南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (5): 941-949.doi: 10.12122/j.issn.1673-4254.2024.05.16
裴蓓1( ), 张艺1, 魏思源1, 梅语1, 宋标1, 董港1, 温子昂2, 李学军1(
), 张艺1, 魏思源1, 梅语1, 宋标1, 董港1, 温子昂2, 李学军1( )
)
收稿日期:2023-09-25
出版日期:2024-05-20
发布日期:2024-06-06
通讯作者:
李学军
E-mail:18356051572@163.com;lixujun0308@126.com
作者简介:裴 蓓,在读博士研究生,E-mail: 18356051572@163.com
基金资助:
Bei PEI1( ), Yi ZHANG1, Siyuan WEI1, Yu MEI1, Biao SONG1, Gang DONG1, Ziang WEN2, Xuejun LI1(
), Yi ZHANG1, Siyuan WEI1, Yu MEI1, Biao SONG1, Gang DONG1, Ziang WEN2, Xuejun LI1( )
)
Received:2023-09-25
Online:2024-05-20
Published:2024-06-06
Contact:
Xuejun LI
E-mail:18356051572@163.com;lixujun0308@126.com
摘要:
目的 探讨肠上皮化生(IM)的潜在关键致病基因。 方法 收集2022年1月~6月在安徽中医药大学第二附属医院脾胃科就诊的21例IM患者,以及同期在我院体检中心接受胃镜检查的21例健康受试者。对所有参与者均行胃镜及病理检查,收集胃组织样本进行转录组学测序以筛选疾病相关差异基因,并通过生物信息学分析明确其生物学功能。同时采用实时荧光定量PCR对结果进行验证。 结果 通过转录组学测序,最终获得了1373个差异基因,其中827个上调的mRNA和546个下调的mRNA。根据差异基因的显著性与平均表达量对其进行了排序,从中选取了6个前20的上调基因进行验证,RT-PCR结果显示,与正常组相比,AGMAT、CCL25、FABP1、CDX1、SPINK4和MUC2表达水平显著上调(P<0.05)。 结论 AGMAT、CCL25、FABP1、SPINK4、CDX1和MUC2可能是诊断肠上皮化生的潜在生物学标志物,参与了IM的发生、发展,对疾病的预测及诊断有一定的价值。
裴蓓, 张艺, 魏思源, 梅语, 宋标, 董港, 温子昂, 李学军. 基于转录组学测序及生物信息学方法鉴定肠上皮化生的潜在致病基因[J]. 南方医科大学学报, 2024, 44(5): 941-949.
Bei PEI, Yi ZHANG, Siyuan WEI, Yu MEI, Biao SONG, Gang DONG, Ziang WEN, Xuejun LI. Identification of potential pathogenic genes of intestinal metaplasia based on transcriptomic sequencing and bioinformatics analysis[J]. Journal of Southern Medical University, 2024, 44(5): 941-949.
| Name | Forward primer | Reverse primer |
|---|---|---|
| AGMAT | 5′-CTCACTCCTAGTCAGGCTC-3′ | 5′-TGAAACTTCGACAAGATCACAG-3′ |
| CCL25 | 5′-CACCCAAGGTGTCTTTGAG-3′ | 5′-CTGGATCCGGTAAGTCCAG-3′ |
| FABP1 | 5′-CATGAAGGCAATCGGTCTG-3′ | 5′-CCATTCTGCACGATTTCCG-3′ |
| SPINK4 | 5′-TCAAGAATGCCCATCTGTG-3′ | 5′-ATATGTGAGCCCATCAGTG-3′ |
| CDX1 | 5′-TCGGACCAAGGACAAGTACC-3′ | 5′-AGATCTTCACCTGCCGTTCA-3′ |
| MUC2 | 5′-GCTGTCCCTTCTACTGGTGT-3′ | 5′-GTTGAGCAGGGTGTTGTTGT-3′ |
表1 关键基因的PCR引物序列
Tab.1 PCR primer sequences of the target genes
| Name | Forward primer | Reverse primer |
|---|---|---|
| AGMAT | 5′-CTCACTCCTAGTCAGGCTC-3′ | 5′-TGAAACTTCGACAAGATCACAG-3′ |
| CCL25 | 5′-CACCCAAGGTGTCTTTGAG-3′ | 5′-CTGGATCCGGTAAGTCCAG-3′ |
| FABP1 | 5′-CATGAAGGCAATCGGTCTG-3′ | 5′-CCATTCTGCACGATTTCCG-3′ |
