Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (10): 2015-2023.doi: 10.12122/j.issn.1673-4254.2024.10.20
Previous Articles Next Articles
Jing ZENG1,2,3( ), Lei HUA1,2, Yong YANG1,2, Xiaomei ZHANG1,2, Jiangping WEI1,2(
), Lei HUA1,2, Yong YANG1,2, Xiaomei ZHANG1,2, Jiangping WEI1,2( ), Lisheng LI3(
), Lisheng LI3( )
)
Received:2024-04-11
Online:2024-10-20
Published:2024-10-31
Contact:
Jiangping WEI, Lisheng LI
E-mail:1589905936@qq.com;sichuanwjp@163.com;medlls@qq.com
Jing ZENG, Lei HUA, Yong YANG, Xiaomei ZHANG, Jiangping WEI, Lisheng LI. Yigong San improves learning and memory functions of APP/PS1 transgenic mice by regulating brain fluid metabolism[J]. Journal of Southern Medical University, 2024, 44(10): 2015-2023.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.10.20
| Group | Dosage (g/kg) | The escape latency (s) | ||||
|---|---|---|---|---|---|---|
| First day | Second day | Third day | Fourth day | Fifth day | ||
| Control | - | 118.95±2.36 | 80.73±28.54^ | 47.48±16.79^^^ | 29.50±10.43^^^ | 14.98±5.30#^^^ |
| APP/PS1 | - | 112.11±23.66 | 99.90±35.32 | 83.94±29.68 | 66.07±23.36^^ | 34.66±9.01*^^^ |
| APP/PS1+Donepezil | 1.5×10-3 | 120.00±0 | 92.39±32.66 | 55.98±19.79^^^ | 38.79±13.71^^^ | 16.10±5.69#^^^ |
| APP/PS1+YGS | 7.5 | 120.00±0 | 102.07±35.20 | 78.92±27.90^ | 42.27±14.94^^^ | 18.23±6.44#^^^ |
Tab.1 Effect of Yigong San (YGS) on learning and memory ability of APP/PS1 mice (Mean±SD, n=8)
| Group | Dosage (g/kg) | The escape latency (s) | ||||
|---|---|---|---|---|---|---|
| First day | Second day | Third day | Fourth day | Fifth day | ||
| Control | - | 118.95±2.36 | 80.73±28.54^ | 47.48±16.79^^^ | 29.50±10.43^^^ | 14.98±5.30#^^^ |
