Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (8): 1758-1767.doi: 10.12122/j.issn.1673-4254.2025.08.20
Xuhui ZHAO1,2( ), Mengran LIU1(
), Mengran LIU1( ), Xi CHEN2,3, Jing HUANG2, Yuan LIU2, Haifeng XU2
), Xi CHEN2,3, Jing HUANG2, Yuan LIU2, Haifeng XU2
Received:2025-03-11
Online:2025-08-20
Published:2025-09-05
Contact:
Mengran LIU
E-mail:xh.zhao@siat.ac.cn;liumengran1991@163.com
Supported by:Xuhui ZHAO, Mengran LIU, Xi CHEN, Jing HUANG, Yuan LIU, Haifeng XU. Synthesis of a temperature-responsive multimodal motion microrobot capable of precise navigation for targeted controllable drug release[J]. Journal of Southern Medical University, 2025, 45(8): 1758-1767.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.08.20
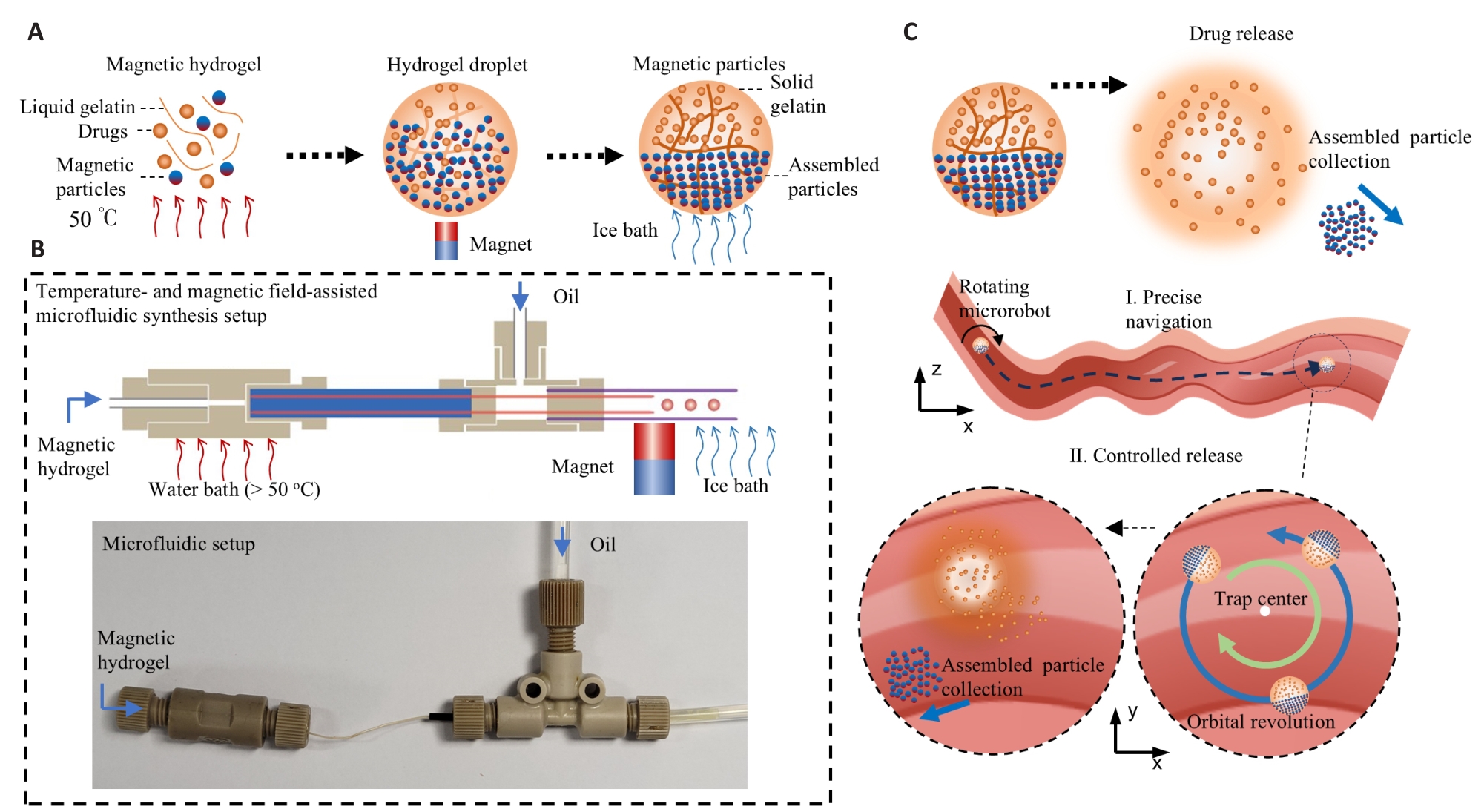
Fig.1 Principle of temperature-responsive multimodal motion microrobot (MMMR) synthesis and drug release. A: Principles of MMMR synthesis. B: Design and appearance of the synthesis device. C: Drug delivery principle of the MMMR capable of precise navigation and controlled drug release.