| SPINK4 | 5′-TCAAGAATGCCCATCTGTG-3′ | 5′-ATATGTGAGCCCATCAGTG-3′ |
| CDX1 | 5′-TCGGACCAAGGACAAGTACC-3′ | 5′-AGATCTTCACCTGCCGTTCA-3′ |
| MUC2 | 5′-GCTGTCCCTTCTACTGGTGT-3′ | 5′-GTTGAGCAGGGTGTTGTTGT-3′ |
| Indexes | Control group | IM group | P |
|---|---|---|---|
| Age (year) | 56.76±10.64 | 60.62±9.02 | 0.2124 |
| Gender | 0.7579 | ||
| Male | 10 | 12 | |
| Female | 11 | 9 | |
| Smoking history | 0.6965 | ||
| Yes | 3 | 5 | |
| No | 18 | 16 | |
| Drinking history | 0.4841 | ||
| Yes | 4 | 7 | |
| No | 17 | 14 | |
| Diet | 0.6060 | ||
| Irregular | 1 | 3 | |
| Regular | 20 | 18 | |
| Diabetes | 0.9999 | ||
| Yes | 0 | 1 | |
| No | 21 | 20 | |
| Hypertension | 0.6965 | ||
| Yes | 3 | 5 | |
| No | 18 | 16 | |
| Hyperlipidemia | 0.7186 | ||
| Yes | 4 | 6 | |
| No | 17 | 15 | |
| Family history of tumors | 0.6060 | ||
| Yes | 1 | 3 | |
| No | 20 | 18 | |
| H. pylori infection | 0.9999 | ||
| Yes | 0 | 0 | |
| No | 21 | 21 |
表2 所有受试者的临床数据分析
Tab.2 Demographic and clinical characteristics of the 21 patients with intestinal metaplasia (IM) and the control subjects
| Indexes | Control group | IM group | P |
|---|---|---|---|
| Age (year) | 56.76±10.64 | 60.62±9.02 | 0.2124 |
| Gender | 0.7579 | ||
| Male | 10 | 12 | |
| Female | 11 | 9 | |
| Smoking history | 0.6965 | ||
| Yes | 3 | 5 | |
| No | 18 | 16 | |
| Drinking history | 0.4841 | ||
| Yes | 4 | 7 | |
| No | 17 | 14 | |
| Diet | 0.6060 | ||
| Irregular | 1 | 3 | |
| Regular | 20 | 18 | |
| Diabetes | 0.9999 | ||
| Yes | 0 | 1 | |
| No | 21 | 20 | |
| Hypertension | 0.6965 | ||
| Yes | 3 | 5 | |
| No | 18 | 16 | |
| Hyperlipidemia | 0.7186 | ||
| Yes | 4 | 6 | |
| No | 17 | 15 | |
| Family history of tumors | 0.6060 | ||
| Yes | 1 | 3 | |
| No | 20 | 18 | |
| H. pylori infection | 0.9999 | ||
| Yes | 0 | 0 | |
| No | 21 | 21 |
| Name | Description | P |
|---|---|---|
| AGMAT | Agmatinase | 3.51×10-13 |
| CCL25 | C-C motif chemokine ligand 25 | 8.86×10-13 |
| FABP1 | Fatty acid binding protein 1 | 6.22×10-11 |
| MUC2 | Mucin 2 | 1.95×10-10 |
| SPINK4 | Serine peptidase inhibitor Kazal type 4 | 1.04×10-9 |
| ANXA13 | Annexin A13 | 1.24×10-9 |
| GIP | Gastric inhibitory polypeptide | 2.01×10-9 |
| KCP | Kielin cysteine rich BMP regulator | 2.48×10-9 |
| DEFA5 | Defensin alpha 5 | 2.55×10-9 |
| ZG16 | Zymogen granule protein 16 | 4.38×10-9 |
