Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (12): 2308-2316.doi: 10.12122/j.issn.1673-4254.2024.12.06
Previous Articles Next Articles
Yanxin ZHONG1,2( ), Yu LIU1,2(
), Yu LIU1,2( ), Weilai TONG1,2, Xinsheng XIE3, Jiangbo NIE1,2, Feng YANG1,2, Zhili LIU1,2, Jiaming LIU1,2(
), Weilai TONG1,2, Xinsheng XIE3, Jiangbo NIE1,2, Feng YANG1,2, Zhili LIU1,2, Jiaming LIU1,2( )
)
Received:2024-07-17
Online:2024-12-20
Published:2024-12-26
Contact:
Jiaming LIU
E-mail:zyx1998033@163.com;ly18296389906@yeah.net;liujiamingdr@hotmail.com
Yanxin ZHONG, Yu LIU, Weilai TONG, Xinsheng XIE, Jiangbo NIE, Feng YANG, Zhili LIU, Jiaming LIU. High expression of AURKB promotes malignant phenotype of osteosarcoma cells by activating nuclear factor-κB signaling via DHX9[J]. Journal of Southern Medical University, 2024, 44(12): 2308-2316.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.12.06
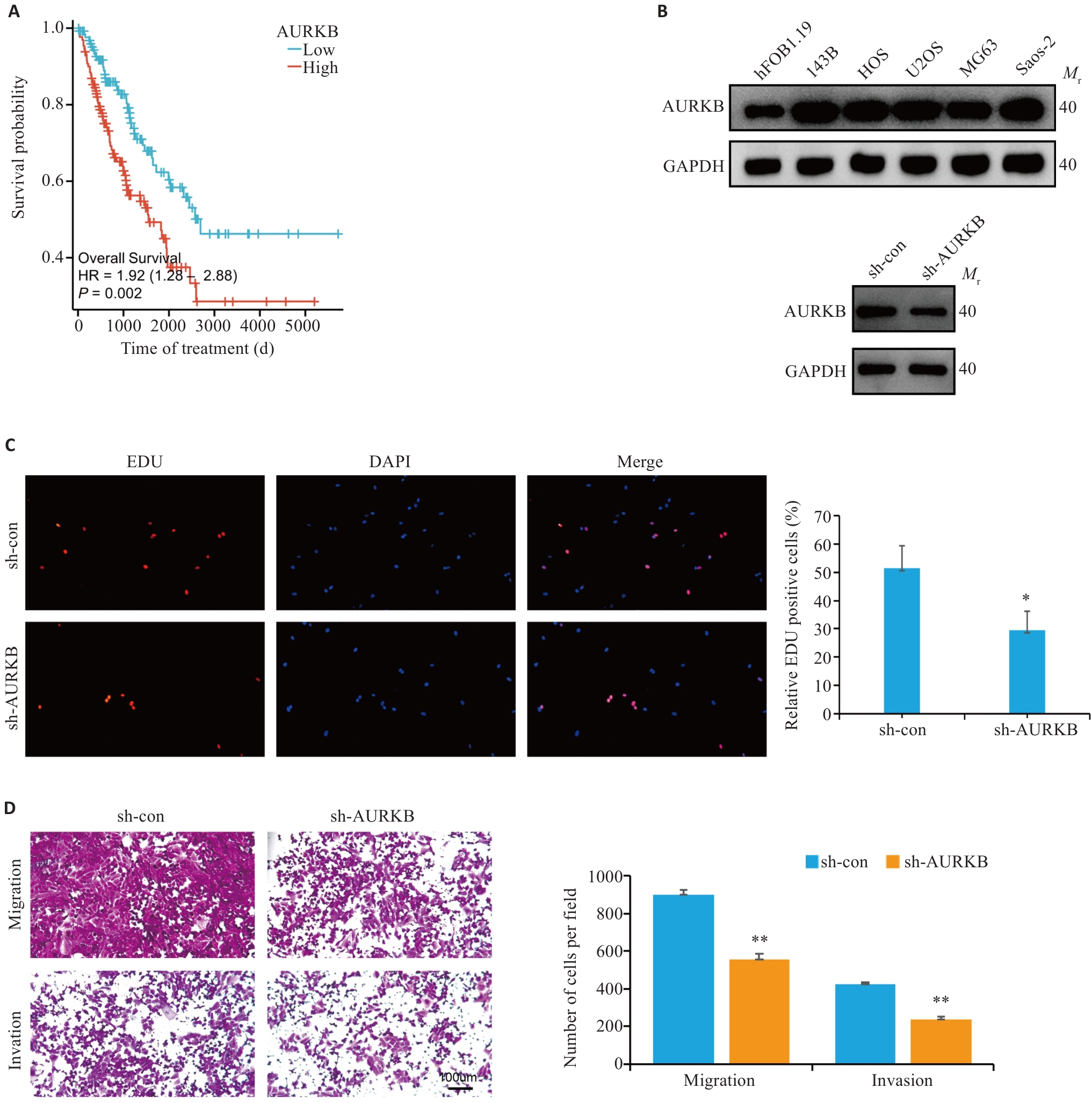
Fig.1 Silencing AURKB inhibits proliferation, migration and invasion of osteosarcoma 143B cells. A: Survival curves of sarcoma patients with different AURKB expression levels. B: Western blotting for detecting AURKB expression levels in osteosarcoma cell lines and for verifying the efficiency of AURKB knockdown in 143B cells. C: EDU assay for detecting the effect of AURKB silencing on proliferation of osteosarcoma cells (Original magnification: ×100). D: Migration and invasion assays of osteosarcoma cells after silencing AURKB (×100). *P<0.05,**P<0.01 vs sh-con.
