南方医科大学学报 ›› 2026, Vol. 46 ›› Issue (1): 34-46.doi: 10.12122/j.issn.1673-4254.2026.01.04
张越1,2( ), 段雨婷1(
), 段雨婷1( ), 张晨1, 喻璐喆1, 刘颖颖1, 邢丽花1,3, 王雷1,3,5, 俞年军1, 彭代银1,3,4,5, 陈卫东1,3,4,5(
), 张晨1, 喻璐喆1, 刘颖颖1, 邢丽花1,3, 王雷1,3,5, 俞年军1, 彭代银1,3,4,5, 陈卫东1,3,4,5( ), 王妍妍1,3,4(
), 王妍妍1,3,4( )
)
收稿日期:2025-07-11
出版日期:2026-01-20
发布日期:2026-01-16
通讯作者:
陈卫东,王妍妍
E-mail:zhangyue@ahtcm.edu.cn;wdchen@ahtcm.edu.cn;wangyanyan@ahtcm.edu.cn
作者简介:张 越,博士后,E-mail: zhangyue@ahtcm.edu.cn基金资助:
Yue ZHANG1,2( ), Yuting DUAN1(
), Yuting DUAN1( ), Chen ZHANG1, Luzhe YU1, Yingying LIU1, Lihua XING1,3, Lei WANG1,3,5, Nianjun YU1, Daiyin PENG1,3,4,5, Weidong CHEN1,3,4,5(
), Chen ZHANG1, Luzhe YU1, Yingying LIU1, Lihua XING1,3, Lei WANG1,3,5, Nianjun YU1, Daiyin PENG1,3,4,5, Weidong CHEN1,3,4,5( ), Yanyan WANG1,3,4(
), Yanyan WANG1,3,4( )
)
Received:2025-07-11
Online:2026-01-20
Published:2026-01-16
Contact:
Weidong CHEN, Yanyan WANG
E-mail:zhangyue@ahtcm.edu.cn;wdchen@ahtcm.edu.cn;wangyanyan@ahtcm.edu.cn
Supported by:摘要:
目的 研究茯苓多糖(PCP)对环磷酰胺(CTX)诱导的肠道粘膜损伤的修复作用,探讨其对肠道微生物群和代谢产物的影响,以及对肠粘膜损伤小鼠的潜在保护机制。 方法 建立CTX诱导的小鼠肠道黏膜损伤模型,观察PCP对肠道屏障和免疫功能的保护作用。小鼠随机分为6组:正常对照组(NC)、CTX模型组(MC)、CTX+谷氨酰胺阳性药物组(PC)、CTX+PCP低剂量组(LD)、CTX+PCP中剂量组(MD)、CTX+PCP高剂量组(HD)。除NC组外,其余各组小鼠连续3 d腹腔注射CTX(80 mg/kg),NC组注射生理盐水。PC组给予谷氨酰胺(300 mg/kg),LD、MD、HD组分别给予PCP(75、150、300 mg/kg),连续灌胃7 d。检测各组小鼠紧密连接蛋白(occludin、ZO-1)表达水平、血清内毒素、D-乳酸盐、二胺氧化酶(DAO)水平、肠道通透性、结肠损伤情况以及细胞因子(IL-4、IL-22、IL-17A、IFN-γ等)水平变化。16S rRNA测序分析肠道微生物群组成变化,气相色谱-质谱法检测短链脂肪酸(SCFAs,如乙酸、丙酸)水平,Western blotting检测GPR41表达。粪菌移植(FMT)实验验证肠道微生物在PCP修复肠道损伤中的作用。 结果 与模型组相比,PCP处理组小鼠结肠组织中紧密连接蛋白Occludin和ZO-1表达显著升高(P<0.001),血清内毒素、D-乳酸盐、DAO水平及肠道通透性均显著降低(P<0.05),结肠组织中IL-4、IL-22、IL-17A和IFN-γ的mRNA表达水平显著升高(P<0.001)。PCP处理组小鼠肠道中Muribaculaceae相对丰度增加(P<0.01),Lactobacillus和Bacteroides相对丰度减少(P<0.05),结肠内容物中乙酸和丙酸含量升高(P<0.05),GPR41蛋白表达水平显著升高(P<0.001)。FMT实验证实了肠道微生物在PCP修复肠粘膜损伤中的关键作用。 结论 PCP能够保护CTX引起的肠粘膜损伤,其机制可能通过调节肠道微生物群和短链脂肪酸的代谢,从而增强肠道的防御能力。
张越, 段雨婷, 张晨, 喻璐喆, 刘颖颖, 邢丽花, 王雷, 俞年军, 彭代银, 陈卫东, 王妍妍. 茯苓多糖通过调节肠道微生物群减轻环磷酰胺引起的肠道屏障和免疫损伤[J]. 南方医科大学学报, 2026, 46(1): 34-46.
Yue ZHANG, Yuting DUAN, Chen ZHANG, Luzhe YU, Yingying LIU, Lihua XING, Lei WANG, Nianjun YU, Daiyin PENG, Weidong CHEN, Yanyan WANG. Poria cocos polysaccharide alleviates cyclophosphamide-induced intestinal barrier dysfunction and inflammation in mice by modulating gut flora[J]. Journal of Southern Medical University, 2026, 46(1): 34-46.