| CDX1 | Caudal type homeobox 1 | 9.33×10-9 |
| RAB3B | RAB3B, member RAS oncogene family | 1.35×10-8 |
| PTAFR | Platelet activating factor receptor | 2.37×10-8 |
| RBP2 | Retinol binding protein 2 | 4.19×10-8 |
| OLFM4 | Olfactomedin 4 | 4.19×10-8 |
| ALPI | Alkaline phosphatase, intestinal | 6.04×10-8 |
| MOCOS | Molybdenum cofactor sulfurase | 8.81×10-8 |
| SLC6A20 | Solute carrier family 6 member 20 | 1.18×10-7 |
| HOXB9 | Homeobox B9 | 2.88×10-7 |
| CPS1 | Carbamoyl-phosphate synthase 1 | 3.00×10-7 |
表3 前20上调差异基因
Tab.3 Top 20 upregulated genes in intestinal metaplasia
| Name | Description | P |
|---|---|---|
| AGMAT | Agmatinase | 3.51×10-13 |
| CCL25 | C-C motif chemokine ligand 25 | 8.86×10-13 |
| FABP1 | Fatty acid binding protein 1 | 6.22×10-11 |
| MUC2 | Mucin 2 | 1.95×10-10 |
| SPINK4 | Serine peptidase inhibitor Kazal type 4 | 1.04×10-9 |
| ANXA13 | Annexin A13 | 1.24×10-9 |
| GIP | Gastric inhibitory polypeptide | 2.01×10-9 |
| KCP | Kielin cysteine rich BMP regulator | 2.48×10-9 |
| DEFA5 | Defensin alpha 5 | 2.55×10-9 |
| ZG16 | Zymogen granule protein 16 | 4.38×10-9 |
| CDX1 | Caudal type homeobox 1 | 9.33×10-9 |
| RAB3B | RAB3B, member RAS oncogene family | 1.35×10-8 |
| PTAFR | Platelet activating factor receptor | 2.37×10-8 |
| RBP2 | Retinol binding protein 2 | 4.19×10-8 |
| OLFM4 | Olfactomedin 4 | 4.19×10-8 |
| ALPI | Alkaline phosphatase, intestinal | 6.04×10-8 |
| MOCOS | Molybdenum cofactor sulfurase | 8.81×10-8 |
| SLC6A20 | Solute carrier family 6 member 20 | 1.18×10-7 |
| HOXB9 | Homeobox B9 | 2.88×10-7 |
| CPS1 | Carbamoyl-phosphate synthase 1 | 3.00×10-7 |
| Name | Description | P |
|---|---|---|
| CARNS1 | Carnosine synthase 1 | 2.38×10-13 |
| SH3GL2 | SH3 domain containing GRB2 like 2, endophilin A1FT | 3.08×10-13 |
| RGMB | Repulsive guidance molecule BMP co-receptor bFT | 2.28×10-10 |
| ZNF334 | Zinc finger protein 334 | 8.71×10-10 |
| WIPF3 | WAS/WASL interacting protein family member 3FT | 4.81×10-9 |
| PM20D1 | Peptidase M20 domain containing 1 | 5.08×10-9 |
| KLHDC8A | Kelch domain containing 8A | 1.34×10-8 |
| RGS7 | Regulator of G protein signaling 7 | 1.50×10-8 |
| RGN | Regucalcin | 2.75×10-8 |
| RPS6KA6 | Ribosomal protein S6 kinase A6 | 5.64×10-8 |
| NCMAP | Non-compact myelin associated protein | 5.99×10-8 |