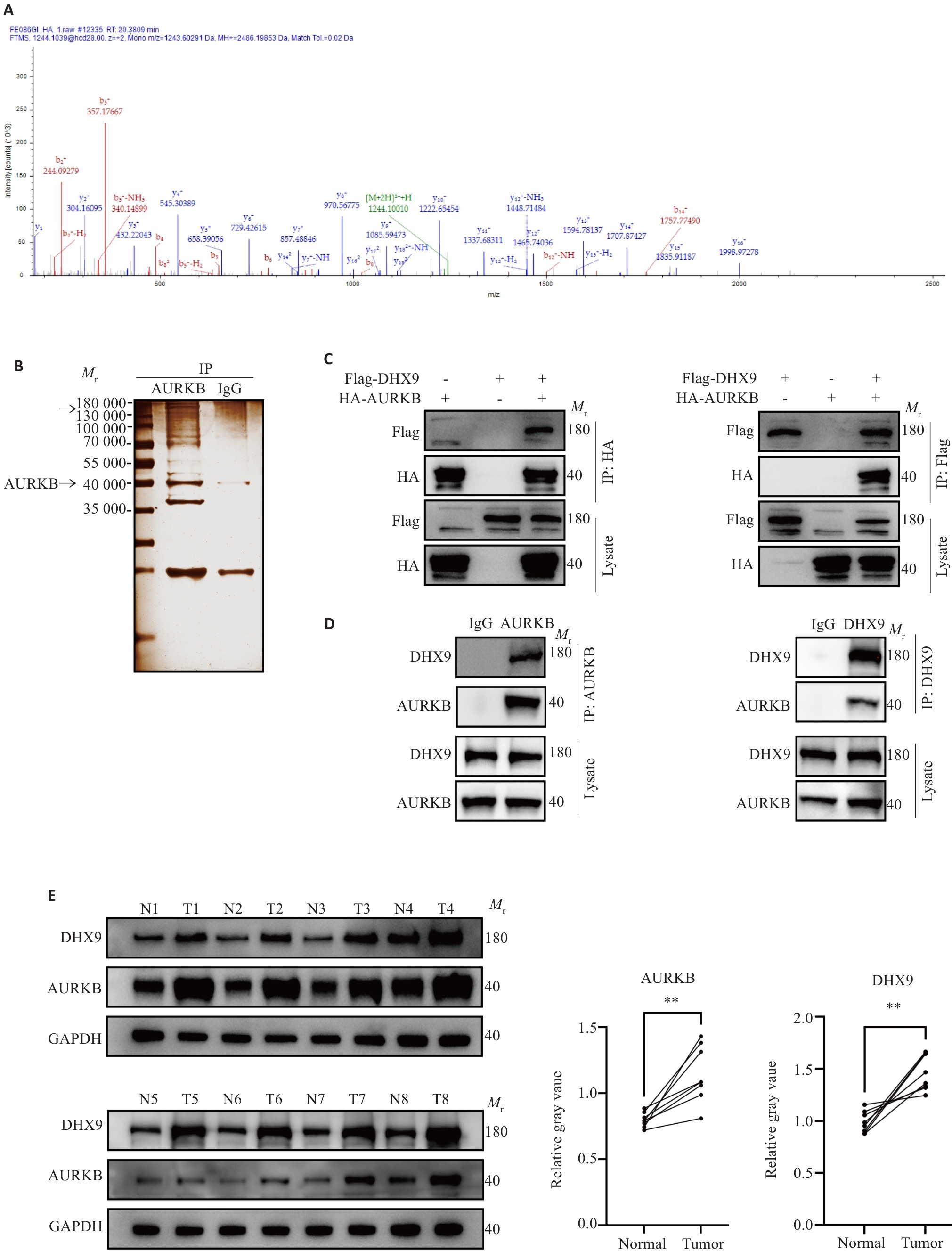
Fig2 AURKB interacts with DHX9. A: DHX9 peptide identified by mass spectrometry. B: Silver-staining assay reveals AURKB-bound proteins. C: CO-IP detection of AURKB and DHX9 interaction in HEK-293 cells. D: CO-IP detection of AURKB and DHX9 interaction in 143B cells. E: Protein expression levels of AURKB and DHX9 in 8 pairs of osteosarcoma tissues and adjacent tissues (T: Tumor; N: Normal). **P<0.01.
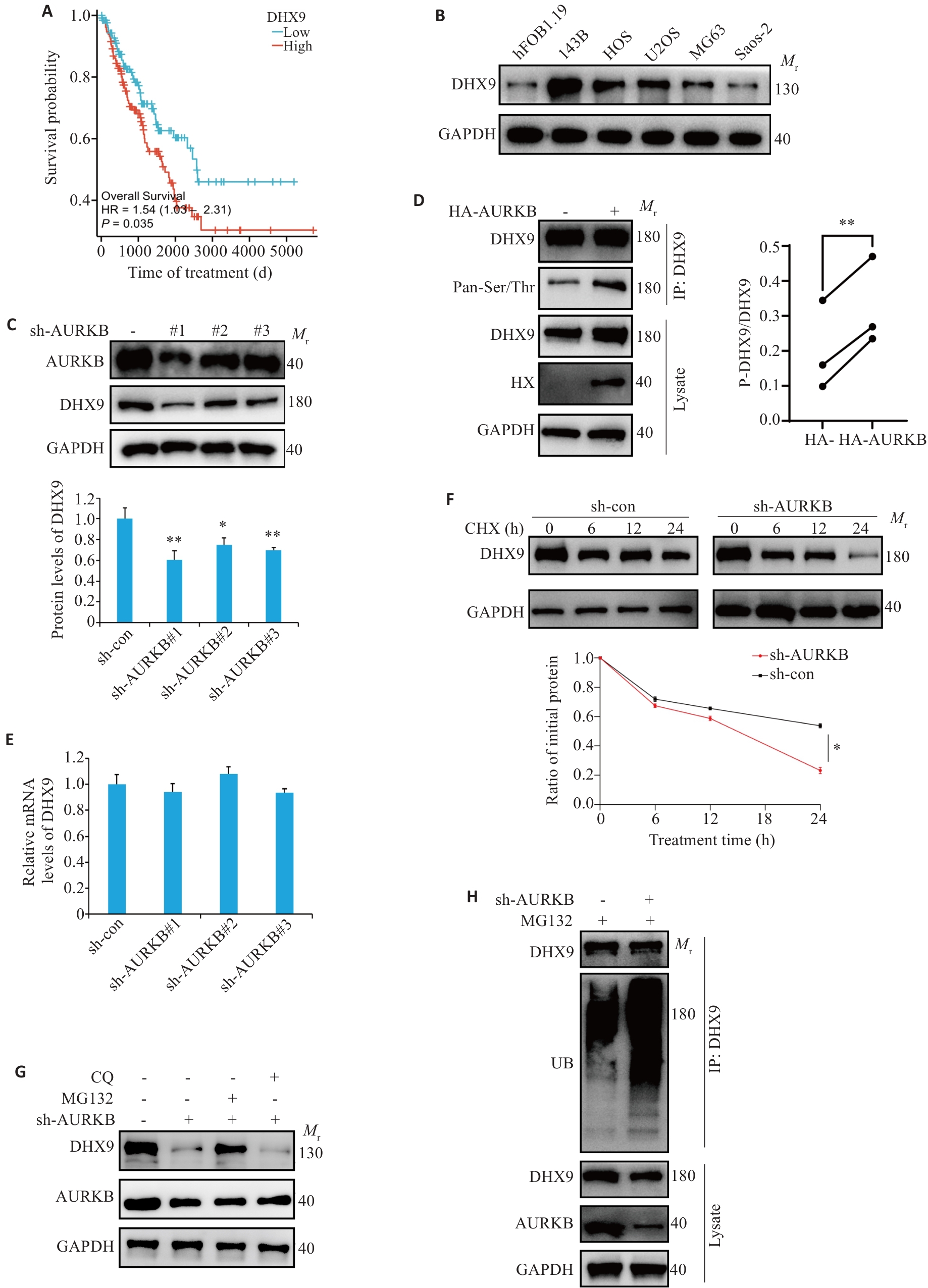
Fig.3 AURKB phosphorylates DHX9 to inhibit its ubiquitinated degradation. A: Survival curves of osteosarcoma patients with low and high AURKB expression. B: Expression of DHX9 in osteosarcoma cell lines. C: Impact of AURKB knockdown on DHX9 protein expression. D: Co-IP analysis of DHX9 protein phosphorylation levels following AURKB overexpression (The diagonal lines represent paired analyses from the same set of experiments). E: DHX9 mRNA expression levels in 143B cells with AURKB silencing. F: Time-dependent analysis of DHX9 protein expression in AURKB-silenced 143B cells treated with cycloheximide. G: Levels of DHX9 and AURKB in AURKB-knockdown 143B cell treated with MG132 (20 μmol/L) or chloroquine (CQ, 20 μmol/L) for 12 h detected by Western blotting. H: Ubiquitination levels of DHX9 in AURKB-knockdown 143B cells. *P<0.05, **P<0.01 vs sh-con.