| APP/PS1 | - | 112.11±23.66 | 99.90±35.32 | 83.94±29.68 | 66.07±23.36^^ | 34.66±9.01*^^^ |
| APP/PS1+Donepezil | 1.5×10-3 | 120.00±0 | 92.39±32.66 | 55.98±19.79^^^ | 38.79±13.71^^^ | 16.10±5.69#^^^ |
| APP/PS1+YGS | 7.5 | 120.00±0 | 102.07±35.20 | 78.92±27.90^ | 42.27±14.94^^^ | 18.23±6.44#^^^ |
| Group | Dosage (g/kg) | Platform quadrant residence time (s) | Platfrom crossing times (times) | Average swimming speed (cm/s) |
|---|---|---|---|---|
| Control | - | 34.37±5.07# | 5.17±1.77 | 19.25±1.21 |
| APP/PS1 | - | 25.69±5.01* | 3.33±1.60 | 20.03±0.98 |
| APP/PS1+Donepezil | 1.5×10-3 | 35.23±5.99# | 4.83±1.07 | 20.34±1.66 |
| APP/PS1+YGS | 7.5 | 34.17±4.08# | 4.83±0.67 | 20.58±1.32 |
Tab.2 Effect of YGS on spatial exploration ability of APP/PS1 mice (Mean±SD, n=8)
| Group | Dosage (g/kg) | Platform quadrant residence time (s) | Platfrom crossing times (times) | Average swimming speed (cm/s) |
|---|---|---|---|---|
| Control | - | 34.37±5.07# | 5.17±1.77 | 19.25±1.21 |
| APP/PS1 | - | 25.69±5.01* | 3.33±1.60 | 20.03±0.98 |
| APP/PS1+Donepezil | 1.5×10-3 | 35.23±5.99# | 4.83±1.07 | 20.34±1.66 |
| APP/PS1+YGS | 7.5 | 34.17±4.08# | 4.83±0.67 | 20.58±1.32 |
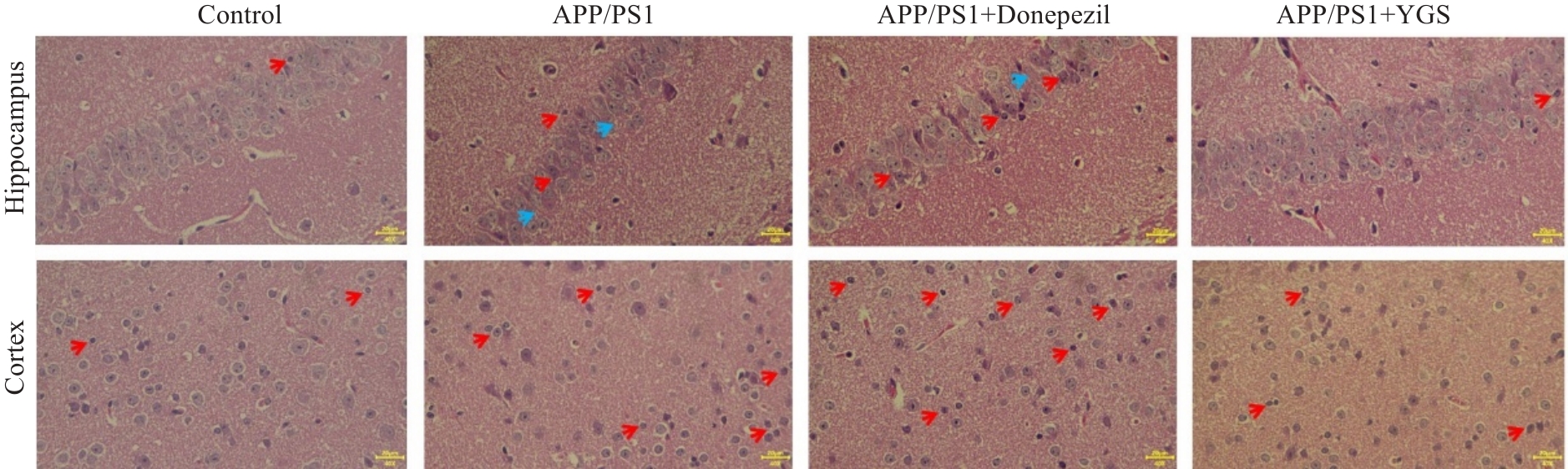
Fig.1 Effect of YGS on hippocampal pathology of APP/PS1 mice (HE staining, original magnification: ×40). Red arrows indicate degenerative or necrotic neurons, and blue arrows indicate cell loss or enlarged intercellular space.
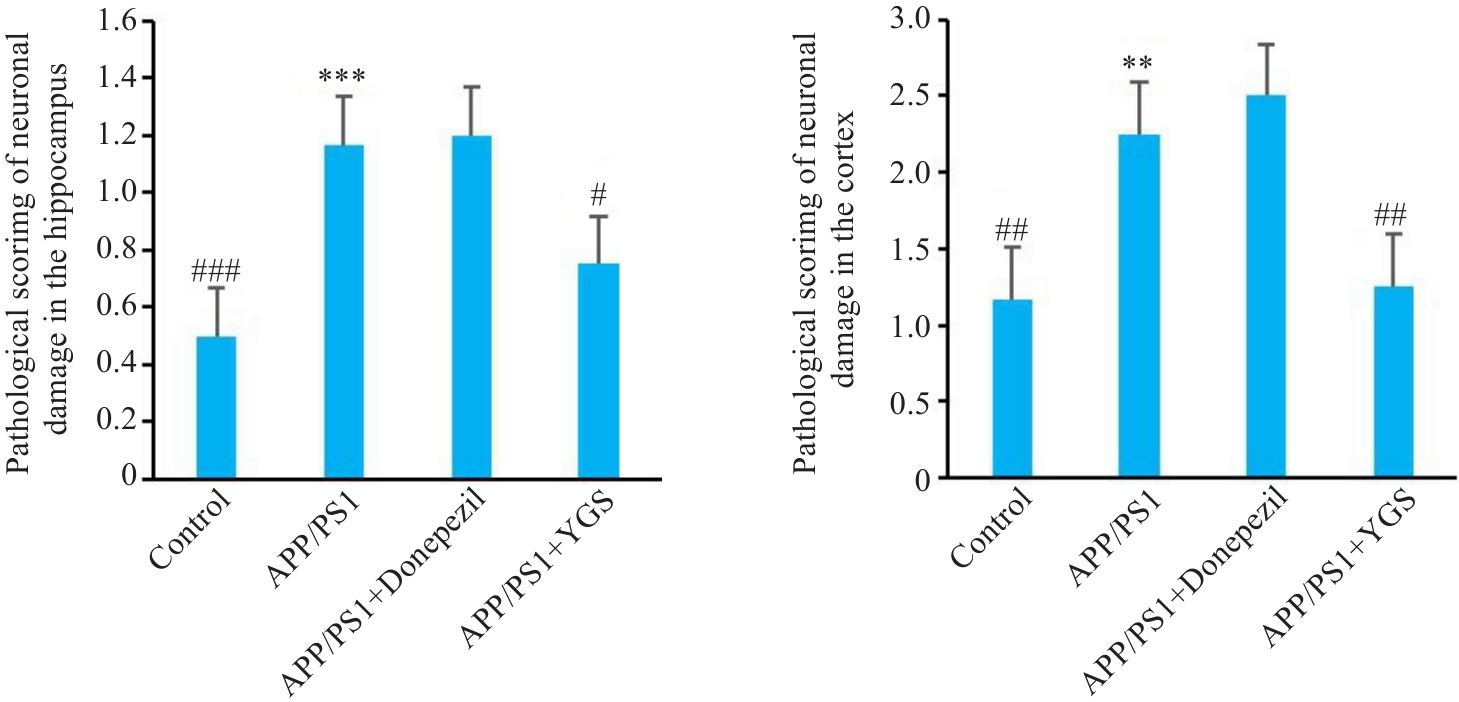
Fig.2 Effect of YGS on pathological score of damaged neurons in the hippocampus and cortex of APP/PS1 mice. **P<0.01, ***P<0.001 vs control group; #P<0.05, ##P<0.01, ###P<0.001 vs APP/PS1 group.