| EDARADD | EDAR associated death domain | 7.13×10-8 |
| GPC3 | Glypican 3 | 7.53×10-8 |
| TMEM151A | Transmembrane protein 151A | 1.07×10-7 |
| PER3 | Period circadian regulator 3 | 1.20×10-7 |
| ZNF677 | Zinc finger protein 677 | 1.55×10-7 |
| RNF217 | Ring finger protein 217 | 1.75×10-7 |
| PWWP3B | PWWP domain containing 3B | 1.79×10-7 |
| ZFP28 | ZFP28 zinc finger protein | 1.91×10-7 |
| CYB5R1 | Cytochrome b5 reductase 1 | 2.74×10-7 |
表4 前20下调差异基因
Tab.4 Top 20 downregulated genes in intestinal metaplasia
| Name | Description | P |
|---|---|---|
| CARNS1 | Carnosine synthase 1 | 2.38×10-13 |
| SH3GL2 | SH3 domain containing GRB2 like 2, endophilin A1FT | 3.08×10-13 |
| RGMB | Repulsive guidance molecule BMP co-receptor bFT | 2.28×10-10 |
| ZNF334 | Zinc finger protein 334 | 8.71×10-10 |
| WIPF3 | WAS/WASL interacting protein family member 3FT | 4.81×10-9 |
| PM20D1 | Peptidase M20 domain containing 1 | 5.08×10-9 |
| KLHDC8A | Kelch domain containing 8A | 1.34×10-8 |
| RGS7 | Regulator of G protein signaling 7 | 1.50×10-8 |
| RGN | Regucalcin | 2.75×10-8 |
| RPS6KA6 | Ribosomal protein S6 kinase A6 | 5.64×10-8 |
| NCMAP | Non-compact myelin associated protein | 5.99×10-8 |
| EDARADD | EDAR associated death domain | 7.13×10-8 |
| GPC3 | Glypican 3 | 7.53×10-8 |
| TMEM151A | Transmembrane protein 151A | 1.07×10-7 |
| PER3 | Period circadian regulator 3 | 1.20×10-7 |
| ZNF677 | Zinc finger protein 677 | 1.55×10-7 |
| RNF217 | Ring finger protein 217 | 1.75×10-7 |
| PWWP3B | PWWP domain containing 3B | 1.79×10-7 |
| ZFP28 | ZFP28 zinc finger protein | 1.91×10-7 |
| CYB5R1 | Cytochrome b5 reductase 1 | 2.74×10-7 |
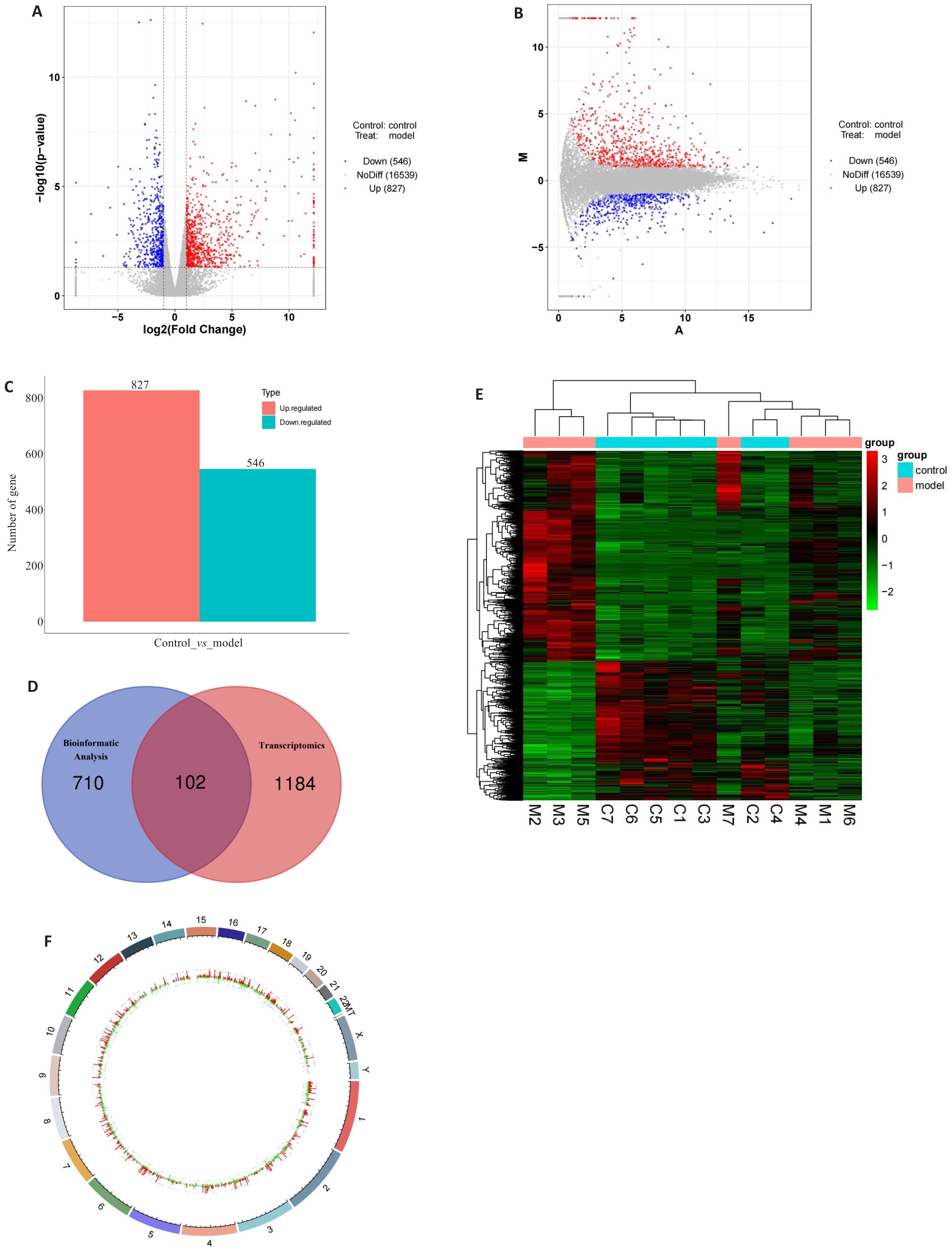
图1 IM相关差异基因的筛选和分析
Fig.1 Screening and analysis of the differentially expressed genes in intestinal metaplasia. A: Volcano plot of the differentially expressed genes. B: MA volcano plot of the differentially expressed genes. C: Histogram of the number of the differentially expressed genes. D: Venn diagram of the differentially expressed genes in transcriptomics sequencing group and the bioinformatic analysis group. E: Heatmap of the distribution of the differentially expressed genes in gastric tissues between the experimental and control groups. F: Genomic circle plot of the differentially expressed genes.