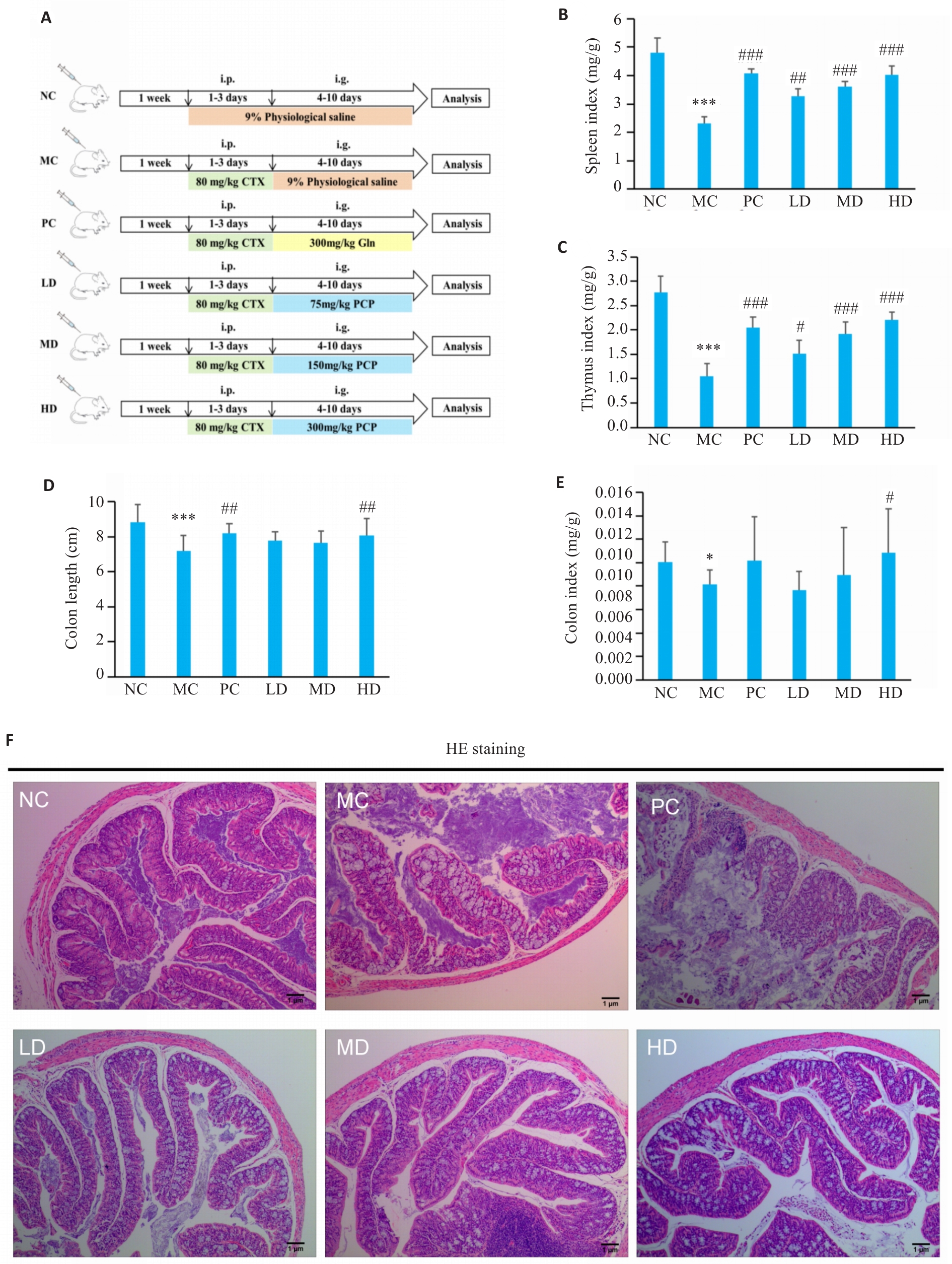
图1 PCP对CTX 治疗小鼠结肠生理状态的影响
Fig.1 Effect of PCP on physiological state of the colon in CTX-treated mice. A: Illustration of the experiment design. i.p.:Intraperitoneal injection,i.g.: Irrigation. B: Spleen index (spleen weight/body weight). C: Thymus index (thymus weight/body weight) of the mice. D: Colon length of the mice. E: Colon index (colon weight/body weight). F: HE staining of the colon tissue (Original magnification: ×7.5). NC: Control group; MC: CTX model group; PC: CTX+Glutamine (positive drug) group; LD: CTX+PCP low dose group; MD: CTX+PCP medium dose group; HD: CTX+PCP high dose group. Data are presented as Mean±SD (n=6). *P<0.05, ***P<0.001 vs NC group; #P<0.05, ##P<0.01, ###P<0.001 vs MC group.
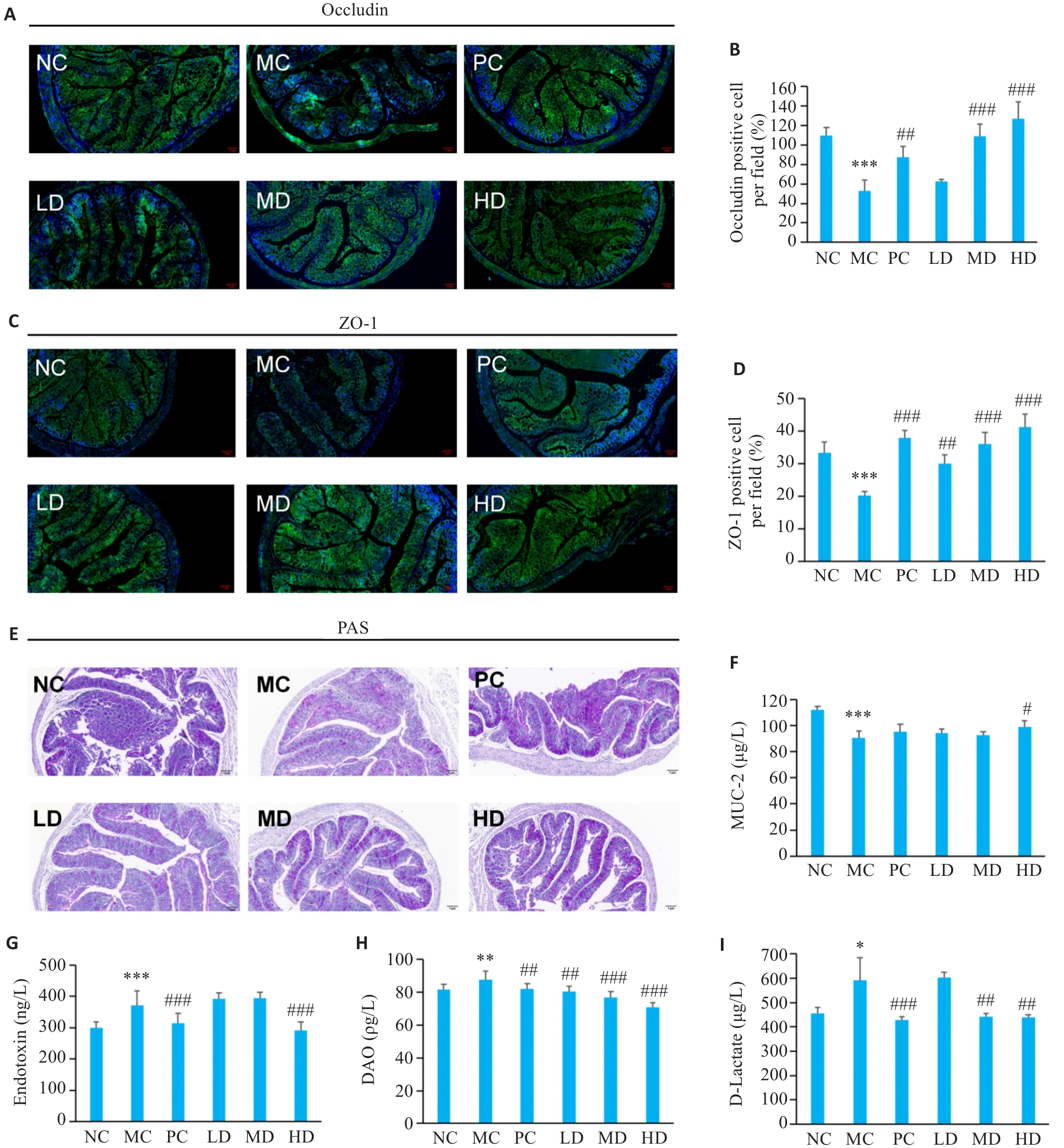
图2 PCP对CTX处理小鼠肠道屏障功能的影响
Fig.2 Effect of PCP on intestinal barrier function in CTX-treated mice. A-D: Immunofluorescence staining of occludin and ZO-1 (green: occludin and ZO-1; blue: nucleus; ×7.5). E: PAS staining showing mucus-secreting epithelial cells (×7.5). F: MUC2 contents in colonic tissues. G: Endotoxin levels in serum. H: DAO levels in serum. I: D-Lactate levels in serum. Data are presented as Mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001 vs NC group; #P<0.05, ##P<0.01, ###P<0.001 vs MC group.
