Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (6): 1270-1279.doi: 10.12122/j.issn.1673-4254.2025.06.16
Yuexuan ZHU( ), Zhangrui ZHU, Peng WU(
), Zhangrui ZHU, Peng WU( )
)
Received:2024-11-27
Online:2025-06-20
Published:2025-06-27
Contact:
Peng WU
E-mail:zhuyuexuan0630@ outlook.com;doctorwupeng @gmail.com
Supported by:Yuexuan ZHU, Zhangrui ZHU, Peng WU. Pentosan polysulfate alleviates cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in mice by modulating gut microbiota and bile acid metabolism[J]. Journal of Southern Medical University, 2025, 45(6): 1270-1279.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.06.16
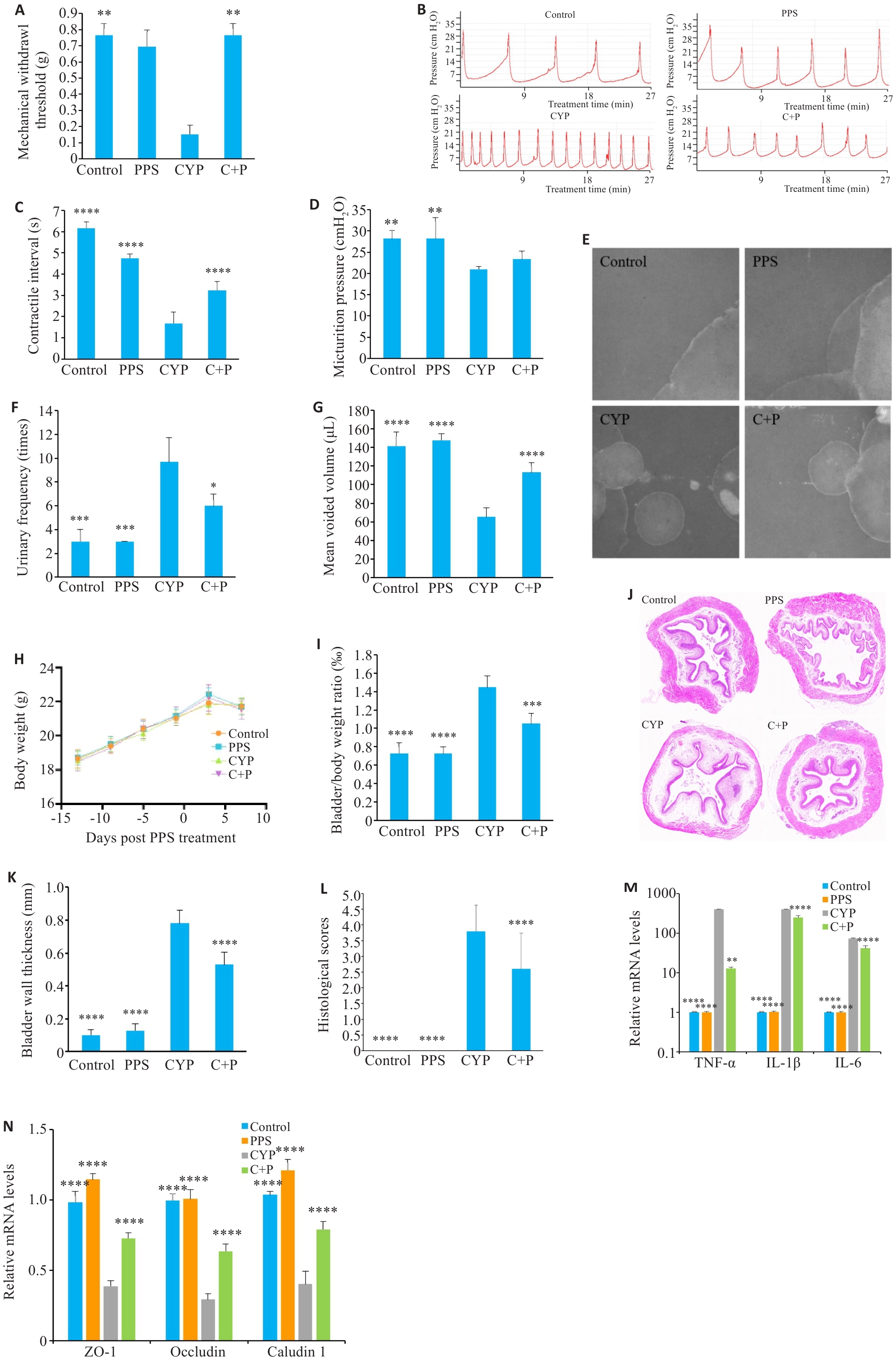
Fig.1 Effect of pentosan polysulfate (PPS) on cyclophosphamide (CYP)‑ induced interstitial cystitis/bladder pain syndrome (IC/BPS) in mice. A: Comparison of mechanical pain thresholds of the mice across the groups. B-D: Urodynamic test results and their parameters in different groups. E-G: Voiding cystometry analysis of changes in voiding behavior of the mice. H: Body weight change curve of the mice. I: Bladder to body weight ratio of the mice. J-L: HE staining of the bladder tissues in different groups (Original magnification: ×10). M: PPS reduces elevated mRNA levels of inflammatory factors induced by CYP. N: PPS lowers mRNA levels of tight junction proteins in CYP-treated mice. Data are presented as Mean±SE. Statistical significance was determined by one-way ANOVA or unpaired Student's t-test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs CYP. C+P: CYP+PPS.
