Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (6): 1163-1173.doi: 10.12122/j.issn.1673-4254.2025.06.06
Previous Articles Next Articles
Jiahao LI( ), Ruiting XIAN, Rong LI(
), Ruiting XIAN, Rong LI( )
)
Received:2025-01-05
Online:2025-06-20
Published:2025-06-27
Contact:
Rong LI
E-mail:731997813@qq.com;nflirong@163.com
Supported by:Jiahao LI, Ruiting XIAN, Rong LI. Down-regulation of ACADM-mediated lipotoxicity inhibits invasion and metastasis of estrogen receptor-positive breast cancer cells[J]. Journal of Southern Medical University, 2025, 45(6): 1163-1173.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.06.06
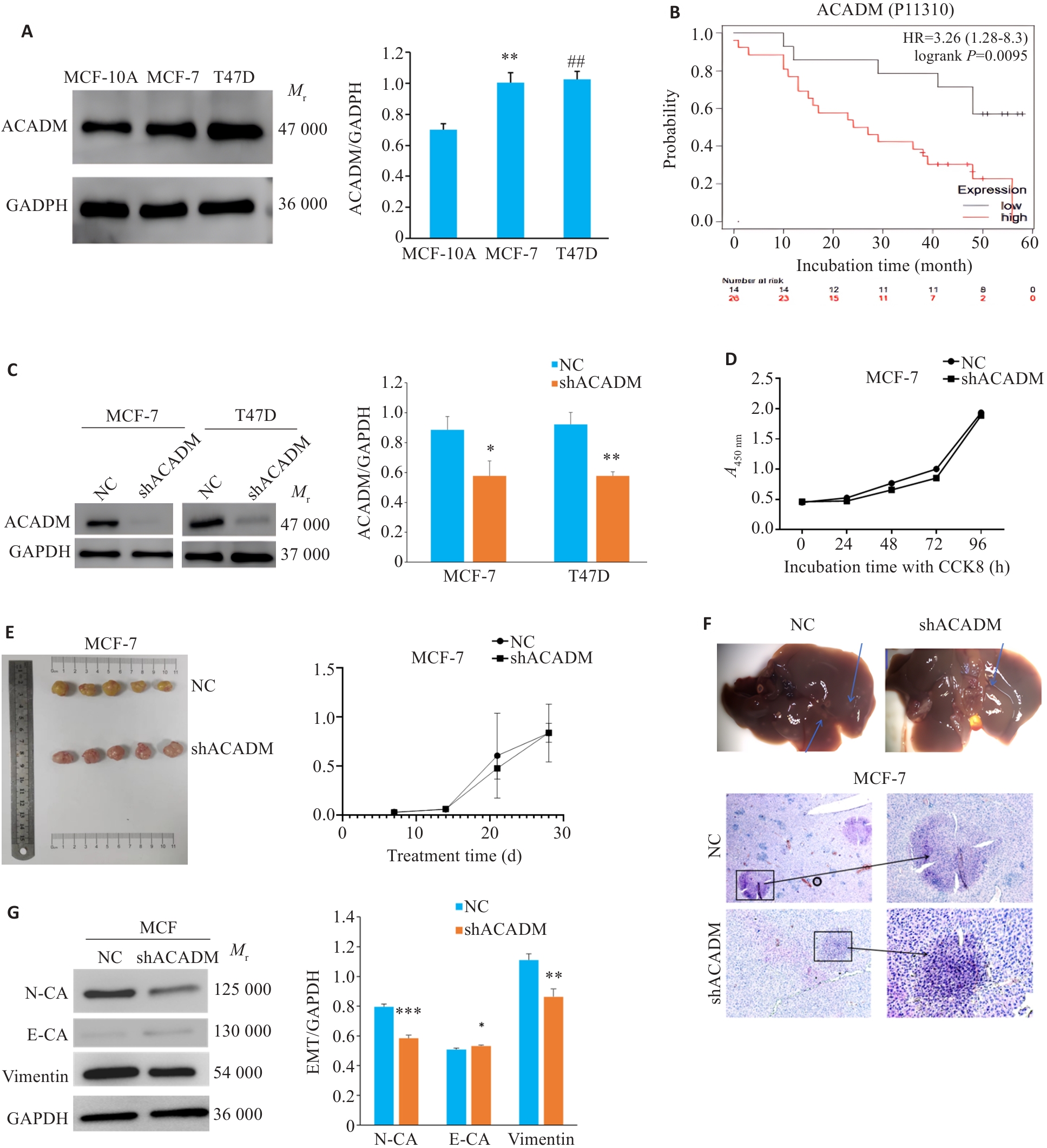
Fig.1 Expression level of ACADM in breast cancer tissues and its effect on malignant behaviors of estrogen receptor (ER)-positive breast cancer cells. A: Expression levels of ACADM detected by Western blotting in normal breast epithelial cells and ER-positive breast cancer cell lines (**P<0.01, ##P<0.01 vs MCF-10A cells). B: Prognosis of breast cancer patients with low and high ACADM expression obtained from the Kaplan-Meier Plotter bioinformatics website. C: Expression of ACADM protein in MCF-7 and T47D cells were detected by Western blotting after down-regulation of ACADM (*P<0.05, **P<0.01 vs NC). D: CCK8 assay for assessing proliferation of MCF-7 cells after down-regulation of ACADM. E: Changes of tumor size of in situ MCF-7 cell xenograft with ACADM knockdown in nude mice. F: HE staining of hepatic metastases in nude mice with tail vein injection MCF-7 cells with or without ACADM knockdown (Original magnification:×200). G: Western blotting for detecting protein expressions in the EMT signaling pathway in MCF-7 cells with ACADM knockdown. *P<0.05, **P<0.01, ***P<0.001 vs NC.

