Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (12): 2300-2307.doi: 10.12122/j.issn.1673-4254.2024.12.05
Previous Articles Next Articles
Xiaoling SU1( ), Daoyong LIAO1(
), Daoyong LIAO1( ), Chao LI1, Li CHEN1, Jingyun WANG1, Tian GAN1, Haodang LUO1, Ning WU3, Jun HE1,2(
), Chao LI1, Li CHEN1, Jingyun WANG1, Tian GAN1, Haodang LUO1, Ning WU3, Jun HE1,2( )
)
Received:2024-05-06
Online:2024-12-20
Published:2024-12-26
Contact:
Jun HE
E-mail:xiaoling2020s@163.com;l1326253952@163.com;junhe@usc.edu.cn
Supported by:Xiaoling SU, Daoyong LIAO, Chao LI, Li CHEN, Jingyun WANG, Tian GAN, Haodang LUO, Ning WU, Jun HE. Protective effect of Streptococcus salivarius K12 against Mycoplasma pneumoniae infection in mice[J]. Journal of Southern Medical University, 2024, 44(12): 2300-2307.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.12.05
| Primer name | Primer sequence 5'-3' |
|---|---|
| Mp 16S rRNA | F:TAACGGCCTACCAAGGCAATGA |
| R:AGTCAAACTCTAGCCATTACCTGC | |
| Mp 16S rRNA probe | ACGGCCCATACTCCTACGGGAGGCAGCAGT |
| P1[ | F:CGCCGCAAAGATGAATGAC |
| R:TGTCCTTCCCCATCTAACAGTTC | |
| CARDS | F:TTCCACTTCAGAAACACCCACAGC |
| R:TCAATCAGGGCACGCAAACG | |
| Cyclophilin[ | F:AGCACTGGAGAGAAAGGATTTGG |
| R:TCTTCTTGCTGGTCTTGCCATT | |
| MUC5ac | F:ACGACACTTTTCAGTACCAATGAC |
| R:GCTTCCTTACAGATGCAGTCCT | |
| MMP-9 | F:CGGATTTGGCCGTATTGGGC |
| R:TGATGGCATGCACTGTGGTC | |
| TNF-α | F:CCACCACGCTCTTCTGTCTAC |
| R:TGGGCTACAGGCTTGTCACT | |
| IL-6 | F:TTCACAAGTCGGAGGCTTA |
| R:CAAGTGCATCATCGTTGTTC | |
| CXCL1 | F:TGGCTGGGATTCACCTCAAG |
| R:CAAGCCTCGCGACCATTCTT | |
| TLR-2[ | F:GCCACCATTTCCACGGACT |
| R:GGCTTCCTCTTGGCCTGG | |
| TLR-4[ | F:TTTATTCAGAGCCGTTGGTG |
| R:CAGAGGATTGTCCTCCCATT | |