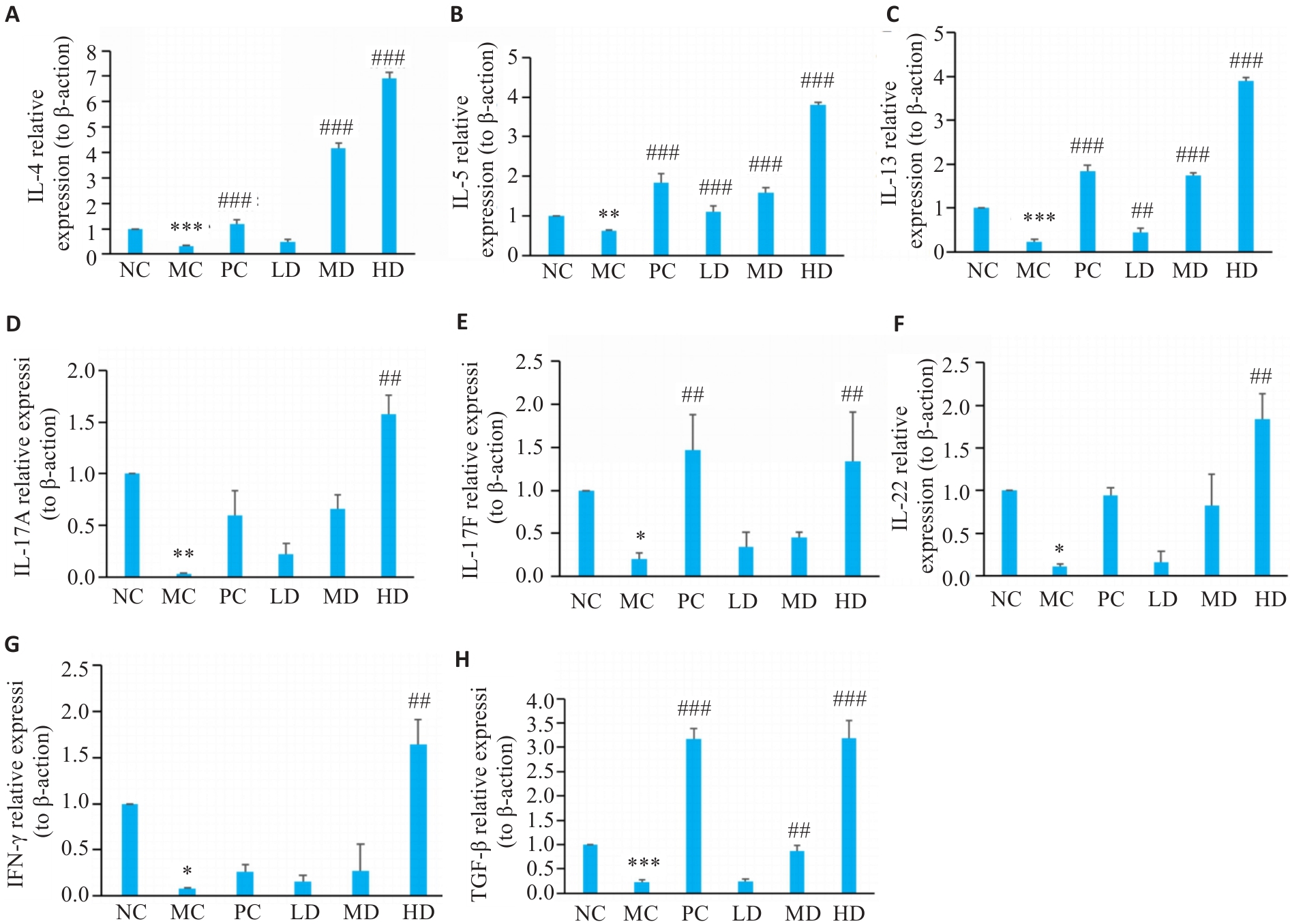
图3 PCP对小鼠结肠黏膜免疫细胞因子的影响
Fig.3 Effect of PCP on immune cytokines in the colonic mucosa of the mice. A-H: Relative mRNA expression levels of IL-4, IL-5, IL-13, IL-17A, IL-17F, IL-22, IFN-γ, and TGF-β detected with RT-qPCR, using β-actin as the reference control. Data are represented as Mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001 vs NC group; ##P<0.01, ###P<0.001 vs MC group.
| Group | Chao1 | ACE | Shannon | Simpson | Goods_coverage |
|---|---|---|---|---|---|
| NC | 195.22±50.78 | 195.79±51.02 | 5.81±0.21 | 0.97±0.00 | 1.00 |
| MC | 182.98±17.05 | 183.05±16.94 | 5.51±0.23 | 0.96±0.01 | 1.00 |
| HD | 275.21±52.61 | 274.09±51.86 | 6.32±0.44 | 0.98±0.01 | 1.00 |
表1 NC组、MC组与HD组肠道菌群多样性指数比较
Tab.1 Diversity indexes of gut microbiota in NC, MC, and HD groups (Mean±SD, n=6)
| Group | Chao1 | ACE | Shannon | Simpson | Goods_coverage |
|---|---|---|---|---|---|
| NC | 195.22±50.78 | 195.79±51.02 | 5.81±0.21 | 0.97±0.00 | 1.00 |
| MC | 182.98±17.05 | 183.05±16.94 | 5.51±0.23 | 0.96±0.01 | 1.00 |
| HD | 275.21±52.61 | 274.09±51.86 | 6.32±0.44 | 0.98±0.01 | 1.00 |
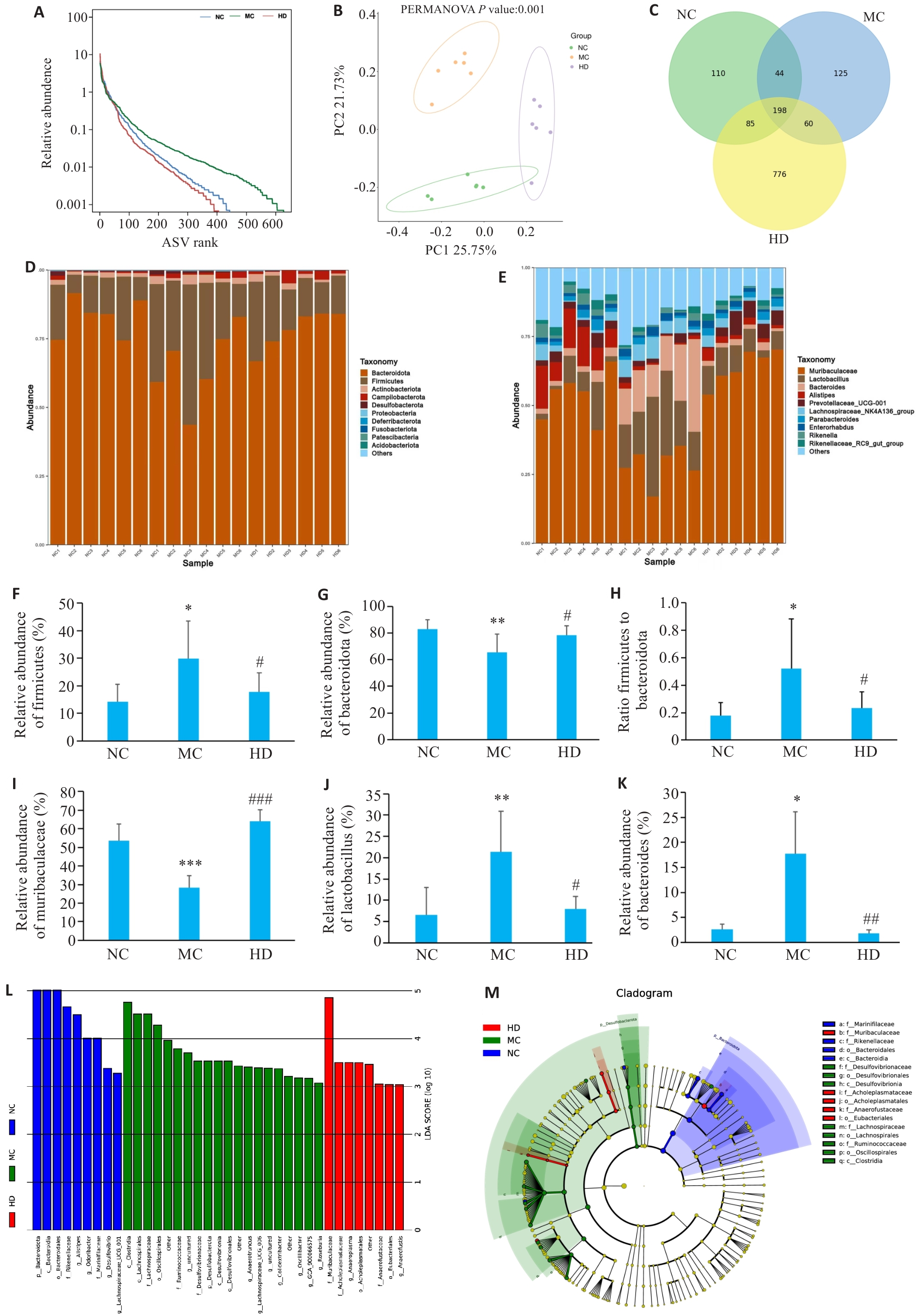
图4 PCP对CTX处理小鼠肠道菌群组成的影响