Fig.2 Down-regulation of ACADM-mediated lipotoxicity attenuates invasiveness of MCF-7 and T47D cells. A: Lipid droplets in shACADM MCF7 and T47D cells detected by Oil red O staining (×100). B: Free fatty acid content in shACADM MCF7 and T47D cells detected using a microplate reader (**P<0.01, ***P<0.001 vs NC). C: Ester CoA contents are increased in shACADM MCF-7 and T47D cells (*P<0.05 vs NC). D:Transwell assay for assessing invasiveness of MCF-7 and T47D cells with ACADM knockdown cultured in 4% serum (**P<0.01 vs shACADM). E: Boyden chamber assay for assessing migration ability of MCF-7 and T47D cells with ACADM knockdown cultured in 4% serum (*P<0.05, **P<0.01 vs shACADM).
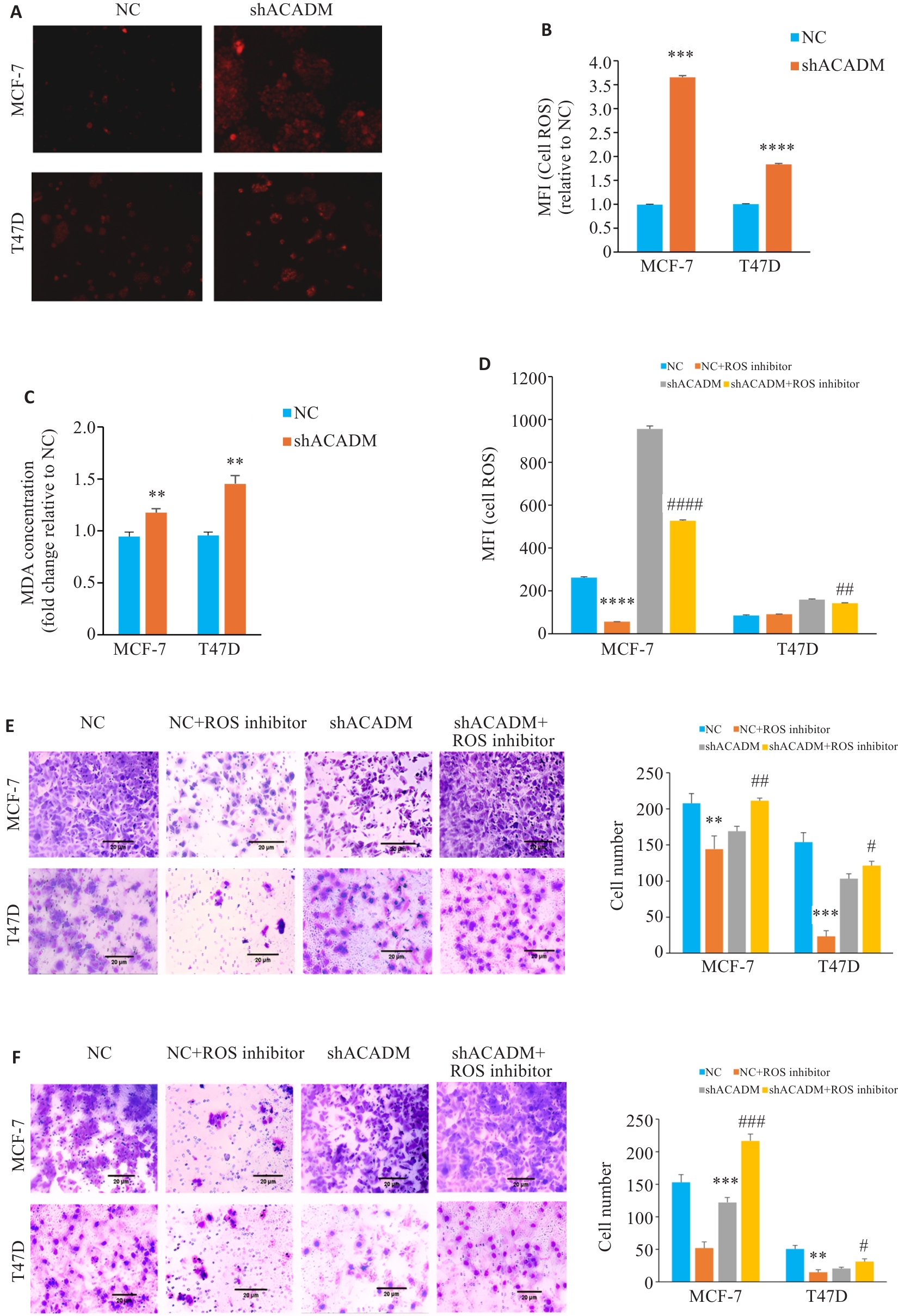
Fig.3 ACADM knockdown results in increased levels of ROS in breast cancer cells. A: ROS levels in MCF-7 and T47D cells with ACADM knockdown detected by fluorescence microscopy (×100). B: ROS levels in MCF-7 and T47D cells with ACADM knockdown detected by flow cytometry (***P<0.001, ****P<0.0001 vs NC). C: Lipid oxidation (MDA) in MCF-7 and T47D cells with ACADM knockdown detected by colorimetric method (**P<0.01 vs NC). D: ROS content in MCF-7 and T47D cells with ACADM knockdown detected by flow cytometry after treatment with ROS scavenger (****P<0.0001 vs NC; ##P<0.01, ####P<0.0001 vs shACADM). E: Invasion and migration of MCF-7 and T47D cells with ACADM knockdown detected by Transwell assay after treatment with ROS scavengers (**P<0.01, ***P<0.001 vs NC; #P<0.05, ##P<0.01 vs shACADM). F: Invasion and migration of MCF-7 and T47D cells with ACADM knockdown detected by Boyden chamber assay after treatment with ROS scavengers (**P<0.01, ***P<0.001 vs NC; #P<0.05, ###P<0.001 vs shACADM).