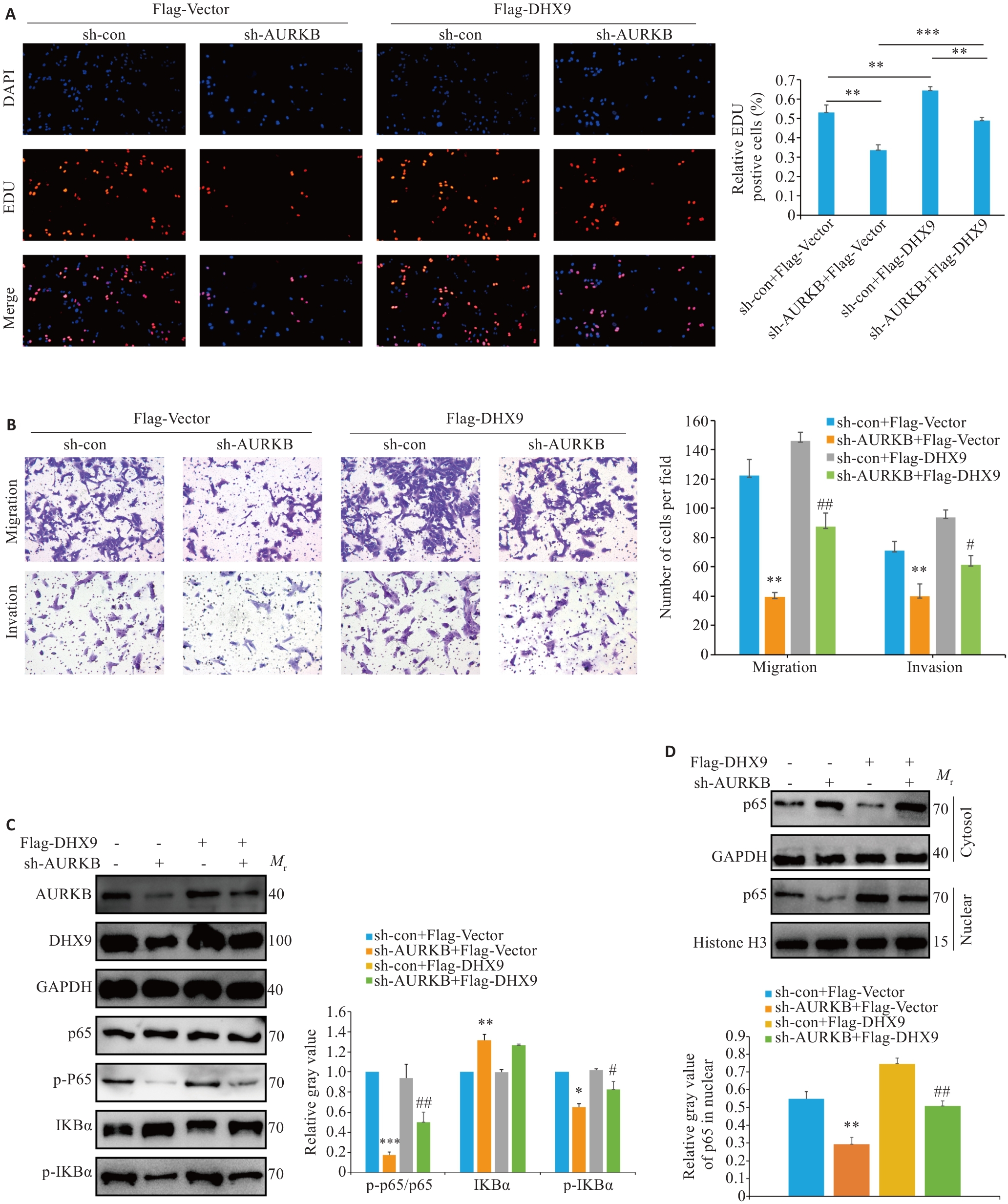
Fig.4 High expression of AURKB enhances malignant phenotype of osteosarcoma cells by activating NF-κB signaling via regulating DHX9. A: EDU experiment for assessing the effects of AURKB silencing and DHX9 overexpression on proliferation of osteosarcoma cells (×100). B: Migration and invasion experiments for assessing the effects of AURKB silencing and DHX9 overexpression on migration and invasion of osteosarcoma cells (×100). C: Western blotting for detecting protein levels of IKBα, p65, phosphorylated IKBα, and phosphorylated p65 in osteosarcoma cells with AURKB silencing and DHX9 overexpression. D: Western blotting for assessing the impact of AURKB silencing and DHX9 overexpression on nuclear translocation of p65. *P<0.05, **P<0.01, ***P<0.001 vs sh-con+Flag-Vector. #P<0.05, ##P<0.01 vs sh-AURKB+Flag-Vector.
| 1 | Friebele JC, Peck J, Pan XL, et al. Osteosarcoma: a meta-analysis and review of the literature[J]. Am J Orthop, 2015, 44(12): 547-53. |
| 2 | Gill J, Ahluwalia MK, Geller D, et al. New targets and approaches in osteosarcoma[J]. Pharmacol Ther, 2013, 137(1): 89-99. |
| 3 | Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment[J]. Ann Oncol, 2015, 26(2): 407-14. |
| 4 | Liu Y, Huang NG, Liao SJ, et al. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma[J]. Cell Prolif, 2021, 54(9): e13102. |