Fig.4 Effect of PCP on gut microbiota composition in CTX-treated mice. A: Species abundance distribution curve. B: Principal coordinates analysis. C: Venn graph showing species overlap. D: Phylum-level microbial composition. E: Genus-level microbial composition. F: Firmicutes abundance. G: Bacteroidetes abundance. H: Firmicutes-to-Bacteroidetes ratio. I: Muribaculaceae abundance. J: Lactobacillus abundance. K: Bacteroides abundance. L: LDA score. M: Phylogenetic distribution of discriminant taxa. Data are represented as Mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001 vs NC group; #P<0.05, ##P<0.01, ###P<0.001 vs MC group.
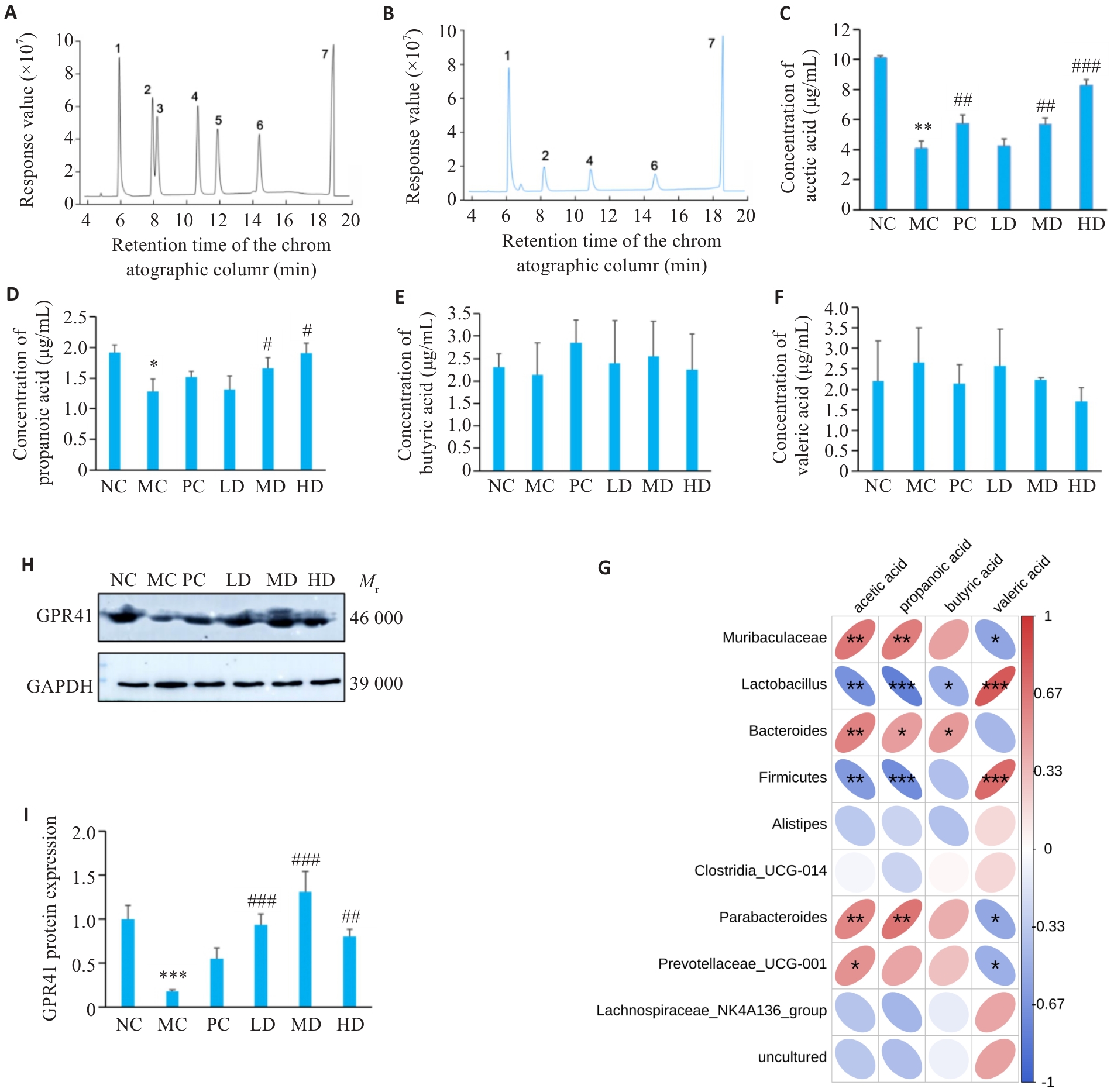
图5 PCP对CTX处理小鼠SCFAs含量的影响
Fig.5 Effect of PCP on SCFAs content in CTX-treated mice. A: Standard curve of each component of SCFAs to be measured. B: GC chromatogram of mixed control solution of SCFAs (1: acetic acid; 2: propionic acid; 3: butyric acid; 4: isobutyric acid; 5: valeric acid; 6: isovaleric acid; 7: 2-ethylbutyric acid). C: Acetate levels. D: Propionate levels. E: Butyrate levels. F: Valerate levels. G: Correlation analysis between gut microbiota and short-chain fatty acids. H: Representative bands of GPR41. I: Protein expression of GPR41. Data are presented as Mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001 vs NC group; #P<0.05, ##P<0.01, ###P<0.001 vs MC group.