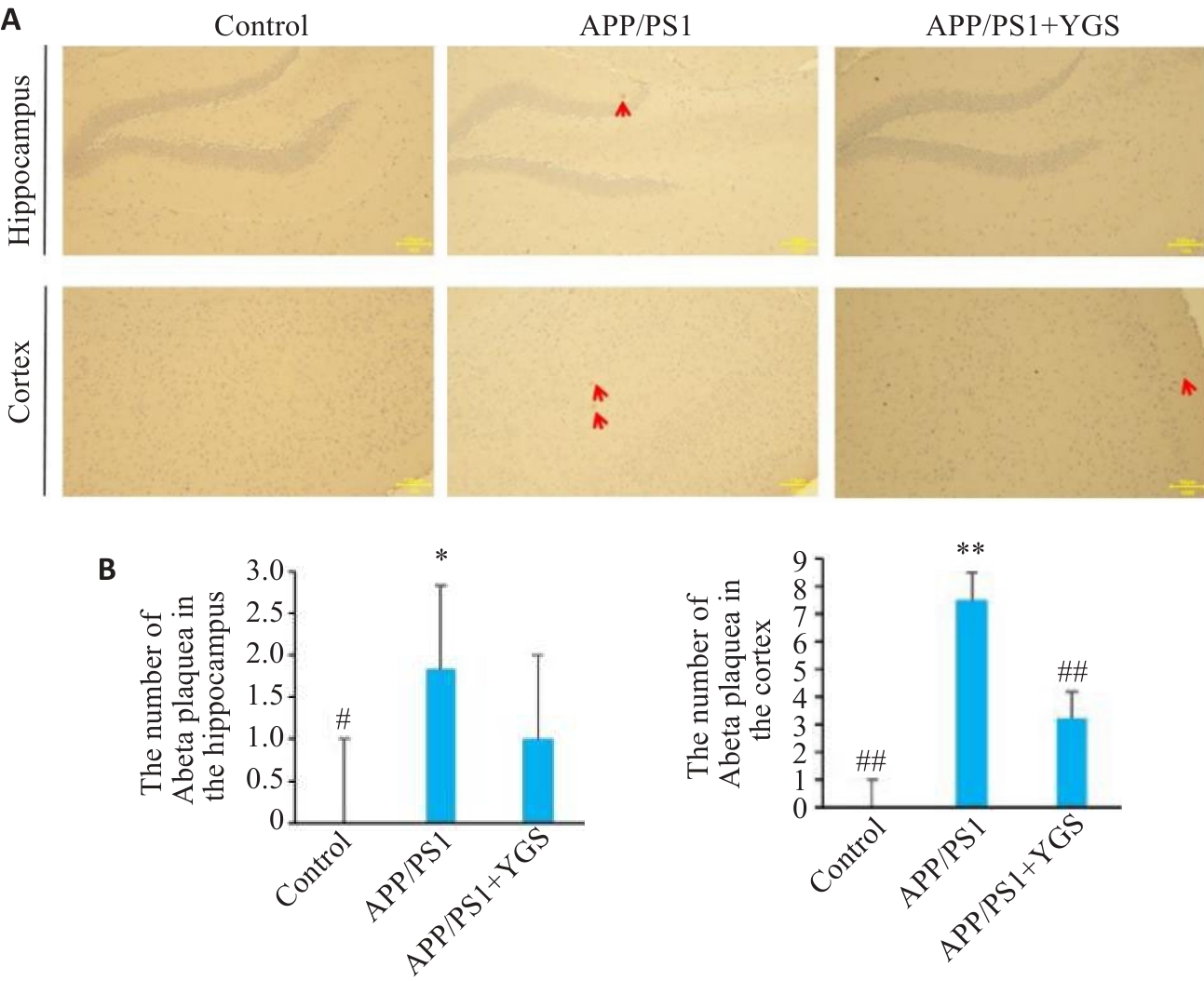
Fig.3 Effect of YGS on the number of Aβ plaques in the hippocampus and cortex of APP/PS1 mice (immunohistochemistry, ×40). A: Immunohistochemical staining ofthe the hippocampal cortex in different groups (red arrows indicate amyloid plaques). B: Number of Aβ plaques in the hippocampus and cortex of the mice.*P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs APP/PS1 group.