| Col3a1[ | F:GCCCACAGCCTTCTACAC |
| R:CCAGGGTCACCATTTCTC | |
| GAPDH | F:AGGTCGGTGTGAACGGATTTG |
| R:TGTAGACCATGTAGTTGAGGTCA |
Tab.1 Primer sequences for RT-qPCR
| Primer name | Primer sequence 5'-3' |
|---|---|
| Mp 16S rRNA | F:TAACGGCCTACCAAGGCAATGA |
| R:AGTCAAACTCTAGCCATTACCTGC | |
| Mp 16S rRNA probe | ACGGCCCATACTCCTACGGGAGGCAGCAGT |
| P1[ | F:CGCCGCAAAGATGAATGAC |
| R:TGTCCTTCCCCATCTAACAGTTC | |
| CARDS | F:TTCCACTTCAGAAACACCCACAGC |
| R:TCAATCAGGGCACGCAAACG | |
| Cyclophilin[ | F:AGCACTGGAGAGAAAGGATTTGG |
| R:TCTTCTTGCTGGTCTTGCCATT | |
| MUC5ac | F:ACGACACTTTTCAGTACCAATGAC |
| R:GCTTCCTTACAGATGCAGTCCT | |
| MMP-9 | F:CGGATTTGGCCGTATTGGGC |
| R:TGATGGCATGCACTGTGGTC | |
| TNF-α | F:CCACCACGCTCTTCTGTCTAC |
| R:TGGGCTACAGGCTTGTCACT | |
| IL-6 | F:TTCACAAGTCGGAGGCTTA |
| R:CAAGTGCATCATCGTTGTTC | |
| CXCL1 | F:TGGCTGGGATTCACCTCAAG |
| R:CAAGCCTCGCGACCATTCTT | |
| TLR-2[ | F:GCCACCATTTCCACGGACT |
| R:GGCTTCCTCTTGGCCTGG | |
| TLR-4[ | F:TTTATTCAGAGCCGTTGGTG |
| R:CAGAGGATTGTCCTCCCATT | |
| Col3a1[ | F:GCCCACAGCCTTCTACAC |
| R:CCAGGGTCACCATTTCTC | |
| GAPDH | F:AGGTCGGTGTGAACGGATTTG |
| R:TGTAGACCATGTAGTTGAGGTCA |
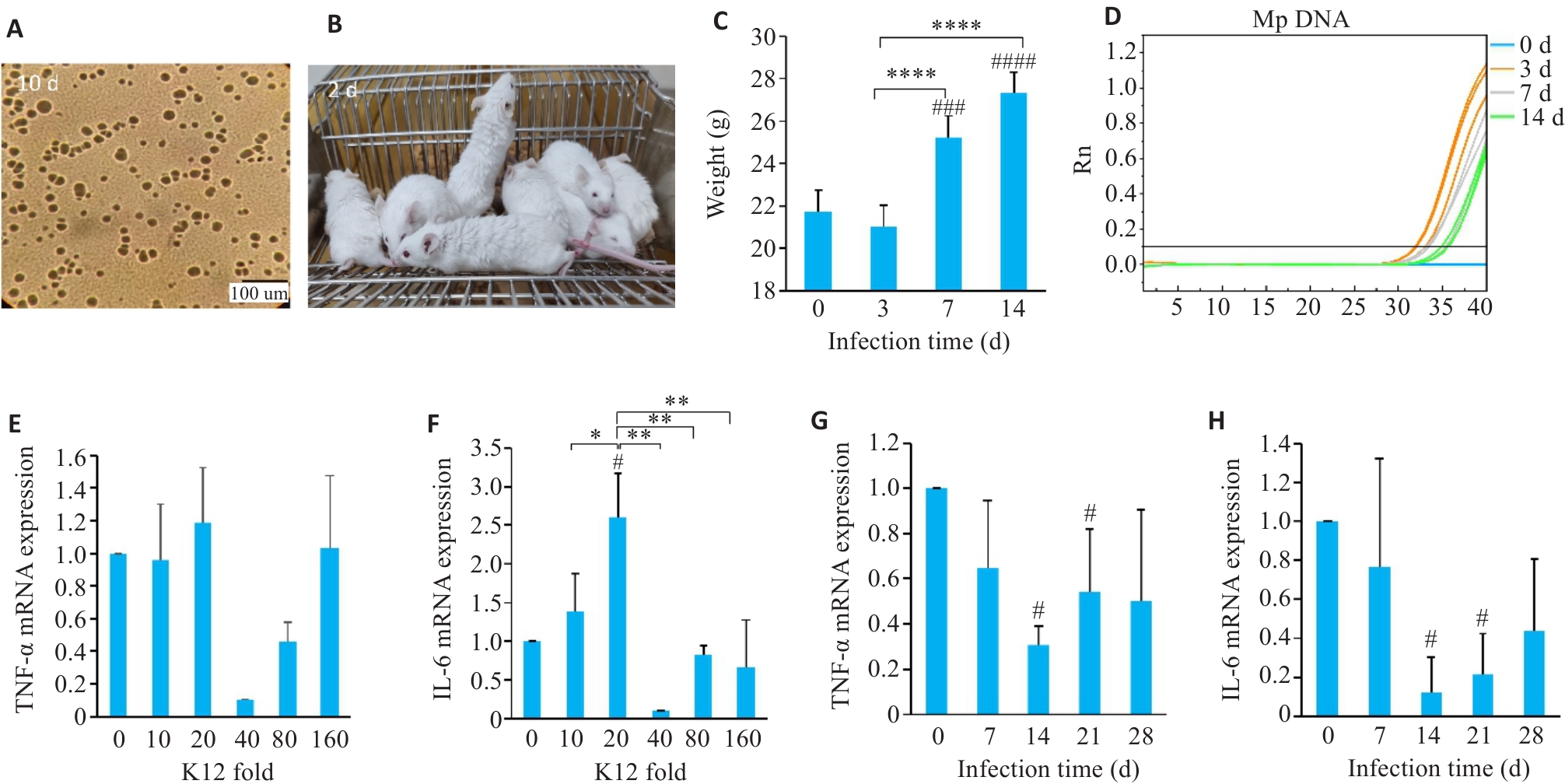
Fig.1 Construction of M. pneumoniae-infected mouse model and optimization of conditions for K12 probiotic treatment. A: Cultivation of M. pneumoniae on solid plate. B: General condition of the mice 3 days after infection with M. pneumoniae. C: Changes in body weight of the mice at different time points after infection. D: M. pneumoniae DNA detection in mice. E-H: Detection of TNF-α and IL-6 mRNA expressions in mouse lung tissues. *P<0.05, **P<0.01, ****P<0.0001. #P<0.05, ###P<0.001, ####P<0.0001 vs 0. (n=3-5).