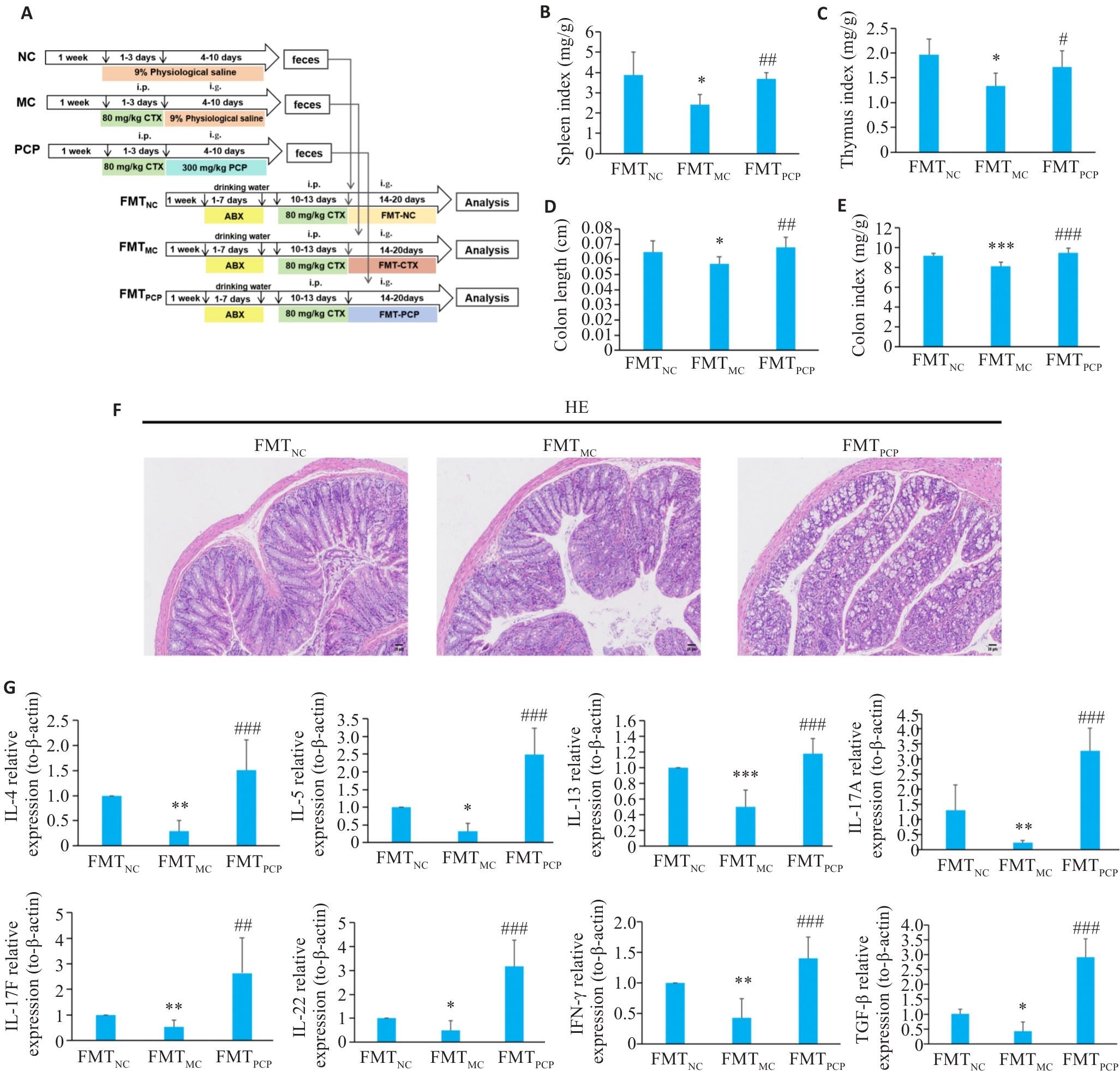
图6 FMT对CTX处理小鼠结肠生理状态的影响
Fig.6 Effect of FMT on colon injury in CTX-treated mice. A: Illustration of the design of fecal microbiota transplantation (FMT) experiment. B: Splenic somatic index (organ-to-body weight ratio). C: Thymus index (thymus weight/body weight). D: Colon length. E: Colon index (colon weight/body weight). F: HE staining (×100). FMTNC: FMT normal group; FMTCTX: FMT CTX model group; FMTPCP: FMT-Poria cocos polysaccharide. G: FMT modulates immune function in CTX-treated mice (Relative mRNA expression levels of cytokines: IL-4, IL-5, IL-13, IL-17A, IL-17F, IL-22, IFN-γ, and TGF-β). Data are presented as Mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001 vs FMTNC group; #P<0.05, ##P<0.01, ###P<0.001 vs FMTMC group.