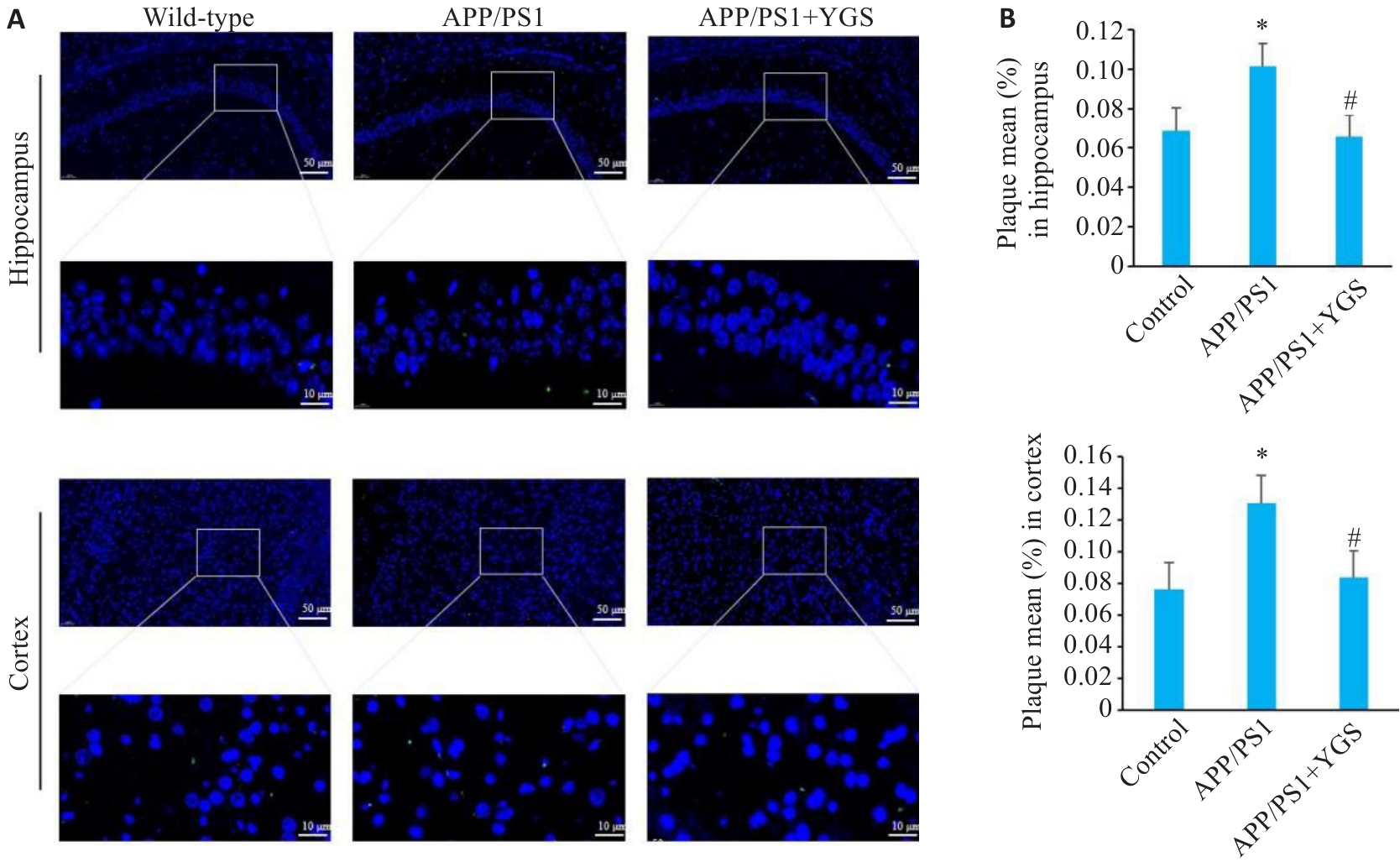
Fig.4 Effects of YGS on Aβ protein expression in the hippocampus and cortex of APP/PS1 mice (immunofluorescence staining, ×20 or ×100). A: Thioflavin-S staining. B: Aβ plaque area in the hippocampus and cortex of the mice. *P<0.05 vs control group; #P<0.05 vs APP/PS1 group.
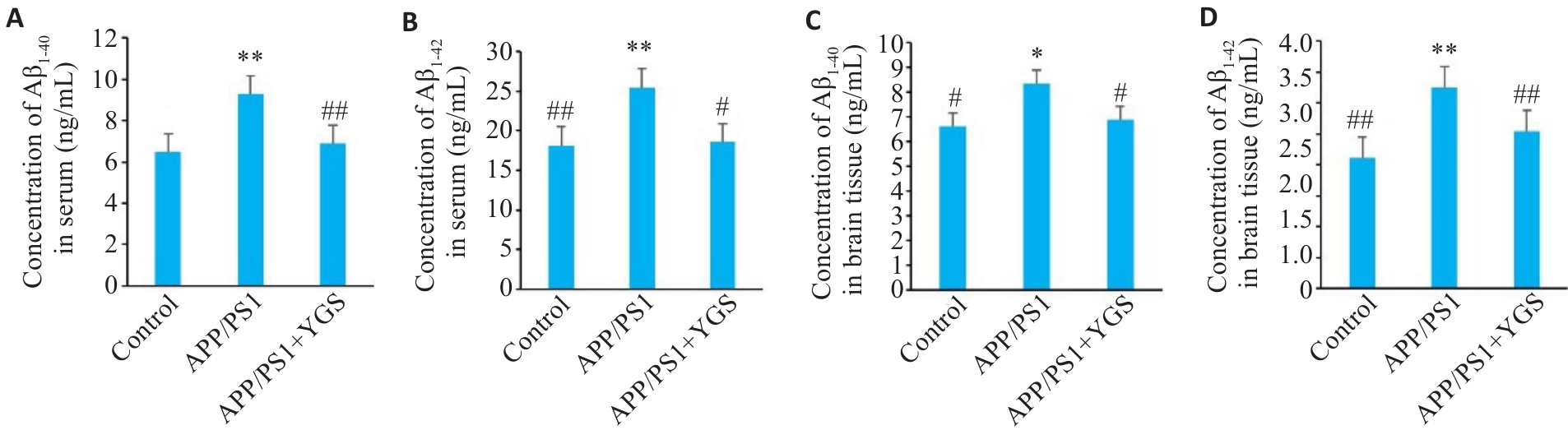
Fig.5 Effect of YGS on Aβ1-40 and Aβ1-42 levels in the serum and brain tissue of APP/PS1 mice. A: Serum Aβ1-40 levels in the mice. B: Serum Aβ1-42 levels in the mice. C: Brain tissue Aβ1-40 level in the mice. D: Brain tissue Aβ1-42 level in the mice. *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs APP/PS1 group.