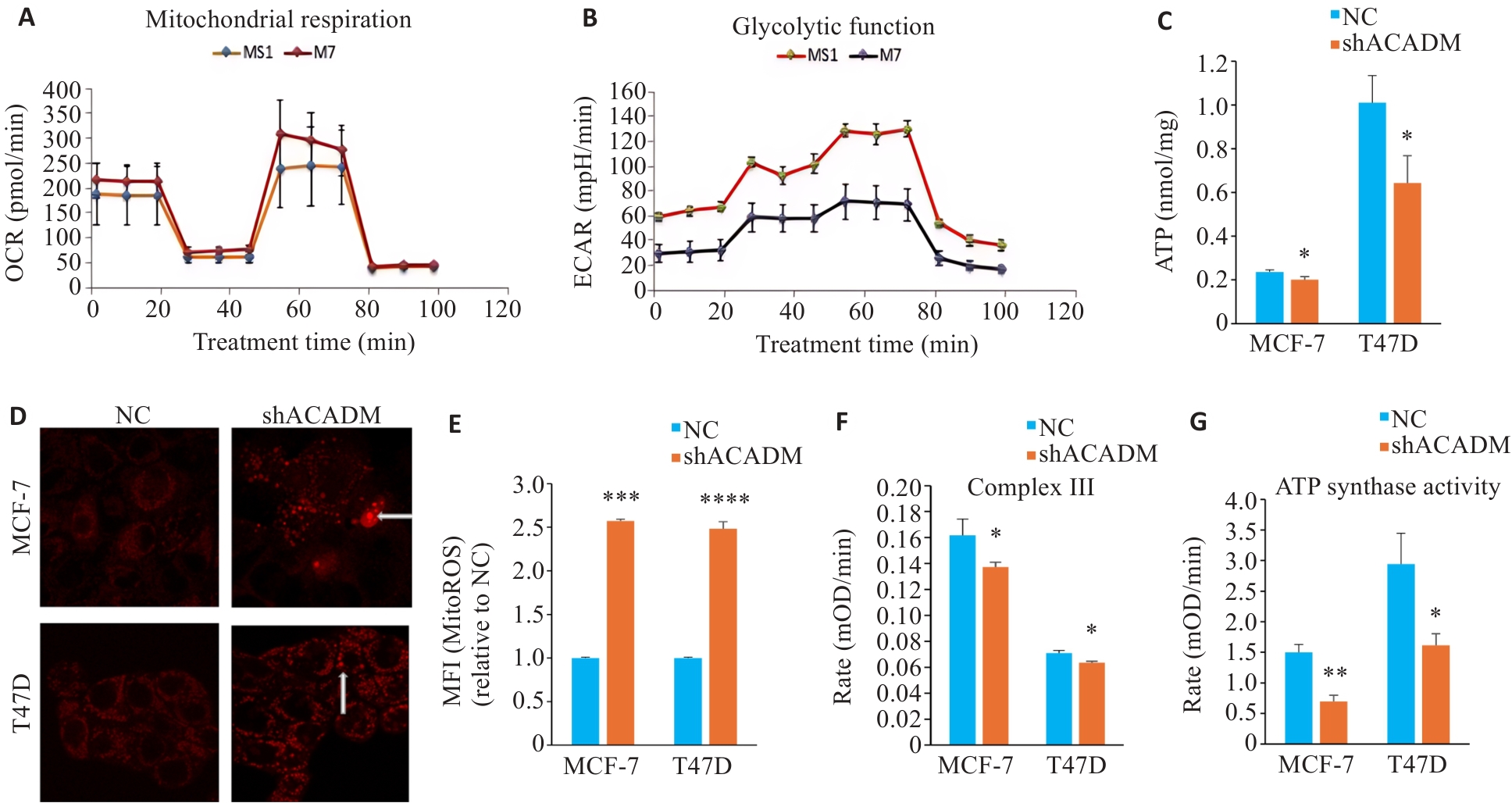
Fig.4 Downregulation of ACADM impairs mitochondrial function in breast cancer cells. A: Seahorse technique for detecting oxygen consumption in MCF-7 cells with ACADM knockdown. B: Glycolysis in MCF-7 cells with ACADM knockdown detected using Seahorse technique. C: ATP content in MCF-7 and T47D cells with ACADM knockdown (*P<0.05 vs NC). D: Mitochondrial ROS in MCF-7 and T47D cells with ACADM knockdown detected by confocal fluorescence microscopy (×100). E: Content of mitochondrial ROS in MCF-7 and T47D with ACADM knockdown detected by flow cytometry (***P<0.001, ****P<0.0001 vs NC). F: Activity of mitochondrial respiratory chain complex III in shACADM MCF-7 and T47D cells (*P<0.05 vs NC). G: Activity of mitochondrial respiratory chain complex V in shACADM MCF-7 and T47D cells (*P<0.05, **P<0.01 vs NC).