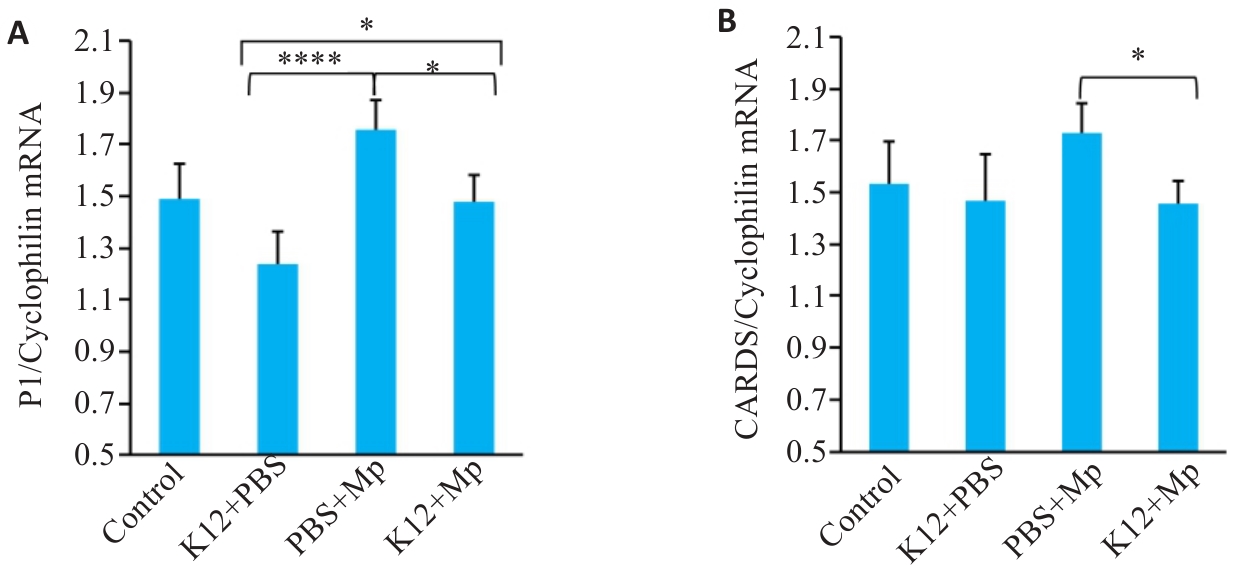
Fig.2 M. pneumoniae load in the lung tissue of the mice after prophylactic administration of K12 probiotics. A,B: qRT-PCR for detecting M. pneumoniae P1 protein and CARDS mRNA in the lung tissue. *P<0.05, ****P<0.0001 (n=5).