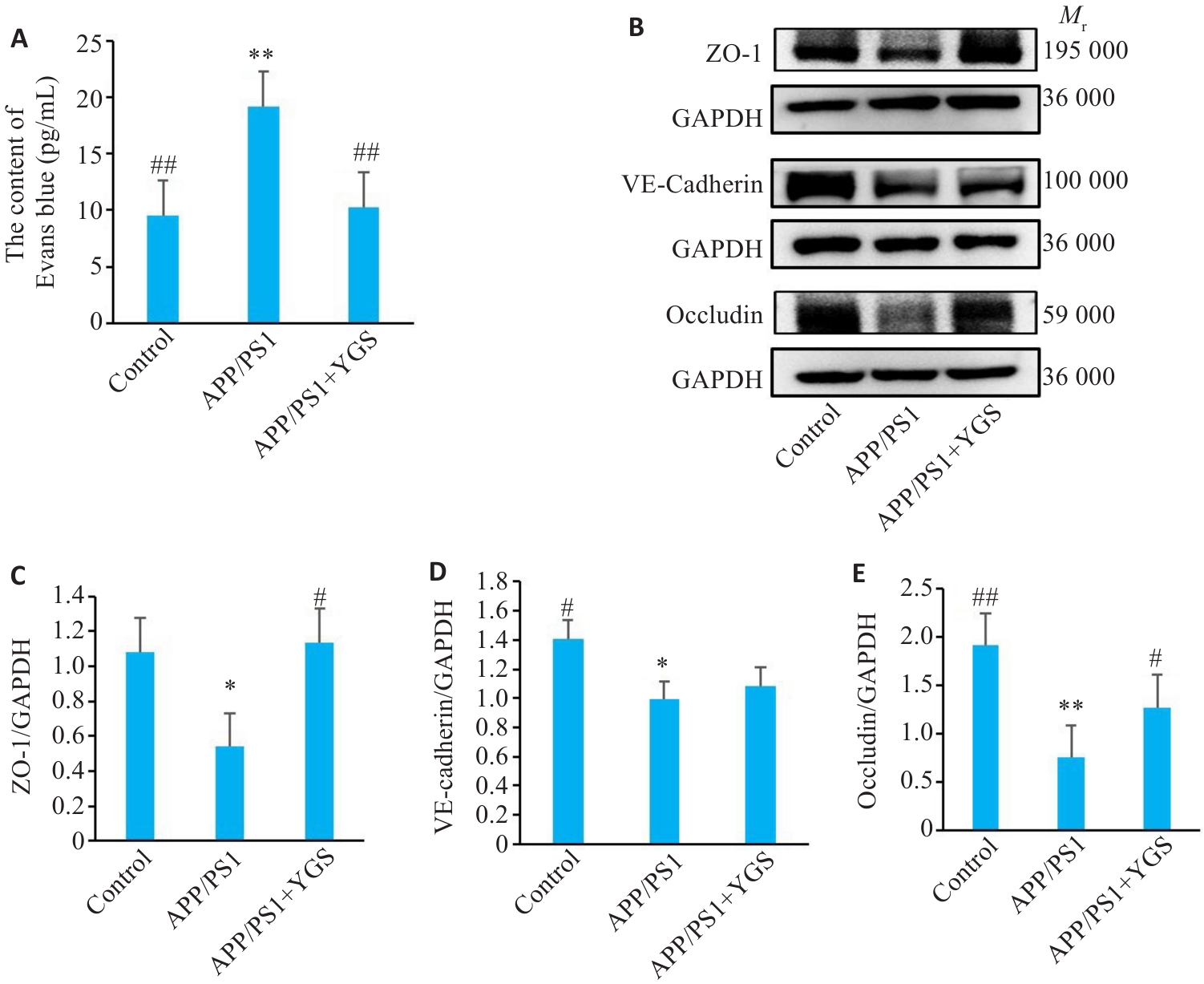
Fig.6 Effects of YGS on BBB permeability and connexin expression in the hippocampus of APP/PS1 mice (n=5). A: Blood-brain barrier permeability of the mice (n=3). B-E: Western blotting for detecting expressions of ZO-1, VE-cadherin and occludin and their relative expression levels. *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs APP/PS1 group.