| 5 | Chen YQ, Liu RZ, Wang W, et al. Advances in targeted therapy for osteosarcoma based on molecular classification[J]. Pharmacol Res, 2021, 169: 105684. |
| 6 | Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints[J]. Nat Rev Mol Cell Biol, 2001, 2(1): 21-32. |
| 7 | Carmena M, Wheelock M, Funabiki H, et al. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis[J]. Nat Rev Mol Cell Biol, 2012, 13(12): 789-803. |
| 8 | Borah NA, Reddy MM. Aurora kinase B inhibition: a potential therapeutic strategy for cancer[J]. Molecules, 2021, 26(7): 1981. |
| 9 | Bertran-Alamillo J, Cattan V, Schoumacher M, et al. AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy[J]. Nat Commun, 2019, 10(1): 1812. |
| 10 | González-Loyola A, Fernández-Miranda G, Trakala M, et al. Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development[J]. Mol Cell Biol, 2015, 35(20): 3566-78. |
| 11 | Chen S, Wang Y, Cai X, et al. AURKB is a key to connect oxidative phosphorylation and immune microenvironment in Gastric Cancer [J]. Res Square, 2023. DOI/10.21203/rs.3.rs-3316505/vi. |
| 12 | Hayama S, Daigo Y, Yamabuki T, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis[J]. Cancer Res, 2007, 67(9): 4113-22. |
| 13 | Wang D, Bu F, Zhang WW. The role of ubiquitination in regulating embryonic stem cell maintenance and cancer development[J]. Int J Mol Sci, 2019, 20(11): 2667. |
| 14 | Jain A, Bacolla A, Chakraborty P, et al. Human DHX9 helicase unwinds triple-helical DNA structures[J]. Biochemistry, 2010, 49(33): 6992-9. |
