺癌细胞-间质细胞的交互作用:基于抑制肿瘤相关成纤维细胞缺氧诱导因子-1α的表达[J]. 南方医科大学学报, 2024, 44(3): 428-436. |
| [9] | 宋端怡, 李赟, 唐雪芳, 李化, 陶康. 地西泮通过let-7a-5p/MYD88轴抑制LPS诱导的细胞焦亡和炎症从而缓解小鼠肺纤维化[J]. 南方医科大学学报, 2024, 44(11): 2092-2101. |
| [10] | 郗雪艳, 邓婷, 杜伯雨. 结直肠成纤维细胞通过激活ERK信号通路促进结直肠癌细胞的恶性生物学行为[J]. 南方医科大学学报, 2024, 44(10): 1866-1873. |
| [11] | 舒萍, 袁孟珂, 杨珂, 何伟志, 刘丽. 槲皮素通过抑制NLRP3/Caspase-1/GSDMD信号通路抑制小鼠成纤维细胞焦亡[J]. 南方医科大学学报, 2024, 44(10): 1874-1880. |
| [12] | 梁梓琛, 余常辉, 梁世秀, 周梓聪, 周子丽, 孟晓静, 邹 飞, 蔡绍曦. 桔梗素D通过下调成纤维细胞TRPC6表达改善小鼠肺纤维化[J]. 南方医科大学学报, 2024, 44(1): 60-69. |
| [13] | 邓 婷, 杜伯雨, 郗雪艳. 结直肠癌细胞通过激活成纤维细胞的ERK通路诱导癌症相关成纤维细胞的形成[J]. 南方医科大学学报, 2023, 43(6): 943-951. |
| [14] | 杨 盼, 王圆圆, 高群伟, 刘 阳, 王 翼, 郭 俣, 刘长青, 刘高峰. 小分子化合物组合将人胚胎成纤维细胞体外编程为神经前体细胞[J]. 南方医科大学学报, 2023, 43(3): 360-367. |
| [15] | 肖姝喆, 成燕玲, 朱 云, 唐 芮, 古建标, 兰 林, 何志华, 刘丹琼, 耿岚岚, 程 旸, 龚四堂. WNT2b高表达的成纤维细胞破坏肠道黏膜屏障[J]. 南方医科大学学报, 2023, 43(2): 206-212. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||