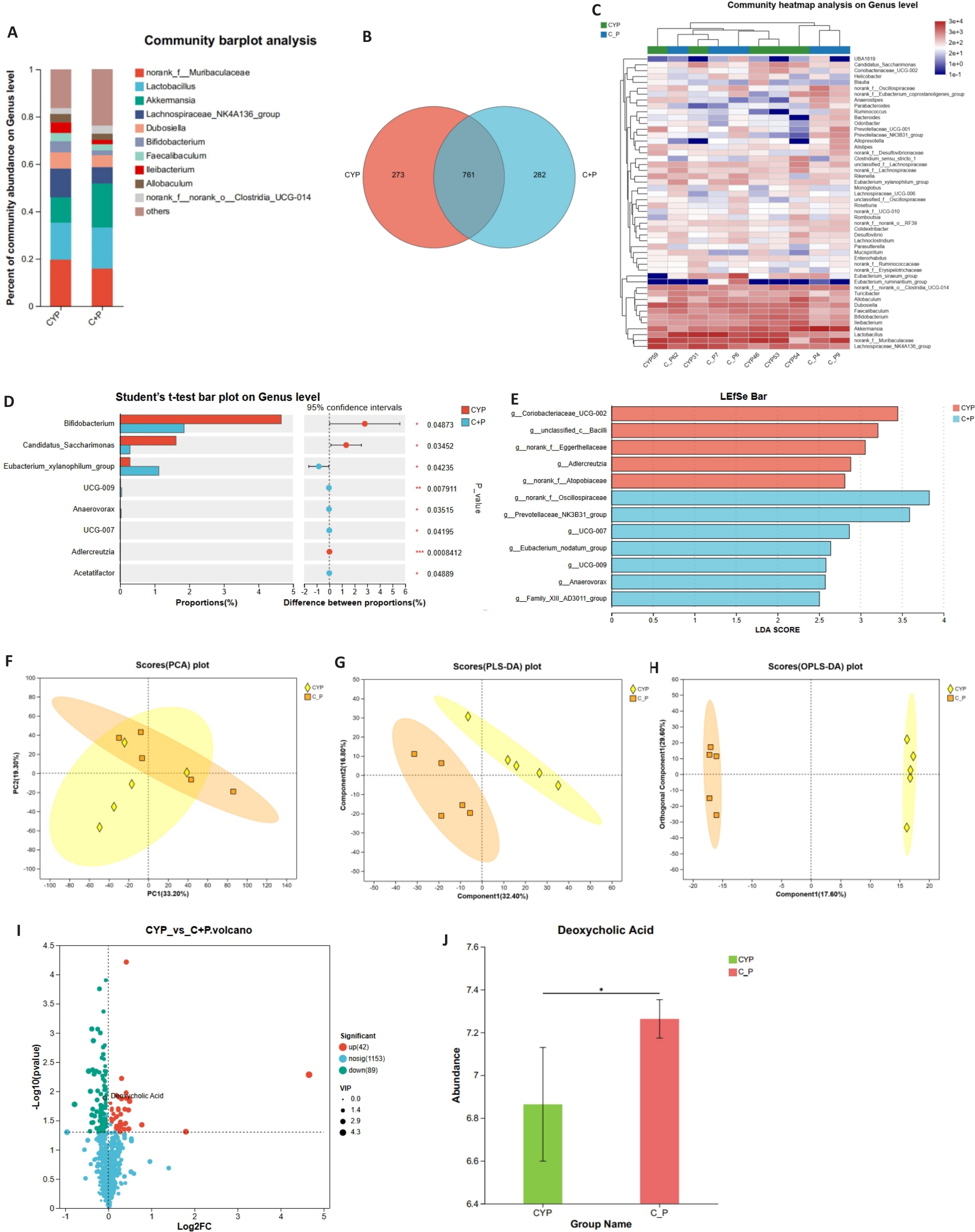
Fig.2 Impact of PPS on gut microbiota and its metabolites in mice with CYP-induced IC/BPS. A: Comparison of gut microbiota composition at the genus level between CYP group and C+P group (n=5). B: Venn diagram showing the shared and unique species of gut microbiota between CYP group and C+P group (n=5). C: Heatmap displaying differences in gut microbiota composition at the genus level (n=5). D: Statistical test for differences in gut microbiota composition at the genus level (n=5). E: LEfSe analysis confirms significant differences in gut microbial community composition between CYP group and C+P group. F-H: Principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA) of non-targeted metabolomics data. I: Volcano plot showing differential metabolites between CYP group and C+P group. J: Comparison of DCA concentration in cecal contents between the two groups. *P<0.05.
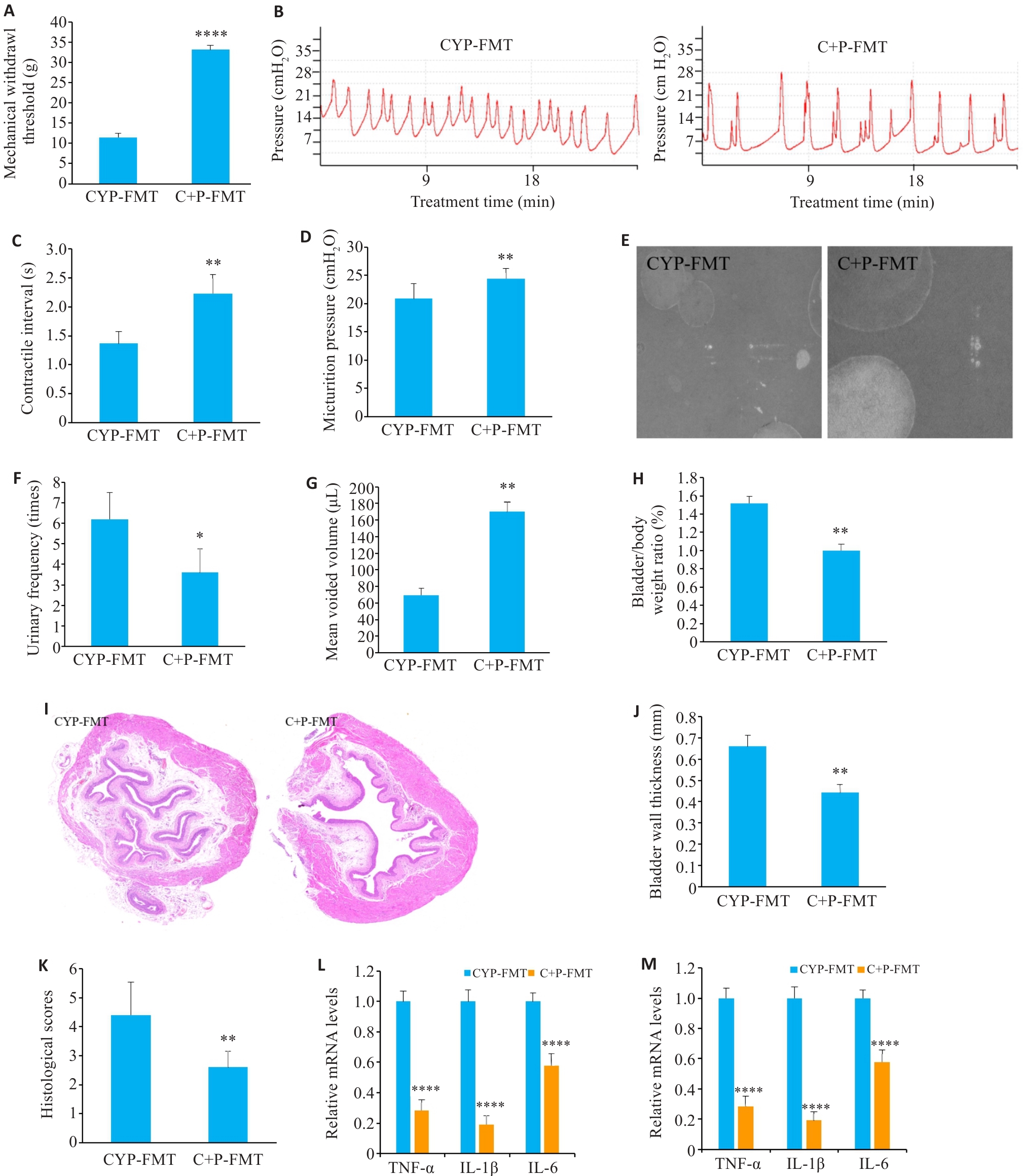
Fig.3 Effect of fecal microbiota transplantation (FMT) from PPS-treated mice on CYP-induced IC/BPS. A: Comparison of mechanical pain thresholds among the groups. B-D: Results of urodynamic testing and their parameters. E-G: Voiding cystometry analysis. H: Bladder to body weight ratio. I-K: HE staining of the bladder tissue in the groups (×10). L: C+P-FMT reduces CYP-induced elevation of mRNA levels of inflammatory factors. M: C+P-FMT restores CYP-induced decrease of mRNA levels of tight junction proteins. Data are presented as Mean±SE. Statistical significance was determined by one-way ANOVA or unpaired Student's t-test. *P<0.05, **P<0.01, ****P<0.0001 vs CYP+FMT.
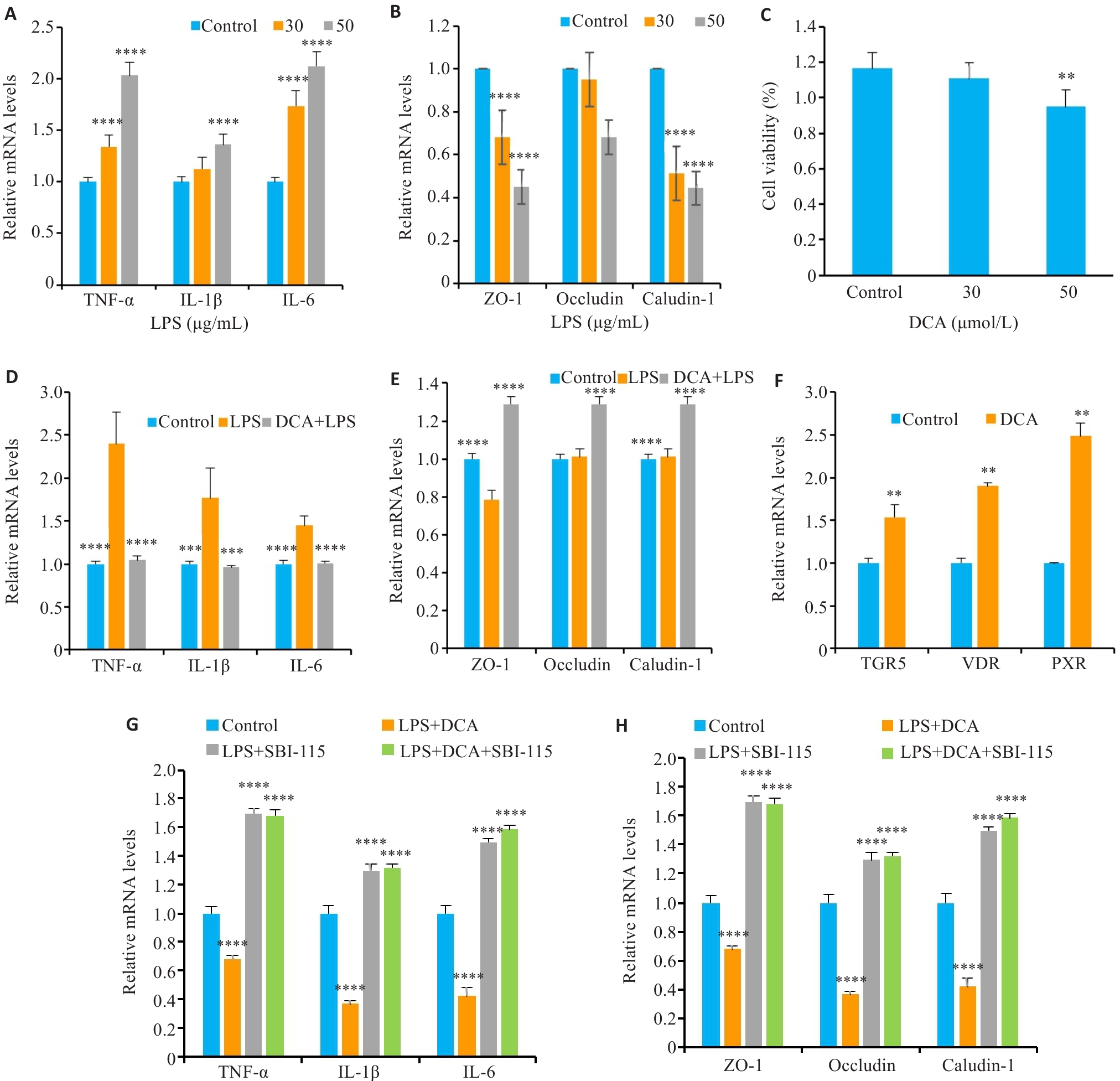
Fig.4 PPS metabolism-related product DCA alleviates bladder epithelial inflammatory injury by activating TGR5. A: Expression levels of inflammatory factors in SV-HUC-1 cells treated with different concentrations of LPS. B: Expression levels of tight junction proteins in SV-HUC-1 cells treated with