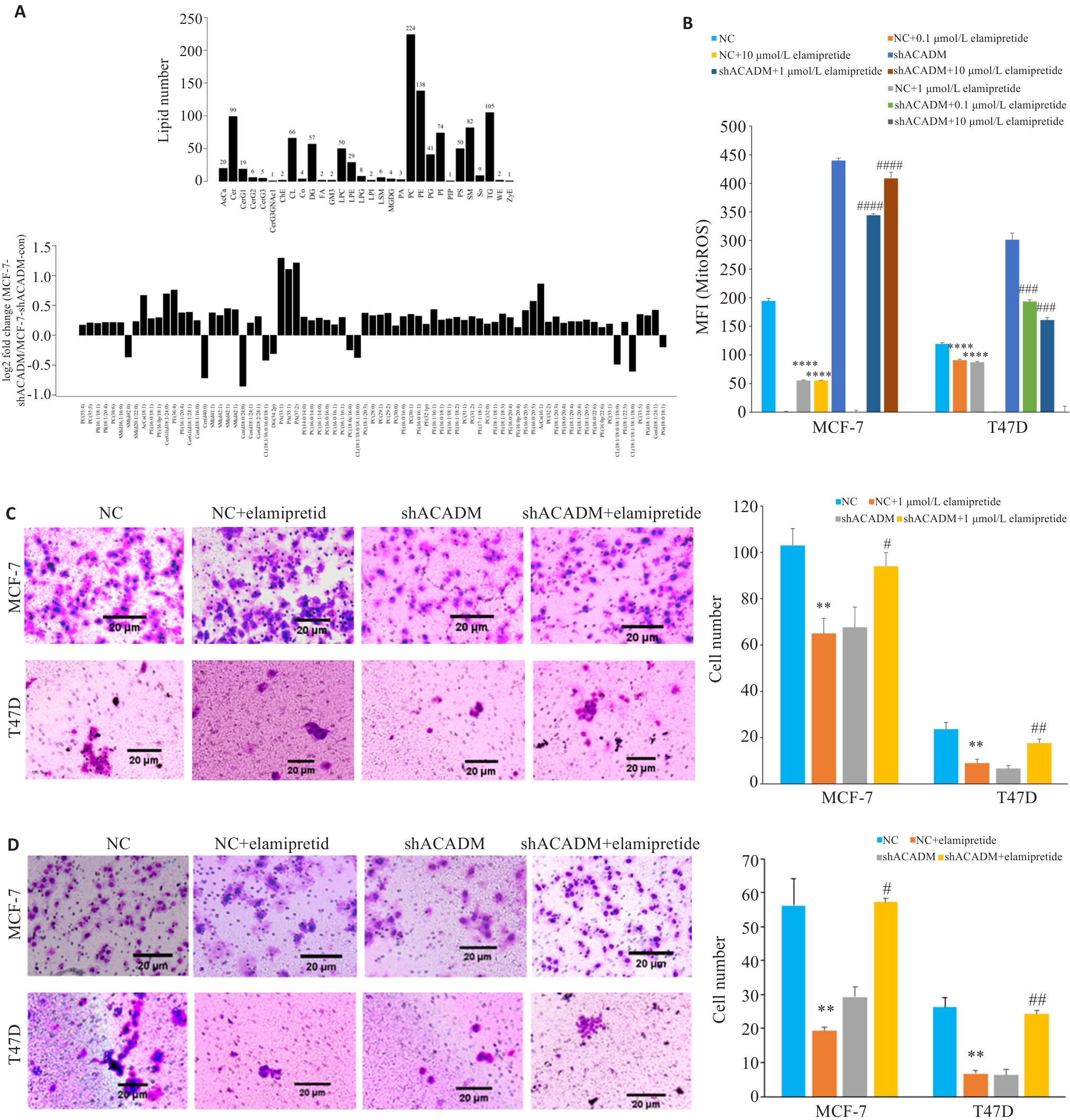
Fig.5 ACADM knockdown reduces mitochondrial cardiolipin to attenuate invasion and migration of breast cancer cells. A: Hierarchical clustering of lipid species in shACADM MCF-7 and T47D cells. B: Contents of mitochondrial ROS detected by flow cytometry in shACADM MCF-7 and T47D cells treated with Elamipretide (****P<0.0001 vs NC; ###P<0.001, ####P<0.0001 vs shACADM). C: Invasion and migration ability of Elamipretide-treated shACADM MCF-7 and T47D cells detected by Transwell assay (**P<0.01 vs NC; #P<0.05, ##P<0.01 vs shACADM). D: Invasion and migration ability of Elamipretide-treated shACADM MCF-7 and T47D cells detected by Boyden chamber assay (**P<0.01 vs NC; #P<0.05, ##P<0.01 vs shACADM).