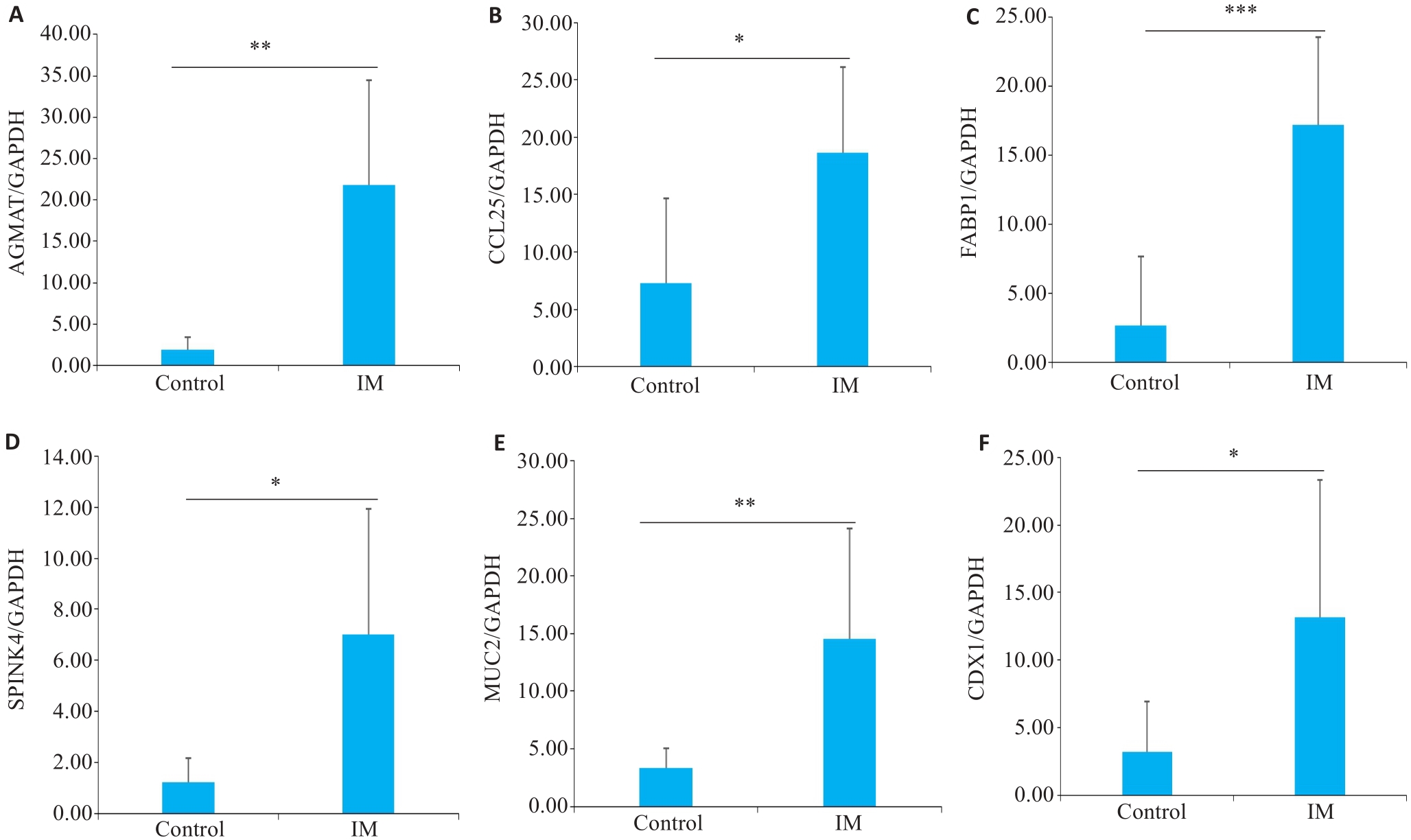
图5 关键基因的mRNA表达水平
Fig.5 Results of qRT-PCR for detecting mRNA expressions of AGMAT, CCL25, FABP1, SPINK4, MUC2 and CDX1 (A-F, respectively) in the gastric tissues of patients with intestinal metaplasia (IM) and the control subjects. *P<0.05, **P<0.01, ***P<0.001.