different concentrations of LPS. C: Viability of SV-HUC-1 cells treated with different concentrations of DCA. D: DCA reduces LPS-induced inflammatory factor expression levels. E: DCA restores LPS-induced reduction of expressions of tight junction proteins in SV-HUC-1 cells. F: DCA stimulates bile acid receptor mRNA expression in SV-HUC-1 cells. G: SBI-115 inhibits the effect of DCA on inflammatory factor expressions in LPS-induced SV-HUC-1 cells. H: SBI-115 inhibits the effect of DCA on tight junction protein expressions in LPS-induced SV-HUC-1 cells. Data are presented as Mean±SE. Statistical significance was determined by one-way ANOVA or unpaired Student's t-test. **P<0.01, ***P<0.001, ****P<0.0001 vs Control (A-C and F-H) or LPS (D-E).
| 1 | Li J, Yi X, Ai JZ. Broaden horizons: the advancement of interstitial cystitis/bladder pain syndrome[J]. Int J Mol Sci, 2022, 23(23): 14594. doi:10.3390/ijms232314594 |
| 2 | Quentin Clemens J, Erickson DR, Varela NP, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome[J]. J Urol, 2022, 208(1): 34-42. doi:10.1097/ju.0000000000002756 |
| 3 | Mohammad A, Laboulaye MA, Shenhar C, et al. Mechanisms of oxidative stress in interstitial cystitis/bladder pain syndrome[J]. Nat Rev Urol, 2024, 21(7): 433-49. doi:10.1038/s41585-023-00850-y |
| 4 | Adak A, Khan MR. An insight into gut microbiota and its functionalities[J]. Cell Mol Life Sci, 2019, 76(3): 473-93. doi:10.1007/s00018-018-2943-4 |
| 5 | El-Salhy M, Hatlebakk JG, Hausken T. Diet in irritable bowel syndrome (IBS): interaction with gut microbiota and gut hormones[J]. Nutrients, 2019, 11(8): 1824. doi:10.3390/nu11081824 |
| 6 | Coelho A, Oliveira R, Antunes-Lopes T, et al. Partners in crime: NGF and BDNF in visceral dysfunction[J]. Curr Neuropharmacol, 2019, 17(11): 1021-38. doi:10.2174/1570159x17666190617095844 |
| 7 | Fu CW, Zhang YW, Liang LH, et al. The microbiota in patients with interstitial cystitis/bladder pain syndrome: a systematic review[J]. BJU Int, 2024, 134(6): 869-80. doi:10.1111/bju.16439 |