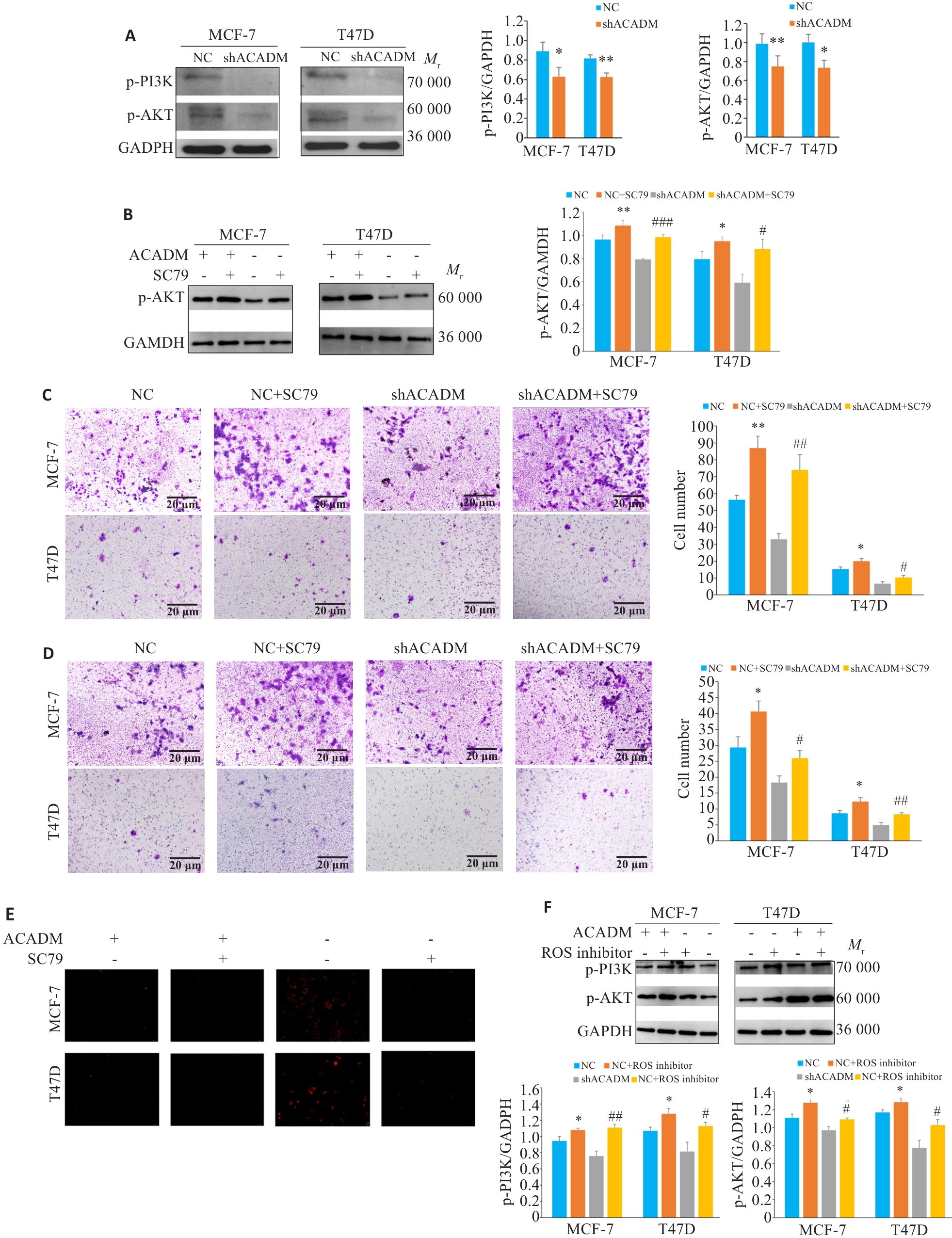
Fig.6 ACADM knockdown affects cell invasion capacity by regulating the PI3K/AKT pathway. A: Expressions of PI3K-AKT pathway proteins in shACADM MCF-7 cells and T47D cells detected by Western blotting (*P<0.05, **P<0.01 vs NC). B: Expression of p-AKT protein in shACADM MCF-7 cells and T47D cells with SC79 treatment detected by Western blotting (*P<0.05, **P<0.01 vs NC; #P<0.05, ###P<0.001 vs shACADM). C: Invasion and metastasis ability of shACADM MCF-7 and T47D cells detected by Transwell assay after SC79 treatment (*P<0.05, **P<0.01vs NC; #P<0.05, ##P<0.01 vs shACADM). D: Invasion and migration ability of shACADM MCF-7 and T47D cells detected by Boyden chamber assay after SC79 treatment (*P<0.05 vs NC; #P<0.05, ##P<0.01 vs shACADM). E: ROS levels in shACADM MCF-7 and T47D cells detected by fluorescence microscope after ROS inhibitor treatment (×100). F: Expressions of PI3K-AKT pathway proteins in shACADM MCF-7 cells and T47D cells treated with ROS inhibitor detected by Western blotting (*P<0.05 vs NC; #P<0.05, ##P<0.01 vs shACADM).