| 15 | Zhang S, Grosse F. Nuclear DNA helicase II unwinds both DNA and RNA[J]. Biochemistry, 1994, 33(13): 3906-12. |
| 16 | Lee T, Pelletier J. The biology of DHX9 and its potential as a therapeutic target[J]. Oncotarget, 2016, 7(27): 42716-39. |
| 17 | Murayama T, Nakayama J, Jiang XP, et al. Targeting DHX9 triggers tumor-intrinsic interferon response and replication stress in small cell lung cancer[J]. Cancer Discov, 2024, 14(3): 468-91. |
| 18 | Cheng DD, Zhang HZ, Yuan JQ, et al. Minichromosome maintenance protein 2 and 3 promote osteosarcoma progression via DHX9 and predict poor patient prognosis[J]. Oncotarget, 2017, 8(16): 26380-93. |
| 19 | Hochegger H, Hégarat N, Pereira-Leal JB. Aurora at the pole and equator: overlapping functions of Aurora kinases in the mitotic spindle[J]. Open Biol, 2013, 3(3): 120185. |
| 20 | Li LZ, Jiang PC, Hu WM, et al. AURKB promotes bladder cancer progression by deregulating the p53 DNA damage response pathway via MAD2L2[J]. J Transl Med, 2024, 22(1): 295. |
| 21 | 吴 昕, 刘家明, 宋宏海, 等. 抑制Aurora激酶B的表达可促进骨肉瘤143B细胞凋亡[J]. 南方医科大学学报, 2020, 40(9): 1273-9. |
| 22 | Yu JJ, Pi WS, Cao Y, et al. Let-7a inhibits osteosarcoma cell growth and lung metastasis by targeting Aurora-B[J]. Cancer Manag Res, 2018, 10: 6305-15. |
| 23 | Gully CP, Velazquez-Torres G, Shin JH, et al. Aurora B kinase phosphorylates and instigates degradation of p53[J]. Proc Natl Acad Sci U S A, 2012, 109(24): E1513-22. |