| 1 | Jonaitis P, Kupcinskas L, Kupcinskas J. Molecular alterations in gastric intestinal Metaplasia [J]. Int J Mol Sci, 2021, 22(11): 5758. DOI: 10.3390/ijms22115758 |
| 2 | Aumpan N, Vilaichone RK, Pornthisarn B, et al. Predictors for regression and progression of intestinal metaplasia (IM): a large population-based study from low prevalence area of gastric cancer (IM-predictor trial)[J]. PLoS One, 2021, 16(8): e0255601. DOI: 10.1371/journal.pone.0255601 |
| 3 | Lee JWJ, Zhu F, Srivastava S, et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: a prospective multicentre cohort study (GCEP)[J]. Gut, 2022, 71(5): 854-63. DOI: 10.1136/gutjnl-2021-324057 |
| 4 | Gawron AJ, Shah SC, Altayar O, et al. AGA technical review on gastric intestinal Metaplasia-natural history and clinical outcomes[J]. Gastroenterology, 2020, 158(3): 705-31. e5. DOI: 10.1053/j.gastro.2019.12.001 |
| 5 | Hrdlickova R, Toloue M, Tian B. RNA-Seq methods for transcriptome analysis[J]. Wiley Interdiscip Rev RNA, 2017, 8(1): 10.1002/wrna.1364. DOI: 10.1002/wrna.1364 |
| 6 | Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics[J]. Nat Rev Genet, 2009, 10(1): 57-63. DOI: 10.1038/nrg2484 |
| 7 | Saeidian AH, Youssefian L, Vahidnezhad H, et al. Research techniques made simple: whole-transcriptome sequencing by RNA-seq forDiagnosis of monogenic disorders[J]. J Investig Dermatol, 2020, 140(6): 1117-26. e1. DOI: 10.1016/j.jid.2020.02.032 |
| 8 | Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator[J]. Database, 2010, 2010: baq020. DOI: 10.1093/database/baq020 |
| 9 | Piñero J, Ramírez-Anguita JM, Saüch-Pitarch J, et al. The DisGeNET knowledge platform for disease genomics: 2019 update[J]. Nucleic Acids Res, 2020, 48(D1): D845-55. |
| 10 | Tjandra D, Busuttil RA, Boussioutas A. Gastric intestinal Metaplasia: challenges and the opportunity for precision prevention[J]. Cancers, 2023, 15(15): 3913. DOI: 10.3390/cancers15153913 |
| 11 | Akbari M, Tabrizi R, Kardeh S, et al. Gastric cancer in patients with gastric atrophy and intestinal metaplasia: a systematic review and meta-analysis[J]. PLoS One, 2019, 14(7): e0219865. DOI: 10.1371/journal.pone.0219865 |
| 12 | Shin SY, Kim JH, Chun J, et al. Chronic atrophic gastritis and intestinal metaplasia surrounding diffuse-type gastric cancer: are they just bystanders in the process of carcinogenesisJ]PLoS One?, 2019, 14(12): e0226427. DOI: 10.1371/journal.pone.0226427 |
| 13 | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: first American Cancer Society Award Lecture on Cancer Epidemiology and Prevention[J]. Cancer Res, 1992, 52(24): 6735-40. |
| 14 | Pei B, Wen ZA, Yang Q, et al. Risk factors analysis and prediction model establishment of intestinal Metaplasia or dysplasia in patients with chronic atrophic gastritis: a multi-center retrospective study[J]. Front Med, 2022, 9: 912331. DOI: 10.3389/fmed.2022.912331 |
| 15 | Ogutmen Koc D, Bektas S. Serum pepsinogen levels and OLGA/OLGIM staging in the assessment of atrophic gastritis types[J]. Postgrad Med J, 2022, 98(1160): 441-5. DOI: 10.1136/postgradmedj-2020-139183 |
| 16 | 李娜娜, 齐 涛, 朱黎明. 血清胃蛋白酶原、胃泌素17和幽门螺杆菌IgG抗体在胃部疾病初筛中的临床价值[J]. 诊断学理论与实践, 2022, 21(4): 509-13. |
| 17 | Zhang Y, Cao LJ, Xie YY, et al. Agmatinase facilitates the tumorigenesis of pancreatic adenocarcinoma through the TGFβ/Smad pathway[J]. Exp Ther Med, 2022, 24(2): 490. DOI: 10.3892/etm.2022.11417 |
| 18 | Zhu HE, Yin JY, Chen DX, et al. Agmatinase promotes the lung adenocarcinoma tumorigenesis by activating the NO-MAPKs-PI3K/Akt pathway[J]. Cell Death Dis, 2019, 10(11): 854. DOI: 10.1038/s41419-019-2082-3 |