| 1 | International Agency for Research on Cancer. Breast cancer fact sheet[EB/OL]. 2020[2024-07-21]. doi:10.1037/e533512012-001 |
| 2 | Katsura C, Ogunmwonyi I, Kankam HK, et al. Breast cancer: presentation, investigation and management[J]. Br J Hosp Med, 2022, 83(2): 1-7. doi:10.12968/hmed.2021.0459 |
| 3 | Kashyap D, Pal D, Sharma R, et al. Global increase in breast cancer incidence: risk factors and preventive measures[J]. Biomed Res Int, 2022, 2022:9605439. doi:10.1155/2022/9605439 |
| 4 | ydlowskiSP, PoirotM. Lipids, lipid oxidation, and cancer: from biology to therapeutics[J]. Front Oncol, 2024, 14:1414992. doi:10.3389/fonc.2024.1414992 |
| 5 | Dos Santos DZ, de Souza JC, Pimenta TM, et al. The impact of lipid metabolism on breast cancer: a review about its role in tumorigenesis and immune escape[J]. Cell Commun Signal, 2023, 21(1): 161. doi:10.1186/s12964-023-01178-1 |
| 6 | World Health Organization. New global breast cancer initiative highlights renewed commitment to improve survival[EB/OL].2021-03-08[2024-07-21]. |
| 7 | Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, et al. The role of lipids in cancer progression and metastasis[J]. Cell Metab, 2022, 34(11): 1675-99. doi:10.1016/j.cmet.2022.09.023 |
| 8 | Balaban S, Shearer RF, Lee LS, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration[J]. Cancer Metab, 2017, 5: 1. doi:10.1186/s40170-016-0163-7 |
| 9 | Zeng F, Yao MK, Wang Y, et al. Fatty acid β-oxidation promotes breast cancer stemness and metastasis via the miRNA-328-3p-CPT1A pathway[J]. Cancer Gene Ther, 2022, 29(3/4): 383-95. doi:10.1038/s41417-021-00348-y |
| 10 | Dinarvand N, Khanahmad H, Hakimian SM, et al. Evaluation of long-chain acyl-coenzyme A synthetase 4 (ACSL4) expression in human breast cancer[J]. Res Pharm Sci, 2020, 15(1): 48-56. doi:10.4103/1735-5362.278714 |
| 11 | Chen CI, Kuo DY, Chuang HY. FASN inhibition shows the potential for enhancing radiotherapy outcomes by targeting glycolysis, AKT, and ERK pathways in breast cancer[J]. Int J Radiat Biol, 2025, 101(3): 292-303. doi:10.1080/09553002.2024.2446585 |
| 12 | Lu Y, Tian LP, Peng CC, et al. ACLY-induced reprogramming of glycolytic metabolism plays an important role in the progression of breast cancer[J]. Acta Biochim Biophys Sin, 2023, 55(5): 878-81. doi:10.3724/abbs.2023084 |
| 13 | Wang J, Zhang WF, Liu C, et al. Reprogramming of lipid metabolism mediates crosstalk, remodeling, and intervention of microenvironment components in breast cancer[J]. Int J Biol Sci, 2024, 20(5): 1884-904. doi:10.7150/ijbs.92125 |
| 14 | Zipinotti Dos Santos D, de Souza JC, Pimenta TM, et al. The impact of lipid metabolism on breast cancer: a review about its role in tumorigenesis and immune escape[J]. Cell Commun Signal, 2023,21(1):161. doi:10.1186/s12964-023-01178-1 |
| 15 | Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway[J]. Cancer Res, 2006, 66(12): 5977-80. doi:10.1158/0008-5472.can-05-4673 |
| 16 | Kapur P, Rakheja D, Roy LC, et al. Fatty acid synthase expression in cutaneous melanocytic neoplasms[J]. Mod Pathol, 2005, 18(8): 1107-12. doi:10.1038/modpathol.3800395 |
| 17 | Takahiro T, Shinichi K, Toshimitsu S. Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas[J]. Clin Cancer Res, 2003, 9(6): 2204-12. |
| 18 | 俞殷珏, 赵林丰, 李 荣. 中链酰基辅酶A脱氢酶增强乳腺癌细胞的侵袭和转移能力[J]. 南方医科大学学报, 2019, 39(6): 650-6. |
| 19 | Ma APY, Yeung CLS, Tey SK, et al. Suppression of ACADM-mediated fatty acid oxidation promotes hepatocellular carcinoma via aberrant CAV1/SREBP1 signaling[J]. Cancer Res, 2021, 81(13): 3679-92. doi:10.1158/0008-5472.can-20-3944 |
| 20 | Puca F, Yu F, Bartolacci C, et al. Medium-chain acyl-CoA dehydrogenase protects mitochondria from lipid peroxidation in glioblastoma[J]. Cancer Discov, 2021, 11(11): 2904-23. doi:10.1158/2159-8290.cd-20-1437 |
| 21 | Liu YN, Zhu HX, Li TY, et al. Lipid nanoparticle encapsulated oleic acid induced lipotoxicity to hepatocytes via ROS overload and the DDIT3/BCL2/BAX/Caspases signaling in vitro and in vivo [J]. Free Radic Biol Med, 2024, 222: 361-70. doi:10.1016/j.freeradbiomed.2024.06.024 |
| 22 | Mason E, Hindmarch CCT, Dunham-Snary KJ. Medium-chain acyl-COA dehydrogenase deficiency: pathogenesis, diagnosis, and treatment[J]. Endocrinol Diabetes Metab, 2023, 6(1): e385. doi:10.1002/edm2.385 |