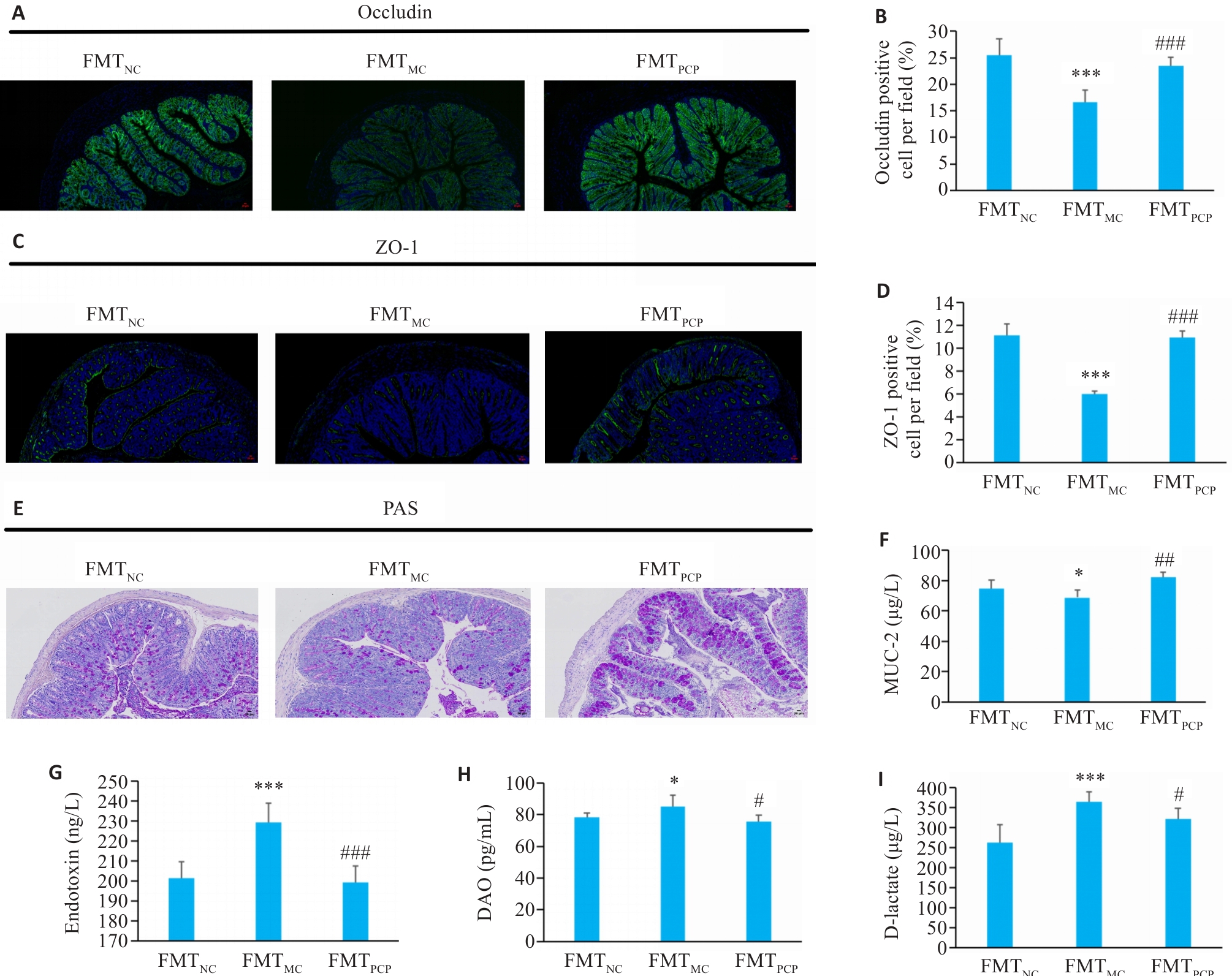
图7 FMT对小鼠结肠通透性的影响
Fig.7 Effect of FMT on intestinal permeability of the mice. A-D: Immunofluorescence staining of occludin and ZO-1. (green: Occludin and ZO-1; blue: nucleus; ×100). E: PAS staining showing mucus-secreting epithelial cells (×100). F: MUC2 content in colonic tissue. G: Endotoxin levels in serum. H: DAO levels in serum. I: D-Lactate levels in serum. Data are presented as Mean±SD (n=6). *P<0.05, ***P<0.001 vs FMTNC group; #P<0.05, ##P<0.01, ###P<0.001 vs FMTMC group.
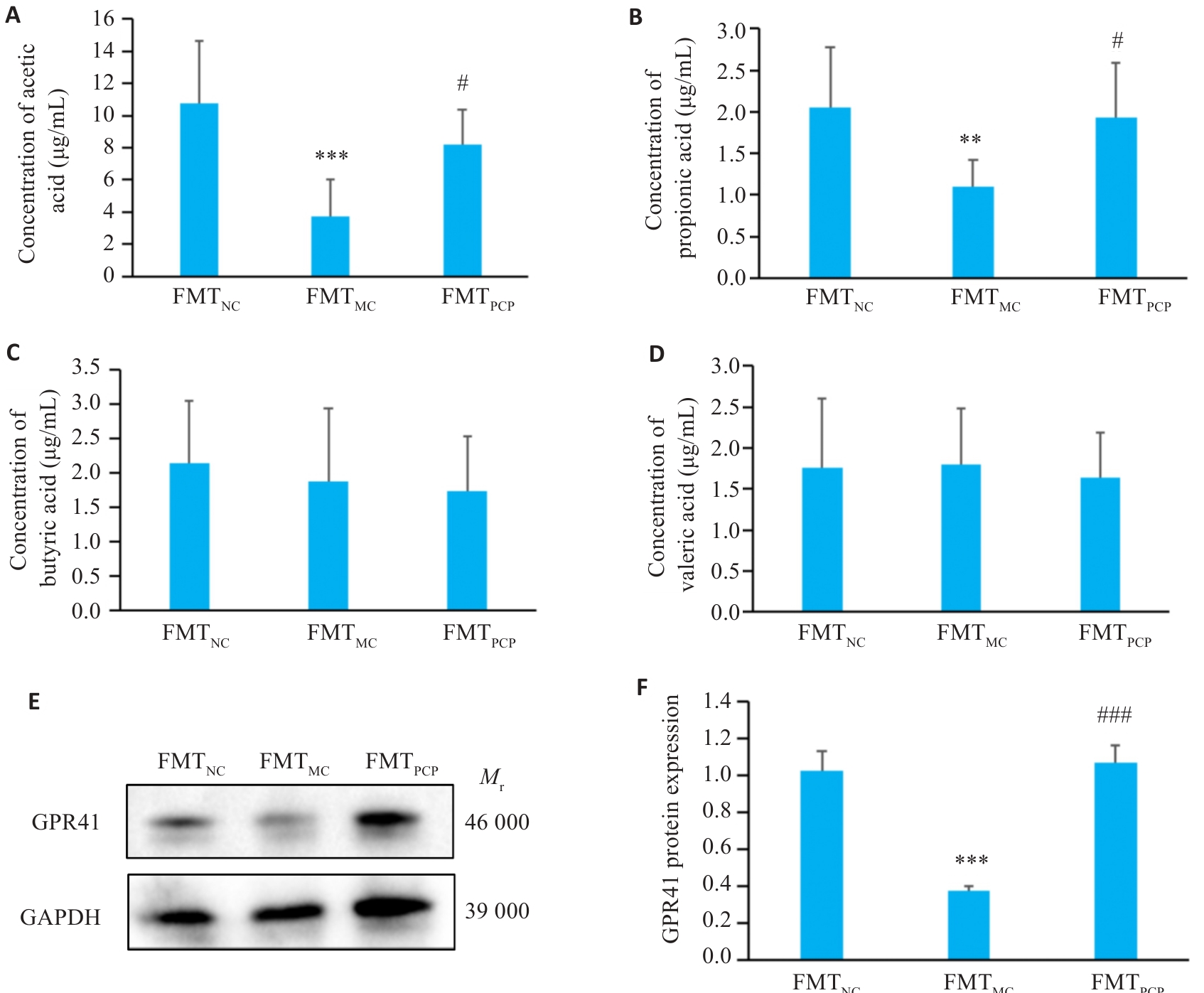
图8 FMT对小鼠结肠SCFAs含量和GPR41的影响
Fig. 8 Effect of FMT on SCFAs content and GPR41 in the colon of the mice. A: Acetate. B: Propionate. C: Butyrate. D: Valerate concentrations. E: Representative bands of GPR41. F: Quantitative analysis of GPR41 protein expression normalized to β-actin. Data are presented as Mean±SD (n=6). **P<0.01, ***P<0.001 vs FMTNC group; #P<0.05, ###P<0.001 vs FMTMC group.