| 8 | Lindeke-Myers A, Hanif AM, Jain N. Pentosan polysulfate maculopathy[J]. Surv Ophthalmol, 2022, 67(1): 83-96. doi:10.1016/j.survophthal.2021.05.005 |
| 9 | Anderson VR, Perry CM. Pentosan polysulfate: a review of its use in the relief of bladder pain or discomfort in interstitial cystitis[J]. Drugs, 2006, 66(6): 821-35. doi:10.2165/00003495-200666060-00006 |
| 10 | Smith MM, Melrose J. Xylan prebiotics and the gut microbiome promote health and wellbeing: potential novel roles for pentosan polysulfate[J]. Pharmaceuticals, 2022, 15(9): 1151. doi:10.3390/ph15091151 |
| 11 | Augé C, Gamé X, Vergnolle N, et al. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats[J]. Front Pharmacol, 2020, 11: 1305. doi:10.3389/fphar.2020.01305 |
| 12 | Wu J, Guan TJ, Zheng SR, et al. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice[J]. Lab Invest, 2011, 91(10): 1459-71. doi:10.1038/labinvest.2011.93 |
| 13 | Mroz MS, Lajczak NK, Goggins BJ, et al. The bile acids, deoxycholic acid and ursodeoxycholic acid, regulate colonic epithelial wound healing[J]. Am J Physiol Gastrointest Liver Physiol, 2018, 314(3): G378-87. doi:10.1152/ajpgi.00435.2016 |
| 14 | Chambers LM, Esakov Rhoades EL, Bharti R, et al. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer[J]. Cancer Res, 2022, 82(24): 4654-69. doi:10.1158/0008-5472.can-22-0455 |
| 15 | Zhou ZP, Qiu YF, Li K, et al. Unraveling the impact of Lactobacillus spp. and other urinary microorganisms on the efficacy of mirabegron in female patients with overactive bladder[J]. Front Cell Infect Microbiol, 2022, 12: 1030315. doi:10.3389/fcimb.2022.1030315 |
| 16 | Bon K, Lichtensteiger CA, Wilson SG, et al. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences[J]. J Urol, 2003, 170(3): 1008-12. doi:10.1097/01.ju.0000079766.49550.94 |