| Group | WBC (106/L) | MN# (106/L) | PMN# (106/L) | MN (%) | PMN (%) |
|---|---|---|---|---|---|
| Control | 157.60±92.75 | 129.60±91.60 | 28.00±21.52 | 79.50±19.33 | 20.50±19.33 |
| K12+PBS | 171.00±55.67 | 157.20±55.83 | 13.80±0.44 | 91.08±3.30 | 8.92±3.30 |
| PBS+Mp | 2032.20±399.44**** | 685.40±185.17**** | 1346.80±291.22**** | 33.88±6.19 | 66.12±6.19 |
| K12+Mp | 905.60±665.89## | 372.60±217.14# | 533.00±468.64## | 53.90±26.18 | 46.10±26.18 |
Tab.2 Leukocyte classification in BALF of M. pneumoniae mice treated with K12 probiotic
| Group | WBC (106/L) | MN# (106/L) | PMN# (106/L) | MN (%) | PMN (%) |
|---|---|---|---|---|---|
| Control | 157.60±92.75 | 129.60±91.60 | 28.00±21.52 | 79.50±19.33 | 20.50±19.33 |
| K12+PBS | 171.00±55.67 | 157.20±55.83 | 13.80±0.44 | 91.08±3.30 | 8.92±3.30 |
| PBS+Mp | 2032.20±399.44**** | 685.40±185.17**** | 1346.80±291.22**** | 33.88±6.19 | 66.12±6.19 |
| K12+Mp | 905.60±665.89## | 372.60±217.14# | 533.00±468.64## | 53.90±26.18 | 46.10±26.18 |
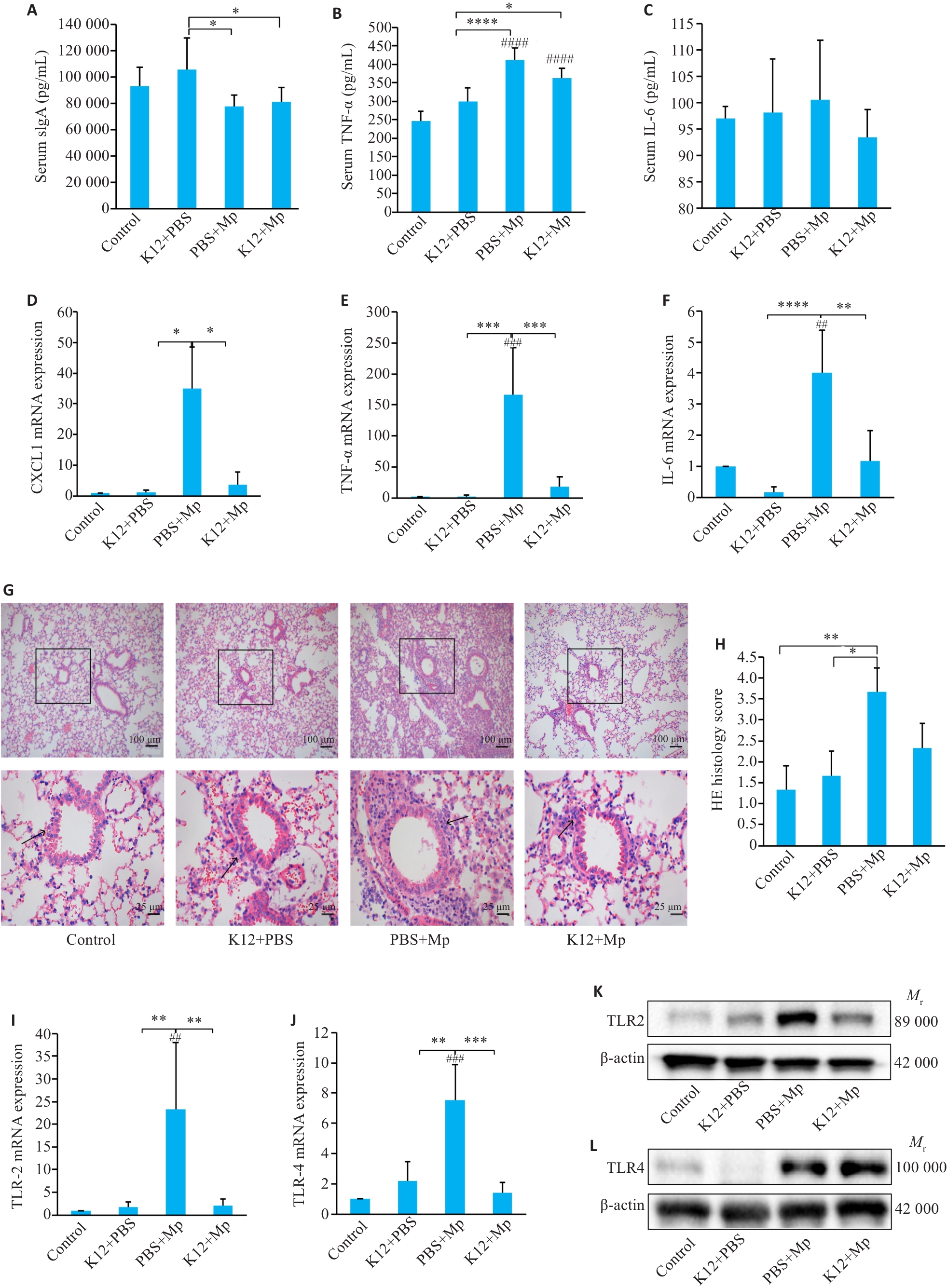
Fig.3 Changes in levels of inflammatory factors in serum and lung tissue of M. pneumoniae mice with K12 pretreatment. A-C: ELISA for detecting serum levels of sIgA, TNF‑α and IL-6. D-F: qRT-PCR for detecting CXCL1, TNF‑α and IL-6 mRNA expressions in the lung tissue. G-H: HE staining of lung tissues and the pathological scores. I-L: qRT-PCR and Western blotting for TLR2 and TLR4 expression. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. ##P<0.01, ###P<0.001, ####P<0.0001 vs Control. (n=5).

Fig.4 Transcriptional levels of airway remodeling factors in lung tissues and AB/PAS staining of mouse lung tissue. A-C: mRNA levels of MMP9, MUC5ac and COL3A1 in the lung tissues detected by qRT-PCR. D: AB/PAS staining. E: AB/PAS staining score. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; ###P<0.001 vs Control. (n=5).
| 1 | Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the respiratory tract and beyond[J]. Clin Microbiol Rev, 2017, 30(3): 747-809. |
| 2 | Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection[J]. J Clin Med Res, 2018, 10(7): 535-44. |
| 3 | Zhang ZK, Wan RJ, Yuan Q, et al. Cell damage and neutrophils promote the infection of Mycoplasma pneumoniae and inflammatory response[J]. Microb Pathog, 2022, 169: 105647. |
| 4 | Tamiya S, Yoshikawa E, Ogura M, et al. Vaccination using inactivated Mycoplasma pneumoniae induces detrimental infiltration of neutrophils after subsequent infection in mice[J]. Vaccine, 2020, 38(32): 4979-87. |