| [1] | Chu Q, Zhang YR, Chen W, et al. Apios americana Medik flowers polysaccharide (AFP) alleviate Cyclophosphamide-induced imm-unosuppression in ICR mice[J]. Int J Biol Macromol, 2020, 144: 829-36. doi:10.1016/j.ijbiomac.2019.10.035 |
| [2] | Day W, Gabriel C, Kelly RE, et al. Juvenile dermatomyositis resembling late-stage Degos disease with gastrointestinal perfo-rations successfully treated with combination of cyclophosphamide and rituximab: case-based review[J]. Rheumatol Int, 2020, 40(11): 1883-90. doi:10.1007/s00296-019-04495-2 |
| [3] | Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide[J]. Science, 2013, 342(6161): 971-6. doi:10.1126/science.1240537 |
| [4] | Allaire JM, Crowley SM, Law HT, et al. The intestinal epithelium: central coordinator of mucosal immunity[J]. Trends Immunol, 2018, 39(9): 677-96. doi:10.1016/j.it.2018.04.002 |
| [5] | Chistiakov DA, Bobryshev YV, Kozarov E, et al. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance[J]. Front Microbiol, 2015, 5: 781. doi:10.3389/fmicb.2014.00781 |
| [6] | Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability[J]. Gut, 2009, 58(8): 1091-103. doi:10.1136/gut.2008.165886 |
| [7] | Yang WJ, Yu TM, Huang XS, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity[J]. Nat Commun, 2020, 11(1): 4457. doi:10.1038/s41467-020-18262-6 |
| [8] | Kayama H, Okumura R, Takeda K. Interaction between the microbiota, epithelia, and immune cells in the intestine[J]. Annu Rev Immunol, 2020, 38: 23-48. doi:10.1146/annurev-immunol-070119-115104 |
| [9] | 聂文律, 王东鹏, 钟丽姣, 等. 麸炒苍术精制多糖通过调节亚油酸代谢改善环磷酰胺诱导的小鼠免疫抑制与肠道损伤[J/OL].中国中药杂志,1-14 [2025-10-15]. . |
| [10] | 郭林霞, 马可为, 冯志华, 等. 枣多糖提取物对环磷酰胺所致的蛋雏鸡肠黏膜免疫屏障功能下降的缓解作用[J]. 中国兽医学报, 2022, 42(1): 107-13. |
| [11] | Xu TR, Zhang HM, Wang SG, et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications[J]. Int J Biol Macromol, 2022, 217: 536-51. doi:10.1016/j.ijbiomac.2022.07.070 |
| [12] | Zhao MH, Guan ZY, Tang N, et al. The differences between the water- and alkaline-soluble Poria cocos polysaccharide: a review[J]. Int J Biol Macromol, 2023, 235: 123925. doi:10.1016/j.ijbiomac.2023.123925 |
| [13] | Jiang YH, Wang L, Chen WD, et al. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness[J]. Front Nutr, 2022, 9: 963598. doi:10.3389/fnut.2022.963598 |
| [14] | Duan YT, Huang JJ, Sun MJ, et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice[J]. Int J Biol Macromol, 2023, 249: 125953. doi:10.1016/j.ijbiomac.2023.125953 |
| [15] | Sun MJ, Yao L, Yu QM, et al. Screening of Poria cocos polysaccharide with immunomodulatory activity and its activation effects on TLR4/MD2/NF-κB pathway[J]. Int J Biol Macromol, 2024, 273(Pt 1): 132931. doi:10.1016/j.ijbiomac.2024.132931 |
| [16] | 房 悦, 黄佳静, 张 越, 等. 基于BTLA/HVEM通路研究茯苓多糖对免疫抑制小鼠的免疫调节作用[J]. 中南药学, 2025, 23(5): 1183-9. |
| [17] | 王灿红, 霍小位, 何晓山, 等. 羧甲基茯苓多糖对肠癌小鼠生命延长及对环磷酰胺的减毒作用[J]. 食品科学, 2016, 37(21): 229-33. |
| [18] | Cheng Y, Xie Y, Ge JC, et al. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos [J]. Carbohydr Polym, 2021, 263: 117979. doi:10.1016/j.carbpol.2021.117979 |
| [19] | Wang J, Li MH, Gao YW, et al. Effects of exopolysaccharides from Lactiplantibacillus plantarum JLAU103 on intestinal immune response, oxidative stress, and microbial communities in cyclophosphamide-induced immunosuppressed mice[J]. J Agric Food Chem, 2022, 70(7): 2197-210. doi:10.1021/acs.jafc.1c06502 |
| [20] | Wu ZH, Huang SM, Li TT, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis[J]. Microbiome, 2021, 9(1): 184. doi:10.1186/s40168-021-01115-9 |
| [21] | Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota[J]. Nat Commun, 2015, 6: 7489. doi:10.1038/ncomms8489 |
| [22] | Jädert C, Phillipson M, Holm L, et al. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease[J]. Redox Biol, 2013, 2: 73-81. doi:10.1016/j.redox.2013.12.012 |
| [23] | Mao XM, Wu S, Huang DD, et al. Complications and comorbidities associated with antineoplastic chemotherapy: Rethinking drug design and delivery for anticancer therapy[J]. Acta Pharm Sin B, 2024, 14(7): 2901-26. doi:10.1016/j.apsb.2024.03.006 |
| [24] | Chen L, Wang D, Liu W, et al. Immunomodulation of exopo-lysaccharide produced by Lacticaseibacillus rhamnosus ZFM216 in cyclophosphamide-induced immunosuppressed mice by modulating gut microbiota[J]. Int J Biol Macromol, 2024, 283(Pt 2): 137619. doi:10.1016/j.ijbiomac.2024.137619 |
| [25] | Xue HK, Liang BM, Wang Y, et al. The regulatory effect of polysaccharides on the gut microbiota and their effect on human health: a review[J]. Int J Biol Macromol, 2024, 270(Pt 2): 132170. doi:10.1016/j.ijbiomac.2024.132170 |
| [26] | Ying MX, Yu Q, Zheng B, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice[J]. Carbohydr Polym, 2020, 235: 115957. doi:10.1016/j.carbpol.2020.115957 |