| 24 | Li L, Xie K, Xie HH, et al. AURKB promotes colorectal cancer progression by triggering the phosphorylation of histone H3 at serine 10 to activate CCNE1 expression[J]. Aging, 2024, 16(9): 8019-30. |
| 25 | Garlapati C, Joshi S, Bhattarai S, et al. PLK1 and AURKB phosphorylate survivin differentially to affect proliferation in racially distinct triple-negative breast cancer[J]. Cell Death Dis, 2023, 14(1): 12. |
| 26 | Shen BC, Chen YM, Hu J, et al. Hepatitis B virus X protein modulates upregulation of DHX9 to promote viral DNA replication[J]. Cell Microbiol, 2020, 22(3): e13148. |
| 27 | Liu SL, He LM, Wu JH, et al. DHX9 contributes to the malignant phenotypes of colorectal cancer via activating NF‑κB signaling pathway[J]. Cell Mol Life Sci, 2021, 78(24): 8261-81. |
| 28 | Lee T, Paquet M, Larsson O, et al. Tumor cell survival dependence on the DHX9 DExH-box helicase[J]. Oncogene, 2016, 35(39): 5093-105. |
| 29 | Shan YN, Wei SS, Xiang XH, et al. SNORA42 promotes oesophageal squamous cell carcinoma development through triggering the DHX9/p65 axis[J]. Genomics, 2021, 113(5): 3015-29. |
| 30 | Huang TT, Chiang CY, Nair JR, et al. AKT1 interacts with DHX9 to mitigate R loop-induced replication stress in ovarian cancer[J]. Cancer Res, 2024, 84(6): 887-904. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||