| 23 | Wang SS, Fernhoff PM, Hannon WH, et al. Medium chain acyl-CoA dehydrogenase deficiency human genome epidemiology review[J]. Genet Med, 1999, 1(7): 332-9. doi:10.1097/00125817-199911000-00004 |
| 24 | Nishida R, Nukaga S, Kawahara I, et al. Differential effects of three medium-chain fatty acids on mitochondrial quality control and skeletal muscle maturation[J]. Antioxidants, 2024, 13(7): 821. doi:10.3390/antiox13070821 |
| 25 | Onkenhout W, Venizelos V, van der Poel PF, et al. Identification and quantification of intermediates of unsaturated fatty acid metabolism in plasma of patients with fatty acid oxidation disorders[J]. Clin Chem, 1995, 41(10): 1467-74. doi:10.1093/clinchem/41.10.1467 |
| 26 | Fauser JK, Matthews GM, Cummins AG, et al. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress[J]. Chemotherapy, 2013, 59(3): 214-24. doi:10.1159/000356067 |
| 27 | Walther TC, Farese RV Jr. Lipid droplets and cellular lipid metabolism[J]. Annu Rev Biochem, 2012, 81: 687-714. doi:10.1146/annurev-biochem-061009-102430 |
| 28 | Kiss E, Kränzlin B, Wagenblaβ K, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors[J]. Am J Pathol, 2013, 182(3): 727-41. doi:10.1016/j.ajpath.2012.11.033 |
| 29 | Peng J, Wang Q, Zhou J, et al. Targeted lipid nanoparticles encapsulating dihydroartemisinin and chloroquine phosphate for suppressing the proliferation and liver metastasis of colorectal cancer[J]. Front Pharmacol, 2021, 12: 720777. doi:10.3389/fphar.2021.720777 |
| 30 | Principe M, Borgoni S, Cascione M, et al. Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis[J]. J Hematol Oncol, 2017, 10(1): 16. doi:10.1186/s13045-016-0385-8 |
| 31 | Zhang ZW, Zhang H, Li DB, et al. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway[J]. J Cell Mol Med, 2021, 25(17): 8159-68. doi:10.1111/jcmm.16574 |
| 32 | Osada S, Sakashita F, Hosono Y, et al. Extracellular signal-regulated kinase phosphorylation due to menadione-induced arylation mediates growth inhibition of pancreas cancer cells[J]. Cancer Chemother Pharmacol, 2008, 62(2): 315-20. doi:10.1007/s00280-007-0610-9 |
| 33 | St-Pierre J, Buckingham JA, Roebuck SJ, et al. Topology of superoxide production from different sites in the mitochondrial electron transport chain[J]. J Biol Chem, 2002, 277(47): 44784-90. doi:10.1074/jbc.m207217200 |
| 34 | Hoffman DL, Brookes PS. Oxygen sensitivity of mitochondrial reactive oxygen species generation depends on metabolic conditions[J]. J Biol Chem, 2009, 284(24): 16236-45. doi:10.1074/jbc.m809512200 |
| 35 | Castelli S, Ciccarone F, De Falco P, et al. Adaptive antioxidant response to mitochondrial fatty acid oxidation determines the proliferative outcome of cancer cells[J]. Cancer Lett, 2023, 554: 216010. doi:10.1016/j.canlet.2022.216010 |
| 36 | Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain[J]. Nat Rev Mol Cell Biol, 2022, 23(2):141-161. [37] OnkenhoutW, VenizelosV, van der PoelPF, et al. Identification and quantification of intermediates of unsaturated fatty acid metabolism in plasma of patients with fatty acid oxidation disorders[J]. Clin Chem, 1995, 41(10):1467-74. doi:10.1038/s41580-021-00415-0 |
| 38 | Chen TH, Wang HC, Chang CJ, et al. Mitochondrial glutathione in cellular redox homeostasis and disease manifestation[J]. Int J Mol Sci, 2024, 25(2): 1314. doi:10.3390/ijms25021314 |
| 39 | Vouilloz A, Bourgeois T, Diedisheim M, et al. Impaired unsaturated fatty acid elongation alters mitochondrial function and accelerates metabolic dysfunction-associated steatohepatitis progression[J]. Metabolism, 2025, 162: 156051. doi:10.1016/j.metabol.2024.156051 |
| 40 | Randall EC, Zadra G, Chetta P, et al. Molecular characterization of prostate cancer with associated gleason score using mass spectrometry imaging[J]. Mol Cancer Res, 2019, 17(5): 1155-65. doi:10.1158/1541-7786.mcr-18-1057 |
| [1] | Xiuying GONG, Shunfu HOU, Miaomiao ZHAO, Xiaona WANG, Zhihan ZHANG, Qinghua LIU, Chonggao YIN, Hongli LI. LncRNA SNHG15 promotes proliferation, migration and invasion of lung adenocarcinoma cells by regulating COX6B1 through sponge adsorption of miR-30b-3p [J]. Journal of Southern Medical University, 2025, 45(7): 1498-1505. |