| 19 | Xie YY, Zhang Y, Liu XJ, et al. MiR-151a-5p promotes the proliferation and metastasis of colorectal carcinoma cells by targeting AGMAT[J]. Oncol Rep, 2023, 49(3): 50. DOI: 10.3892/or.2023.8487 |
| 20 | Wang DL, Su Q, Qu Q, et al. Adeno-associated virus-mediated knockdown of agmatinase attenuates inflammation and tumorigenesis in a mouse model of colitis-associated colorectal cancer[J]. Hum Gene Ther, 2023, 34(11/12): 518-29. DOI: 10.1089/hum.2022.191 |
| 21 | Jarade A, Garcia Z, Marie S, et al. Inflammation triggers ILC3 patrolling of the intestinal barrier[J]. Nat Immunol, 2022, 23(9): 1317-23. DOI: 10.1038/s41590-022-01284-1 |
| 22 | Wu X, Sun M, Yang Z, et al. The roles of CCR9/CCL25 in inflammation and inflammation-associated diseases[J]. Front Cell Dev Biol, 2021, 9: 686548. DOI: 10.3389/fcell.2021.686548 |
| 23 | Yu H, Mei Y, Dong Y, et al. CCR9-CCL25 mediated plasmacytoid dendritic cell homing and contributed the immunosuppressive microenvironment in gastric cancer[J]. Transl Oncol, 2023, 33: 101682. DOI: 10.1016/j.tranon.2023.101682 |
| 24 | Niu YX, Tang DF, Fan LW, et al. CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner[J]. Exp Ther Med, 2020, 19(6): 3571-80. |
| 25 | Du DY, Liu C, Qin MY, et al. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma[J]. Acta Pharm Sin B, 2022, 12(2): 558-80. DOI: 10.1016/j.apsb.2021.09.019 |
| 26 | Liu SY, Ni CX, Li YZ, et al. The involvement of TRIB3 and FABP1 and their potential functions in the dynamic process of gastric cancer[J]. Front Mol Biosci, 2021, 8: 790433. DOI: 10.3389/fmolb.2021.790433 |
| 27 | Hashimoto T, Kusakabe T, Watanabe K, et al. Liver-type fatty acid-binding protein is highly expressed in intestinal metaplasia and in a subset of carcinomas of the stomach without association with the fatty acid synthase status in the carcinoma[J]. Pathobiology, 2004, 71(3): 115-22. DOI: 10.1159/000076465 |
| 28 | Wang XJ, Yu Q, Ghareeb WM, et al. Downregulated SPINK4 is associated with poor survival in colorectal cancer[J]. BMC Cancer, 2019, 19(1): 1258. DOI: 10.1186/s12885-019-6484-5 |
| 29 | Chen TJ, Tian YF, Chou CL, et al. High SPINK4 expression predicts poor outcomes among rectal cancer patients receiving CCRT[J]. Curr Oncol, 2021, 28(4): 2373-84. DOI: 10.3390/curroncol28040218 |
| 30 | Owen RP, White MJ, Severson DT, et al. Single cell RNA-seq reveals profound transcriptional similarity between Barrett’s oesophagus and oesophageal submucosal glands[J]. Nat Commun, 2018, 9(1): 4261. DOI: 10.1038/s41467-018-06796-9 |
| 31 | Brenna Ø, Furnes MW, Drozdov I, et al. Relevance of TNBS-colitis in rats: a methodological study with endoscopic, histologic and Transcriptomic characterization and correlation to IBD[J]. PLoS One, 2013, 8(1): e54543. DOI: 10.1371/journal.pone.0054543 |
| 32 | Häsler R, Feng Z, Bäckdahl L, et al. A functional methylome map of ulcerative colitis[J]. Genome Res, 2012, 22(11): 2130-7. DOI: 10.1101/gr.138347.112 |
| 33 | Liu Y, Yu XJ, Zhao JX, et al. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression[J]. Int J Biol Macromol, 2020, 164: 884-91. DOI: 10.1016/j.ijbiomac.2020.07.191 |
| 34 | Kerckhoffs KGP, Liu DHW, Saragoni L, et al. Mucin expression in gastric- and gastro-oesophageal signet-ring cell cancer: results from a comprehensive literature review and a large cohort study of Caucasian and Asian gastric cancer[J]. Gastric Cancer, 2020, 23(5): 765-79. DOI: 10.1007/s10120-020-01086-0 |