| 17 | Wegner KA, Abler LL, Oakes SR, et al. Void spot assay procedural optimization and software for rapid and objective quantification of rodent voiding function, including overlapping urine spots[J]. Am J Physiol Renal Physiol, 2018, 315(4): F1067-80. doi:10.1152/ajprenal.00245.2018 |
| 18 | Bjorling DE, Wang ZY, Vezina CM, et al. Evaluation of voiding assays in mice: impact of genetic strains and sex[J]. Am J Physiol Renal Physiol, 2015, 308(12): F1369-78. doi:10.1152/ajprenal.00072.2015 |
| 19 | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method[J]. Methods, 2001, 25(4): 402-8. doi:10.1006/meth.2001.1262 |
| 20 | Zhang ML, Chekan JR, Dodd D, et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes[J]. Proc Natl Acad Sci USA, 2014, 111(35): E3708-17. doi:10.1073/pnas.1406156111 |
| 21 | Ridlon JM, Ikegawa S, Alves JMP, et al. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens[J]. J Lipid Res, 2013, 54(9): 2437-49. doi:10.1194/jlr.M038869 |
| 22 | Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions[J]. Exp Mol Med, 2018, 50(2): e450. doi:10.1038/emm.2017.282 |
| 23 | Blacher E, Levy M, Tatirovsky E, et al. Microbiome-modulated metabolites at the interface of host immunity[J]. J Immunol, 2017, 198(2): 572-80. doi:10.4049/jimmunol.1601247 |
| 24 | Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids[J]. J Biol Chem, 2003, 278(11): 9435-40. doi:10.1074/jbc.m209706200 |
| 25 | Masyuk TV, Masyuk AI, Lorenzo Pisarello M, et al. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling[J]. Hepatology, 2017, 66(4): 1197-218. doi:10.1002/hep.29284 |
| 26 | Zhao CJ, Wu KY, Hao HY, et al. Gut microbiota-mediated secondary bile acid alleviates Staphylococcus aureus-induced mastitis through the TGR5-cAMP-PKA-NF-κB/NLRP3 pathways in mice[J]. NPJ Biofilms Microbiomes, 2023, 9(1): 8. doi:10.1038/s41522-023-00374-8 |
| 27 | Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy[J]. Am J Physiol Gastrointest Liver Physiol, 2020, 318(3): G554-73. doi:10.1152/ajpgi.00223.2019 |