| [2] | Shunjie QING, Zhiyong SHEN. High expression of hexokinase 2 promotes proliferation, migration and invasion of colorectal cancer cells by activating the JAK/STAT pathway and regulating tumor immune microenvironment [J]. Journal of Southern Medical University, 2025, 45(3): 542-553. |
| [3] | Di CHEN, Ying LÜ, Yixin GUO, Yirong ZHANG, Ruixuan WANG, Xiaoruo ZHOU, Yuxin CHEN, Xiaohui WU. Dihydroartemisinin enhances doxorubicin-induced apoptosis of triple negative breast cancer cells by negatively regulating the STAT3/HIF-1α pathway [J]. Journal of Southern Medical University, 2025, 45(2): 254-260. |
| [4] | Qiao CHU, Xiaona WANG, Jiaying XU, Huilin PENG, Yulin ZHAO, Jing ZHANG, Guoyu LU, Kai WANG. Pulsatilla saponin D inhibits invasion and metastasis of triple-negative breast cancer cells through multiple targets and pathways [J]. Journal of Southern Medical University, 2025, 45(1): 150-161. |
| [5] | Xiaohua CHEN, Hui LU, Ziliang WANG, Lian WANG, Yongsheng XIA, Zhijun GENG, Xiaofeng ZHANG, Xue SONG, Yueyue WANG, Jing LI, Jianguo HU, Lugen ZUO. Role of Abelson interactor 2 in progression and prognosis of gastric cancer and its regulatory mechanisms [J]. Journal of Southern Medical University, 2024, 44(9): 1653-1661. |
| [6] | Mingzi OUYANG, Jiaqi CUI, Hui WANG, Zheng LIANG, Dajin PI, Liguo CHEN, Qianjun CHEN, Yingchao WU. Kaixinsan alleviates adriamycin-induced depression-like behaviors in mice by reducing ferroptosis in the prefrontal cortex [J]. Journal of Southern Medical University, 2024, 44(8): 1441-1449. |
| [7] | Xiaofan CONG, Teng CHEN, Shuo LI, Yuanyuan WANG, Longyun ZHOU, Xiaolong LI, Pei ZHANG, Xiaojin SUN, Surong ZHAO. Dihydroartemisinin enhances sensitivity of nasopharyngeal carcinoma HNE1/DDP cells to cisplatin-induced apoptosis by promoting ROS production [J]. Journal of Southern Medical University, 2024, 44(8): 1553-1560. |
| [8] | Jincun FANG, Liwei LIU, Junhao LIN, Fengsheng CHEN. Overexpression of CDHR2 inhibits proliferation of breast cancer cells by inhibiting the PI3K/Akt pathway [J]. Journal of Southern Medical University, 2024, 44(6): 1117-1125. |
| [9] | Zhi CUI, Cuijiao MA, Qianru WANG, Jinhao CHEN, Ziyang YAN, Jianlin YANG, Yafeng LÜ, Chunyu CAO. A recombinant adeno-associated virus expressing secretory TGF‑β type II receptor inhibits triple-negative murine breast cancer 4T1 cell proliferation and lung metastasis in mice [J]. Journal of Southern Medical University, 2024, 44(5): 818-826. |
| [10] | Yuanyuan WANG, Teng CHEN, Xiaofan CONG, Yiran LI, Rui CHEN, Pei ZHANG, Xiaojin SUN, Surong ZHAO. Pristimerin enhances cisplatin-induced apoptosis in nasopharyngeal carcinoma cells via ROS-mediated deactivation of the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 904-912. |
| [11] | Yongsheng XIA, Lian WANG, Xiaohua CHEN, Yulu ZHANG, Aofei SUN, Deli CHEN. TSR2 overexpression inhibits proliferation and invasion of gastric cancer cells by downregulating the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2024, 44(5): 913-919. |
| [12] | HUANG Qiuhu, ZHOU Jian, WANG Zizhen, YANG Kun, CHEN Zhenggang. MiR-26-3p regulates proliferation, migration, invasion and apoptosis of glioma cells by targeting CREB1 [J]. Journal of Southern Medical University, 2024, 44(3): 578-584. |
| [13] | ZHONG Weixiong, LIANG Fangrong, YANG Ruimeng, ZHEN Xin. Prediction of microvascular invasion in hepatocellular carcinoma based on multi-phase dynamic enhanced CT radiomics feature and multi-classifier hierarchical fusion model [J]. Journal of Southern Medical University, 2024, 44(2): 260-269. |
| [14] | Huan LI, Zixin QIU, Wenjie XU, Xue CHEN, Diandian WEI, Yun WANG. Luteolin inhibits proliferation of lung cancer A549 cells by increasing ROS production and inhibiting the AKT/mTOR signaling pathway and HO-1 expression [J]. Journal of Southern Medical University, 2024, 44(12): 2367-2374. |
| [15] | Youqin ZENG, Siyu CHEN, Yan LIU, Yitong LIU, Ling ZHANG, Jiao XIA, Xinyu WU, Changyou WEI, Ping LENG. AKBA combined with doxorubicin inhibits proliferation and metastasis of triple-negative breast cancer MDA-MB-231 cells and xenograft growth in nude mice [J]. Journal of Southern Medical University, 2024, 44(12): 2449-2460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||