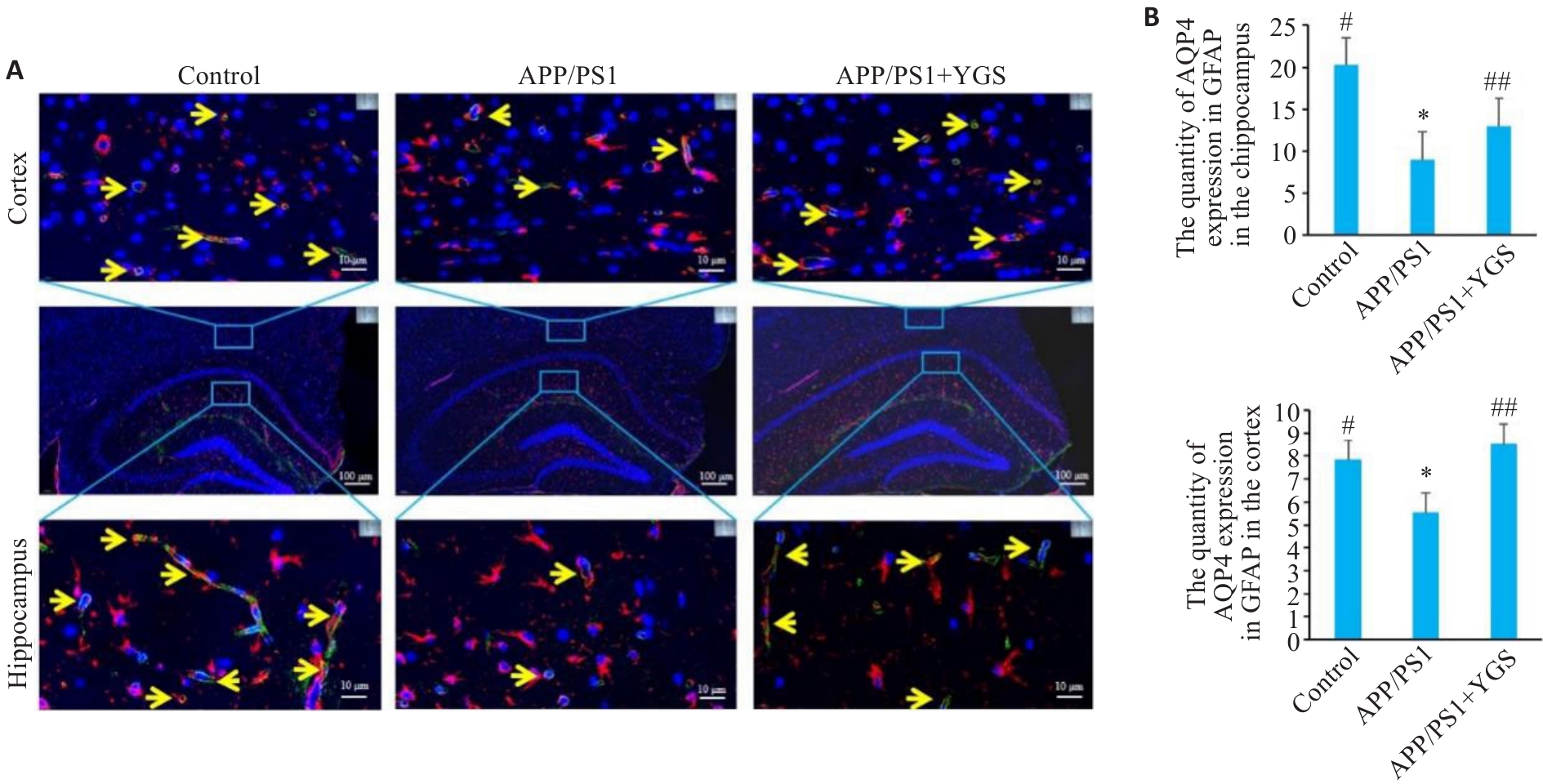
Fig.8 Effect of YGS on AQP4 polarization in the brain tissue of APP/PS1 mice. A: Expression of AQP4 in astrocytes (Red is GFAP; green is AQP4; and blue is DAPI. The yellow arrows indicate AQP4-expressing GFAP-positive cells). B: Numbers of AQP4+GFAP in the brain tissue of the mice (n=5). *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs APP/PS1 group.