| 5 | Meyer Sauteur PM, Beeton ML, European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycoplasma and Chlamydia Infections study group. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions[J]. Lancet Microbe, 2024, 5(2): e100-1. |
| 6 | Chen Y, Zhang Y, Tang QN, et al. Efficacy of doxycycline therapy for macrolide-resistant Mycoplasma pneumoniae pneumonia in children at different periods[J]. Ital J Pediatr, 2024, 50(1): 38. |
| 7 | di Pierro F, Colombo M, Zanvit A, et al. Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children[J]. Drug Healthc Patient Saf, 2014, 6: 15-20. |
| 8 | Mokhtar M, Rismayuddin NAR, Mat Yassim AS, et al. Streptococcus salivarius K12 inhibits Candida albicans aggregation, biofilm formation and dimorphism[J]. Biofouling, 2021, 37(7): 767-76. |
| 9 | Laws GL, Hale JDF, Kemp RA. Human systemic immune response to ingestion of the oral probiotic Streptococcus salivarius BLIS K12[J]. Probiotics Antimicrob Proteins, 2021, 13(6): 1521-9. |
| 10 | di Pierro F, Iqtadar S, Mumtaz SU, et al. Clinical effects of Streptococcus salivarius K12 in hospitalized COVID-19 patients: results of a preliminary study[J]. Microorganisms, 2022, 10(10): 1926. |
| 11 | MacDonald KW, Chanyi RM, Macklaim JM, et al. Streptococcus salivarius inhibits immune activation by periodontal disease pathogens[J]. BMC Oral Health, 2021, 21(1): 245. |
| 12 | 徐叔云. 药理实验方法学[M]. 3版. 北京: 人民卫生出版社, 2002. |
| 13 | 梁珂莹. 应用TaqMan实时荧光定量PCR优化肺炎支原体生长条件的研究[D]. 南华大学,2021. |
| 14 | Iannuzo N, Insel M, Marshall C, et al. CC16 deficiency in the context of early-life Mycoplasma pneumoniae infection results in augmented airway responses in adult mice[J]. Infect Immun, 2022, 90(2): e0054821. |
| 15 | Daniel S, Phillippi D, Schneider LJ, et al. Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet[J]. Part Fibre Toxicol, 2021, 18(1): 3. |
| 16 | Wang T, Sun HM, Lu ZT, et al. The CARDS toxin of Mycoplasma pneumoniae induces a positive feedback loop of type 1 immune response[J]. Front Immunol, 2022, 13: 1054788. |
| 17 | Li YT, Shao FY, Zheng SW, et al. Alteration of Streptococcus salivarius in buccal mucosa of oral lichen planus and controlled clinical trial in OLP treatment[J]. Probiotics Antimicrob Proteins, 2020, 12(4): 1340-8. |
| 18 | Tamiya S, Yoshikawa E, Ogura M, et al. Neutrophil-mediated lung injury both via TLR2-dependent production of IL-1α and IL-12 p40, and TLR2-independent CARDS toxin after Mycoplasma pneumoniae infection in mice[J]. Microbiol Spectr, 2021, 9(3): e0158821. |