| 28 | Yang F, Mao CY, Guo LL, et al. Structural basis of GPBAR activation and bile acid recognition[J]. Nature, 2020, 587(7834): 499-504. doi:10.1038/s41586-020-2569-1 |
| 29 | Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice[J]. Nat Med, 2017, 23(1): 107-13. doi:10.1038/nm.4236 |
| 30 | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis[J]. Annu Rev Biochem, 2003, 72: 137-74. doi:10.1146/annurev.biochem.72.121801.161712 |
| 31 | Dai C, Huang YH, Jiang M. Fecal microbiota transplantation for irritable bowel syndrome: Current evidence and perspectives[J]. World J Gastroenterol, 2024, 30(16): 2179-83. doi:10.3748/wjg.v30.i16.2179 |
| 32 | Zhao SN, Gong ZZ, Zhou JF, et al. Deoxycholic acid triggers NLRP3 inflammasome activation and aggravates DSS-induced colitis in mice[J]. Front Immunol, 2016, 7: 536. doi:10.3389/fimmu.2016.00536 |
| [1] | Anbang ZHANG, Xiuqi SUN, Bo PANG, Yuanhua WU, Jingyu SHI, Ning ZHANG, Tao YE. Electroacupuncture pretreatment alleviates cerebral ischemia-reperfusion injury in rats by inhibiting ferroptosis through the gut-brain axis and the Nrf2/HO-1 signaling pathway [J]. Journal of Southern Medical University, 2025, 45(5): 911-920. |
| [2] | Jiachun LUO, Sodnomjamts Batzaya, Xuefeng GAO, Jingyu CHEN, Zhengying YU, Shasha XIONG, Hong CAO. Akkermansia muciniphila gavage improves gut-brain interaction disorders in gp120 transgenic mice [J]. Journal of Southern Medical University, 2025, 45(3): 554-565. |
| [3] | Jiajin LIU, Changhong MIAO, Jiankang XU, Weijie YU, Jixin CHEN, Haozhi TANG, Aifeng LIU. Causal relationship between gut microbiota and pigmented villonodular synovitis: a Mendelian randomization analysis [J]. Journal of Southern Medical University, 2024, 44(7): 1397-1406. |
| [4] | ZHU Jiwei, LU Manlu, JIAO Qianqian, SUN Yunliang, LIU Lu, DING Honghong, YU Yan, PAN Lei. Analysis of gut target microbiota and species difference in patients with obstructive sleep apnea based on 16S rRNA sequencing [J]. Journal of Southern Medical University, 2024, 44(1): 146-155. |