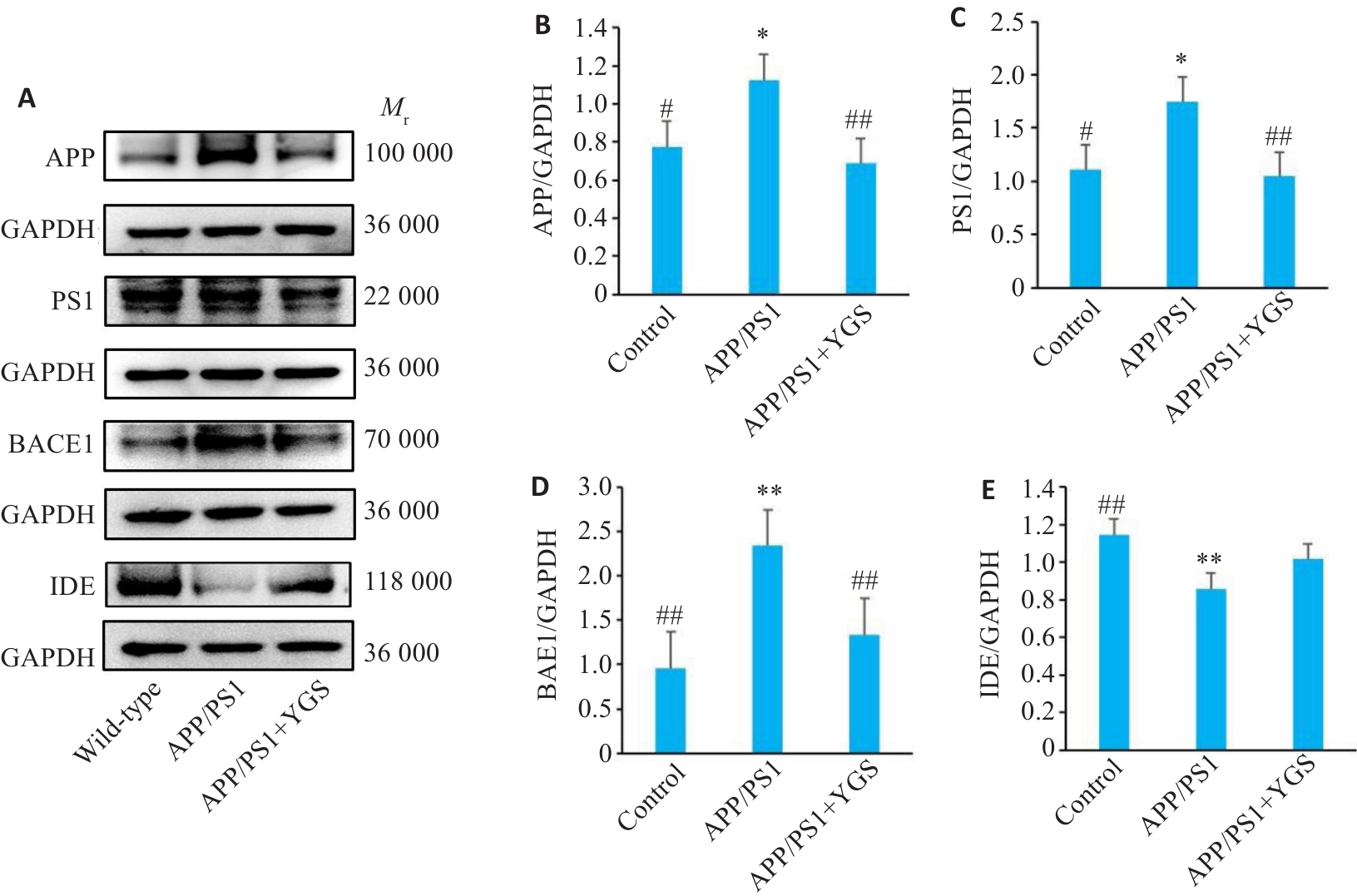
Fig.9 Effect of YGS on protein expressions of APP, PS1, BACE1 and IDE in the brain tissue of APP/PS1 mice (n=5). A-E: Western blotting for detecting expressions of APP, PS1, BACE1 and IDE and their relative expression levels. *P<0.05, **P<0.01 vs control group; #P<0.05, ##P<0.01 vs APP/PS1 group.
| 1 | 张景明, 陈震霖. 论津液与脏腑关系及其抗病御邪作用[J]. 中国中医基础医学杂志, 2007, 13(1): 13-4. |
| 2 | 陈路军, 孙智玲, 陈文静, 等. 从 “津液代谢” 探讨变通小青龙汤在慢性阻塞性肺疾病急性期的应用[J]. 中医药临床杂志, 2022, 34(10): 1811-4. |
| 3 | 徐世军, 赵宜军, 张文生. 从中医脑络功能演变谈轻度认知障碍的病机[J]. 中医杂志, 2011, 52(19): 1627-9. DOI: 10.1038/cdd.2010.68 |
| 4 | 田 恺, 张向宇, 牛博真, 等. 基于 “毒损脑络” 理论中医脑病病因病机和辨证施治的研究进展[J]. 中西医结合心脑血管病杂志, 2021, 19(8): 1308-10. |
| 5 | 魏江平, 赵子瑄, 曾 静, 等. 异功散调控CXCL12/CXCR4信号减少谷氨酸释放改善衰老模型小鼠认知下降[J]. 中国中药杂志, 2023, 48(23): 6483-91. |
| 6 | Mestre H, Kostrikov S, Mehta RI, et al. Perivascular spaces, glymphatic dysfunction, and small vessel disease[J]. Clin Sci, 2017, 131(17): 2257-74. |
| 7 | Mamtilahun M, Wei ZY, Qin C, et al. DL-3n-butylphthalide improves blood-brain barrier integrity in rat after middle cerebral artery occlusion[J]. Front Cell Neurosci, 2020, 14: 610714. |
| 8 | Wei JP, Dai Y, Wen W, et al. Blood-brain barrier integrity is the primary target of alcohol abuse[J]. Chem Biol Interact, 2021, 337: 109400. |
| 9 | Shi ZS, Zhu LH, Li TT, et al. Neuroprotective mechanisms of Lycium barbarum polysaccharides against ischemic insults by regulating NR2B and NR2A containing NMDA receptor signaling pathways[J]. Front Cell Neurosci, 2017, 11: 288. |
| 10 | Gonneaud J, Arenaza-Urquijo EM, Mézenge F, et al. Increased florbetapir binding in the temporal neocortex from age 20 to 60 years[J]. Neurology, 2017, 89(24): 2438-46. |
| 11 | 吴东南, 刘 玲, 明淑萍, 等. 基于“Aβ异常沉积” 浅析中医“从痰论治” 阿尔茨海默病[J]. 中华中医药杂志, 2019, 34(10): 4699-702. |
| 12 | Yang AC, Vest RT, Kern F, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk[J]. Nature, 2022, 603(7903): 885-92. |
| 13 | Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus[J]. Neuron, 2015, 85(2): 296-302. |
| 14 | Wei JP, Qin LX, Fu Y, et al. Long-term consumption of alcohol exacerbates neural lesions by destroying the functional integrity of the blood-brain barrier[J]. Drug Chem Toxicol, 2022, 45(1): 231-8. |
| 15 | Wang Q, Huang XM, Su YX, et al. Activation of Wnt/β-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer's disease[J]. Brain, 2022, 145(12): 4474-88. |
| 16 | Hu MY, Li TM, Ma XM, et al. Macrophage lineage cells-derived migrasomes activate complement-dependent blood-brain barrier damage in cerebral amyloid angiopathy mouse model[J]. Nat Commun, 2023, 14(1): 3945. |
| 17 | Xie P, Kancherla K, Chandramohan S, et al. Involvement of single nucleotide polymorphisms of junction adhesion molecule with small vessel vascular dementia[J]. Aging Med, 2023, 6(4): 347-52. |
| 18 | Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease[J]. Nature, 1996, 382(6593): 685-91. |
| 19 | Sagare A, Deane R, Bell RD, et al. Clearance of amyloid-beta by circulating lipoprotein receptors[J]. Nat Med, 2007, 13(9): 1029-31. |