| 19 | Li G, Fan LP, Wang YQ, et al. High co-expression of TNF‑α and CARDS toxin is a good predictor for refractory Mycoplasma pneumoniae pneumonia[J]. Mol Med, 2019, 25(1): 38. |
| 20 | Mei XZ, Wang J, Zhang C, et al. Apigenin suppresses mycoplasma-induced alveolar macrophages necroptosis via enhancing the methylation of TNF‑α promoter by PPARγ‑Uhrf1 axis[J]. Phytomedicine, 2023, 108: 154504. |
| 21 | Chen M, Deng H, Zhao Y, et al. Toll-like receptor 2 modulates pulmonary inflammation and TNF‑α release mediated by Mycoplasma pneumoniae [J]. Front Cell Infect Microbiol, 2022, 12: 824027. |
| 22 | Luo H, He J, Qin L, et al. Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a proinflammatory response[J]. Clin Exp Immunol, 2021, 203(1): 66-79. |
| 23 | Johnson MDL, Younis US, Menghani SV, et al. CC16 binding to α4β1 integrin protects against Mycoplasma pneumoniae infection[J]. Am J Respir Crit Care Med, 2021, 203(11): 1410-8. |
| 24 | Ma Y, Gu YQ, Zhang XX, et al. High expression of MUC5AC, MUC5B, and layilin plays an essential role in prediction in the development of plastic bronchitis caused by MPP[J]. Front Microbiol, 2022, 13: 911228. |
| 25 | Tunçer S, Karaçam S. Cell-free supernatant of Streptococcus salivarius M18 impairs the pathogenic properties of Pseudomonas aeruginosa and Klebsiella pneumonia [J]. Arch Microbiol, 2020, 202(10): 2825-40. |
| 26 | Vertillo Aluisio G, Spitale A, Bonifacio L, et al. Streptococcus salivarius 24SMBc genome analysis reveals new biosynthetic gene clusters involved in antimicrobial effects on Streptococcus pneumoniae and Streptococcus pyogenes [J]. Microorganisms, 2022, 10(10): 2042. |
| 27 | Garcia-Castillo V, Tomokiyo M, Raya Tonetti F, et al. Alveolar macrophages are key players in the modulation of the respiratory antiviral immunity induced by orally administered Lacticasei-bacillus rhamnosus CRL1505[J]. Front Immunol, 2020, 11: 568636. |
| 28 | Zhang NY, Zeng WW, Du TF, et al. Lacticaseibacillus casei CNRZ1874 supplementation promotes M1 alveolar macrophage activation and attenuates Mycoplasma pneumoniae pneumonia[J]. J Appl Microbiol, 2023, 134(3): lxad022. |
| [1] |
.
Comparison of three approaches to establishing Balb/c mouse models of hind-limb ischemia [J]. Journal of Southern Medical University, 2014, 34(08): 1167-. |
| [2] |
.
Optimization of streptozotocin dosing for establishing tumor-bearing diabetic mouse models [J]. Journal of Southern Medical University, 2014, 34(06): 827-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||