| 35 | Yokoyama S, Hamada T, Higashi M, et al. Predicted prognosis of patients with pancreatic cancer by machine learning[J]. Clin Cancer Res, 2020, 26(10): 2411-21. DOI: 10.1158/1078-0432.ccr-19-1247 |
| 36 | Astashchanka A, Shroka TM, Jacobsen BM. Mucin 2 (MUC2) modulates the aggressiveness of breast cancer[J]. Breast Cancer Res Treat, 2019, 173(2): 289-99. DOI: 10.1007/s10549-018-4989-2 |
| 37 | Ribeirinho-Soares S, Pádua D, Amaral AL, et al. Prognostic significance of MUC2, CDX2 and SOX2 in stage II colorectal cancer patients[J]. BMC Cancer, 2021, 21(1): 359. DOI: 10.1186/s12885-021-08070-6 |
| 38 | Li YX, Jiang LB, Li ZC, et al. Differences in gastric microbiota and mucosal function between patients with chronic superficial gastritis and intestinal metaplasia[J]. Front Microbiol, 2022, 13: 950325. DOI: 10.3389/fmicb.2022.950325 |
| 39 | Battista S, Ambrosio MR, Limarzi F, et al. Molecular alterations in gastric preneoplastic lesions and early gastric cancer[J]. Int J Mol Sci, 2021, 22(13): 6652. DOI: 10.3390/ijms22136652 |
| 40 | Grainger S, Hryniuk A, Lohnes D. Cdx1 and Cdx2 exhibit transcriptional specificity in the intestine[J]. PLoS One, 2013, 8(1): e54757. DOI: 10.1371/journal.pone.0054757 |
| 41 | Almeida R, Silva E, Santos-Silva F, et al. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas[J]. J Pathol, 2003, 199(1): 36-40. DOI: 10.1002/path.1246 |
| 42 | Kang JM, Lee BH, Kim N, et al. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer[J]. J Korean Med Sci, 2011, 26(5): 647-53. DOI: 10.3346/jkms.2011.26.5.647 |
| 43 | Wong NA, Wilding J, Bartlett S, et al. CDX1 is an important molecular mediator of Barrett’s metaplasia[J]. Proc Natl Acad Sci USA, 2005, 102(21): 7565-70. DOI: 10.1073/pnas.0502031102 |
| 44 | Rau TT, Rogler A, Frischauf M, et al. Methylation-dependent activation of CDX1 through NF-κB[J]. Am J Pathol, 2012, 181(2): 487-98. DOI: 10.1016/j.ajpath.2012.04.028 |
| [1] | 周伟, 聂军, 胡佳, 蒋艺枝, 张大发. 内质网应激相关基因在主动脉夹层疾病中的差异性表达及与免疫浸润的相关性[J]. 南方医科大学学报, 2024, 44(5): 859-866. |
| [2] | 王沁智, 宋冰, 郝诗睿, 肖志远, 金连辉, 郑通, 柴芳. 基于生物信息学分析CCND2在甲状腺乳头状癌中的表达及其对免疫浸润的影响[J]. 南方医科大学学报, 2024, 44(5): 981-988. |
| [3] | 王梓凝, 杨 明, 李双磊, 迟海涛, 王军惠, 肖苍松. 心肌梗死后心肌纤维化小鼠心肌线粒体功能和能量代谢重塑相关性的转录组学分析[J]. 南方医科大学学报, 2024, 44(4): 666-674. |
| [4] | 梁一豪, 赖颖君, 袁燕文, 袁 炜, 张锡波, 张拔山, 卢志锋. 基于GEO数据库筛选胃癌差异表达基因及其功能和通路富集分析[J]. 南方医科大学学报, 2024, 44(3): 605-616. |
| [5] | 成佳聪, 李智慧, 刘 鳐, 李 成, 黄 鑫, 田颖鑫, 沈富兵. 早幼粒细胞白血病蛋白与TAK1结合蛋白相互作用的生物信息学分析与验证[J]. 南方医科大学学报, 2024, 44(1): 179-186. |
| [6] | 吴秀华, 范应静, 叶永浓, 李 萍, 朱青安, 陈泽森, 李 博, 王 文, 郑 磊. 生酮饮食导致小鼠骨质疏松的转录组学[J]. 南方医科大学学报, 2023, 43(8): 1440-1446. |
| [7] | 王秋生, 张 震, 王 炼, 汪 煜, 姚新宇, 王月月, 张小凤, 葛思堂, 左芦根. 高表达DAP5促进胃癌细胞的糖代谢并与不良预后相关[J]. 南方医科大学学报, 2023, 43(7): 1063-1070. |
| [8] | 张伟健, 邹琸玥, 朱永娜, 王 敏, 马彩云, 武峻捷, 石 昕, 刘 茜. IL-34在舌鳞状细胞癌中的表达及意义[J]. 南方医科大学学报, 2023, 43(12): 2111-2117. |
| [9] | 辛宝山, 刘 刚, 张成雄, 汪 兵, 史良会. LncRNA LINC00342通过靶向 miR-596 促进胃癌细胞的增殖、迁移和侵袭[J]. 南方医科大学学报, 2023, 43(10): 1761-1770. |
| [10] | 洪海宁, 朱浩楠, 李 超, 臧 超, 桑海威, 陈力维, 王安生. FNDC1在肺腺癌中高表达并与不良预后相关[J]. 南方医科大学学报, 2022, 42(8): 1182-1190. |
| [11] | 黄 平, 朱 晶, 李 华, 王艳召, 汤益民, 刘 强. 奥氮平连续处理后大鼠中缝背核组织差异表达蛋白的生物信息学分析[J]. 南方医科大学学报, 2022, 42(8): 1221-1229. |
| [12] | 王 康, 张 军, 邓牧文, 剧永乐, 欧阳满照. 甲基转移酶样蛋白27是结肠癌预后的生物标志物并与免疫浸润相关[J]. 南方医科大学学报, 2022, 42(4): 486-497. |
| [13] | 张晓宁, 张晓瑜, 刘 鹏, 刘 阔, 李文文, 陈倩倩, 马万山. 白三烯B4受体在急性髓系白血病中的预后价值和功能富集分析[J]. 南方医科大学学报, 2022, 42(3): 309-320. |
| [14] | 朱雅莉, 章述军, 阳 成, 薛 薇, 张 佳, 李佳俊, 赵金秋, 徐 静, 黄文祥. 非酒精性脂肪性肝病患者的肝组织差异蛋白的定量分析:基于iTRAQ技术[J]. 南方医科大学学报, 2021, 41(9): 1381-1387. |
| [15] | 文智慧, 梁炜祺, 钟依秀, 孙 菲, 张庆玲. 烟酰胺-N-甲基转移酶在胃癌中高表达并与预后负相关:基于生物信息学方法[J]. 南方医科大学学报, 2021, 41(6): 828-838. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||