| 20 | Lee J, Lee H, Lee H, et al. ANKS1A regulates LDL receptor-related protein 1 (LRP1)‑mediated cerebrovascular clearance in brain endothelial cells[J]. Nat Commun, 2023, 14(1): 8463. |
| 21 | Kanekiyo T, Cirrito JR, Liu CC, et al. Neuronal clearance of amyloid‑β by endocytic receptor LRP1[J]. J Neurosci, 2013, 33(49): 19276-83. |
| 22 | Burfeind KG, Murchison CF, Westaway SK, et al. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer's disease[J]. Alzheimers Dement, 2017, 3(3): 348-59. |
| 23 | Zeppenfeld DM, Simon M, Haswell JD, et al. Association of perivascular localization of aquaporin-4 with cognition and alzheimer disease in aging brains[J]. JAMA Neurol, 2017, 74(1): 91-9. |
| 24 | Iliff JJ, Wang MH, Liao YH, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid Β[J]. Sci Transl Med, 2012, 4(147): 147ra111. |
| 25 | Galvão F Jr, Grokoski KC, da Silva BB, et al. The amyloid precursor protein (APP) processing as a biological link between Alzheimer's disease and cancer[J]. Ageing Res Rev, 2019, 49: 83-91. |
| 26 | Rammes G. Molecular mechanism of Alzheimer’s disease[J]. Int J Mol Sci, 2023, 24(23): 16837. |
| [1] | Jing ZENG, Rong CHEN, Xiangyi REN, Lei HUA, Yong YANG, Jiangping WEI, Xiaomei ZHANG. Yigong San improves cognitive decline in a rat model of Alzheimer's disease by regulating intestinal microorganisms [J]. Journal of Southern Medical University, 2024, 44(7): 1297-1305. |
| [2] | . Expression of aquaporin-4 protein in the brain of preeclampsia model rats [J]. Journal of Southern Medical University, 2013, 33(09): 1329-. |
| [3] | CHEN Jing1,HUANG Yong1,WANG Sheng-xu1,LI Qiu-shi1,LIANG Yong-jiu2,GUO Yong-ning3 1Department of Traditional Chinese Medicine,Southern Medical University,Guangzhou 510515,China;2Medical Department,Millennium Rest home,Guangzhou 510407,China;3Medical Department,Xingguang Rest home,Guangzhou 510407,China. ~(18)FDG PET cerebral function imaging in 10 vascular dementia patients receiving needling at Baihui(DU20),Shuigou(DU26) and Shenmen(HT7) [J]. Journal of Southern Medical University, 2006, 26(05): 610-612. |
| [4] | HUANG Yong1, CHEN Jing1, LAI Xin-sheng2, TANG An-wu3, LI Dong-jiang3. Effects of needling in Baihui (DU20),Shuigou (DU26) and Shenmen (HT7) on glucose metabolism in the lentiform nuclus in patients with vascular dementia [J]. Journal of Southern Medical University, 2005, 25(11): 1405-1407. |
| [5] | LIU Lin, LI Tao-ping. Expression of aquaporin-4 in isolated and purified rat alveolar typeⅡcells [J]. Journal of Southern Medical University, 2005, 25(07): 784-786. |
| [6] | OUYANG Shi, SUN Li-sha, GUO Sheng-lan, LIU Xu, XU Jiang-ping. Effects of timosaponins on learning and memory abilities of rats with dementia induced by lateral cerebral ventricular injection of amyloid β-peptide [J]. Journal of Southern Medical University, 2005, 25(02): 121-126. |
| [7] | YANG Guo-feng1, WANG Lu-ning1, ZHAO Xin2, NIE Yong-hui1. Two-dimensional electrophoregram for proteomic analysis of rat brain with intrahippocampal amyloid β injection and normal rat brain [J]. Journal of Southern Medical University, 2004, 24(05): 553-555. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||