| [5] | LIN Qiongxi, LUN Jingxian, ZHANG Jimin, HE Xiaolong, GONG Zelong, GAO Xuefeng, CAO Hong. Gut microbiome composition in pre-adolescent children with different meat consumption patterns [J]. Journal of Southern Medical University, 2021, 41(12): 1801-1808. |
| [6] | . Inhibitory effect of ketogenic diet on neuroblastoma in BALB/c-nu mouse models [J]. Journal of Southern Medical University, 2020, 40(08): 1155-1164. |
| [7] | . Gut microbiota—an important contributor to liver diseases [J]. Journal of Southern Medical University, 2020, 40(04): 595-600. |
| [8] | . Effect of cyclophosphamide on hematopoietic stem cells in mice with iron overload [J]. Journal of Southern Medical University, 2020, 40(01): 110-117. |
| [9] | . Effects of low-intensity pulsed ultrasound on hematopoietic function in rats after combined chemotherapy with doxorubicin and cyclophosphamide [J]. Journal of Southern Medical University, 2019, 39(07): 836-. |
| [10] | . Gut microbiota in renal transplant recipients, patients with chronic kidney disease and healthy subjects [J]. Journal of Southern Medical University, 2018, 38(12): 1401-. |
| [11] | . Establishment of New Zealand rabbit models of aplastic anemia [J]. Journal of Southern Medical University, 2017, 37(12): 1660-. |
| [12] | . Effect of intermittent fasting on physiology and gut microbiota in presenium rats [J]. Journal of Southern Medical University, 2017, 37(04): 423-. |
| [13] | . A machine learning model using gut microbiome data for predicting changes of trimethylamine-N-oxide in healthy volunteers after choline consumption [J]. Journal of Southern Medical University, 2017, 37(03): 290-. |
| [14] | . Gut microbiota and osteoporosis [J]. Journal of Southern Medical University, 2017, 37(02): 278-. |
| [15] | . Distribution characteristics of trimethylamine N-oxide and its association with gut microbiota [J]. Journal of Southern Medical University, 2016, 36(04): 455-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||