| [27] | Zhu ZP, Luo YR, Lin LT, et al. Modulating effects of turmeric polysaccharides on immune response and gut microbiota in cyclophosphamide-treated mice[J]. J Agric Food Chem, 2024, 72(7): 3469-82. doi:10.1021/acs.jafc.3c05590 |
| [28] | Li N, Wang D, Wen XJ, et al. Effects of polysaccharides from Gastrodia elata on the immunomodulatory activity and gut microbiota regulation in cyclophosphamide-treated mice[J]. J Sci Food Agric, 2023, 103(7): 3390-401. doi:10.1002/jsfa.12491 |
| [29] | Wang DP, Dong Y, Xie Y, et al. Atractylodes lancea rhizome polysaccharide alleviates immunosuppression and intestinal mucosal injury in mice treated with cyclophosphamide[J]. J Agric Food Chem, 2023. doi:10.1021/acs.jafc.3c05173 |
| [30] | Chopyk DM, Grakoui A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159(3): 849-63. doi:10.1053/j.gastro.2020.04.077 |
| [31] | Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease[J]. Cold Spring Harb Perspect Biol, 2018, 10(1): a029314. doi:10.1101/cshperspect.a029314 |
| [32] | Nyström EEL, Martinez-Abad B, Arike L, et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function[J]. Science, 2021, 372(6539): eabb1590. doi:10.1126/science.abb1590 |
| [33] | Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis[J]. Nat Rev Gastroenterol Hepatol, 2022, 19(12): 785-803. doi:10.1038/s41575-022-00675-x |
| [34] | Pizarro TT, Dinarello CA, Cominelli F. Editorial: cytokines and intestinal mucosal immunity[J]. Front Immunol, 2021, 12: 698693. doi:10.3389/fimmu.2021.698693 |
| [35] | Wu XC, Huang XJ, Ma WN, et al. Bioactive polysaccharides promote gut immunity via different ways[J]. Food Funct, 2023, 14(3): 1387-400. doi:10.1039/d2fo03181g |
| [36] | Knox NC, Forbes JD, Peterson CL, et al. The gut microbiome in inflammatory bowel disease: lessons learned from other immune-mediated inflammatory diseases[J]. Am J Gastroenterol, 2019, 114(7): 1051-70. doi:10.14309/ajg.0000000000000305 |
| [37] | Fu YP, Feng B, Zhu ZK, et al. The polysaccharides from Codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice[J]. Molecules, 2018, 23(7): 1801. doi:10.3390/molecules23071801 |
| [38] | Zhu YQ, Chen BR, Zhang XY, et al. Exploration of the Muribaculaceae family in the gut microbiota: diversity, metabolism, and function[J]. Nutrients, 2024, 16(16): 2660. doi:10.3390/nu16162660 |
| [39] | Wastyk HC, Fragiadakis GK, Perelman D, et al. Gut-microbiota-targeted diets modulate human immune status[J]. Cell, 2021, 184(16): 4137-53. e14. doi:10.1016/j.cell.2021.06.019 |
| [40] | Han F, Wang Y, Han YY, et al. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats[J]. J Agric Food Chem, 2018, 66(25): 6326-35. doi:10.1021/acs.jafc.8b01891 |
| [41] | Wang H, Li ML, Jiao FR, et al. Soluble dietary fibers from solid-state fermentation of wheat bran by the fungus Cordyceps cicadae and their effects on colitis mice[J]. Food Funct, 2024, 15(2): 516-29. doi:10.1039/d3fo03851c |
| [42] | de Vos WM, Tilg H, Van Hul M, et al. Gut microbiome and health: mechanistic insights[J]. Gut, 2022, 71(5): 1020-32. doi:10.1136/gutjnl-2021-326789 |
| [1] | 邓楚玉, 王雪莹, 甘立祥, 王大禹, 郑晓燕, 唐纯志. 电针足三里改善高脂血症小鼠的血脂紊乱:基于肠道菌群结构的改善[J]. 南方医科大学学报, 2025, 45(8): 1633-1642. |
| [2] | 韩瑞敏, 赵曼可, 袁俊芳, 史振红, 王珍, 王德峰. 枯草杆菌二联活菌肠溶胶囊调节2型糖尿病合并昼夜节律紊乱小鼠糖脂代谢的作用机制[J]. 南方医科大学学报, 2025, 45(7): 1490-1497. |
| [3] | 黄凯悦, 齐景馨, 罗文谦, 林怡萱, 陈梅妹, 甘慧娟. 温胆汤通过调控肠道菌群-胆汁酸轴改善代谢综合征痰证大鼠的代谢表型[J]. 南方医科大学学报, 2025, 45(6): 1174-1184. |
| [4] | 罗嘉纯, Sodnomjamts Batzaya, 高雪锋, 陈晶宇, 余政颖, 熊莎莎, 曹虹. Akkermansia muciniphila改善gp120转基因小鼠的肠-脑相互作用障碍[J]. 南方医科大学学报, 2025, 45(3): 554-565. |
| [5] | 许欣筑, 郭丽娜, 郑康帝, 马燕, 林淑娴, 何盈犀, 盛雯, 许素哗, 邱峰. 副干酪乳酪杆菌E6通过其代谢物改善长春瑞滨诱导的斑马鱼免疫抑制[J]. 南方医科大学学报, 2025, 45(2): 331-339. |
| [6] | 刘莹, 李柏睿, 李永财, 常禄博, 王娇, 杨琳, 颜永刚, 屈凯, 刘继平, 张岗, 沈霞. 加味逍遥丸通过神经递质调节、抗炎抗氧化及肠道菌群调控改善大鼠的抑郁样行为[J]. 南方医科大学学报, 2025, 45(2): 347-358. |
| [7] | 喻珍妮, 高竟哲, 孙惠, 冯芹, 那效旗, 张宁, 沈昆双, 王媛媛, 王喜军. 肠道菌群、T细胞在结直肠癌发病中的因果关联:孟德尔随机化分析[J]. 南方医科大学学报, 2025, 45(12): 2756-2766. |
| [8] | 潘兴旭, 张秉祺, 张智华, 曹秋实. 戈登杆菌属丰度降低与肾结石风险增加相关:一项孟德尔随机化分析与动物实验研究[J]. 南方医科大学学报, 2025, 45(11): 2405-2415. |
| [9] | 林淑娴, 郭丽娜, 马燕, 熊尧, 何盈犀, 许欣筑, 盛雯, 许素哗, 邱峰. 植物乳植杆菌ZG03通过其代谢物短链脂肪酸缓解斑马鱼的氧化应激[J]. 南方医科大学学报, 2025, 45(10): 2223-2230. |
| [10] | 刘佳进, 缪长宏, 徐健康, 余伟杰, 陈继鑫, 唐好知, 刘爱峰. 肠道菌群与色素沉着绒毛结节性滑膜炎之间的因果关系:基于孟德尔随机化分析[J]. 南方医科大学学报, 2024, 44(7): 1397-1406. |
| [11] | 李新翼, 刘玉杰, 邓克崇, 胡义奎. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-410. |
| [12] | 朱继伟, 卢曼路, 焦倩倩, 孙运良, 刘 璐, 丁红红, 于 燕, 潘 磊. 基于16S rRNA测序分析阻塞性睡眠呼吸暂停患者肠道靶标菌群的变化[J]. 南方医科大学学报, 2024, 44(1): 146-155. |
| [13] | 库尔班乃木·卡合曼, 赵健锋, 穆凯代斯·艾合买提, 王汉铭, 朱稷蔚, 潘文涛, 卡思木江·阿西木江. 屎肠球菌QH06能减轻溃疡性结肠炎大鼠的结肠黏膜损伤[J]. 南方医科大学学报, 2022, 42(7): 976-987. |
| [14] | 王展强, 徐开宇, 周宏伟. 卒中患者肠道宏病毒组的组成和菌群特征[J]. 南方医科大学学报, 2021, 41(6): 862-869. |
| [15] | 肖 瑶, 牛 玥, 毛明慧, 林 涵, 汪佰丽, 乌恩奇, 赵焕虎, 李树春. 2型糖尿病与肠道核心菌群的相关性[J]. 南方医科大学学报, 2021, 41(3): 358-369. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||