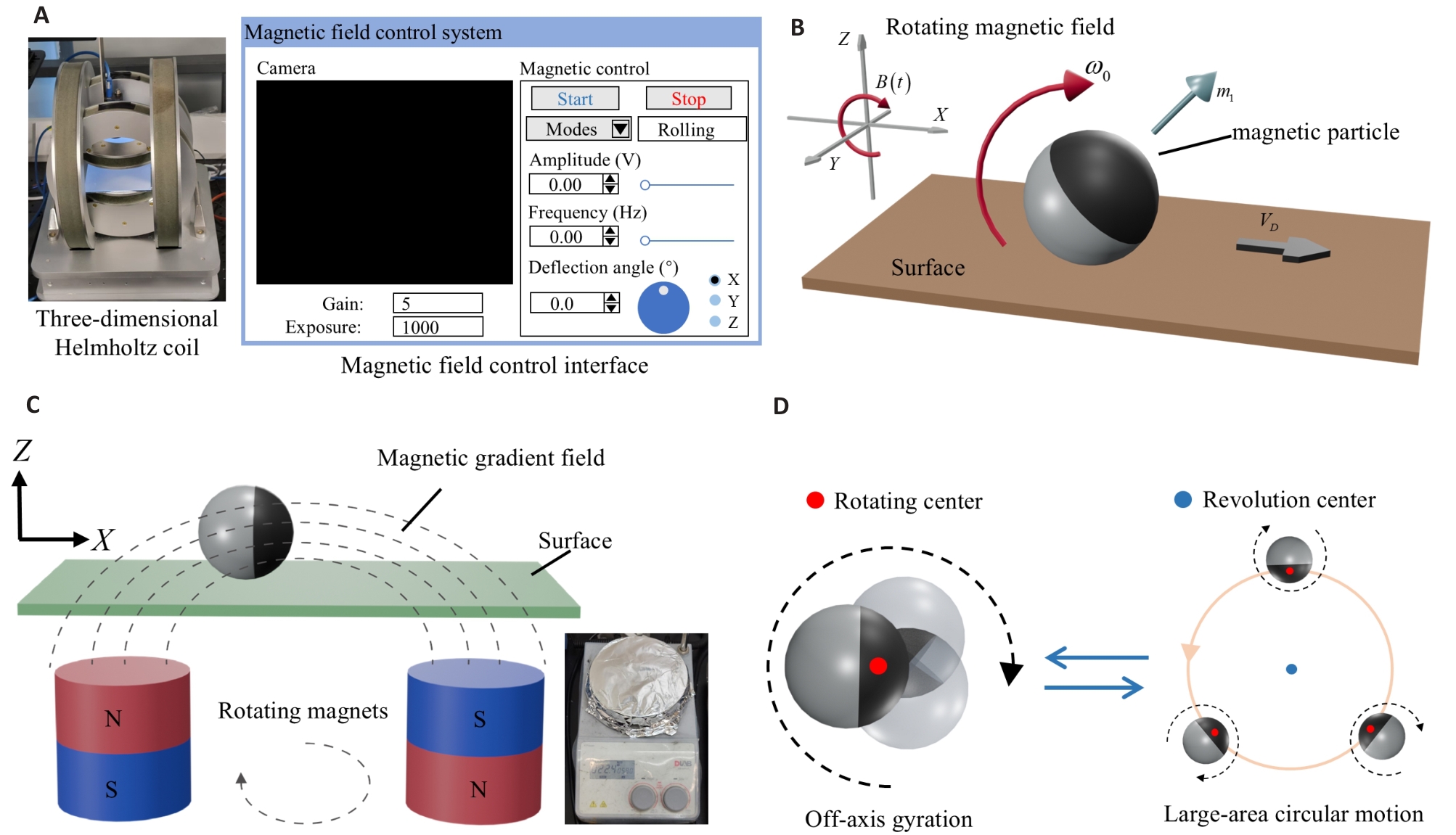
Fig.2 Multimodal motion control of the MMMR. A: Magnetic control platform and control interface for linear motion control. B: Schematic diagram of linear motion of the MMMR. C: Schematic illustration of the rotating gradient magnetic field and a photograph of the device. D: Schematic diagram of circular motion of MMMR.
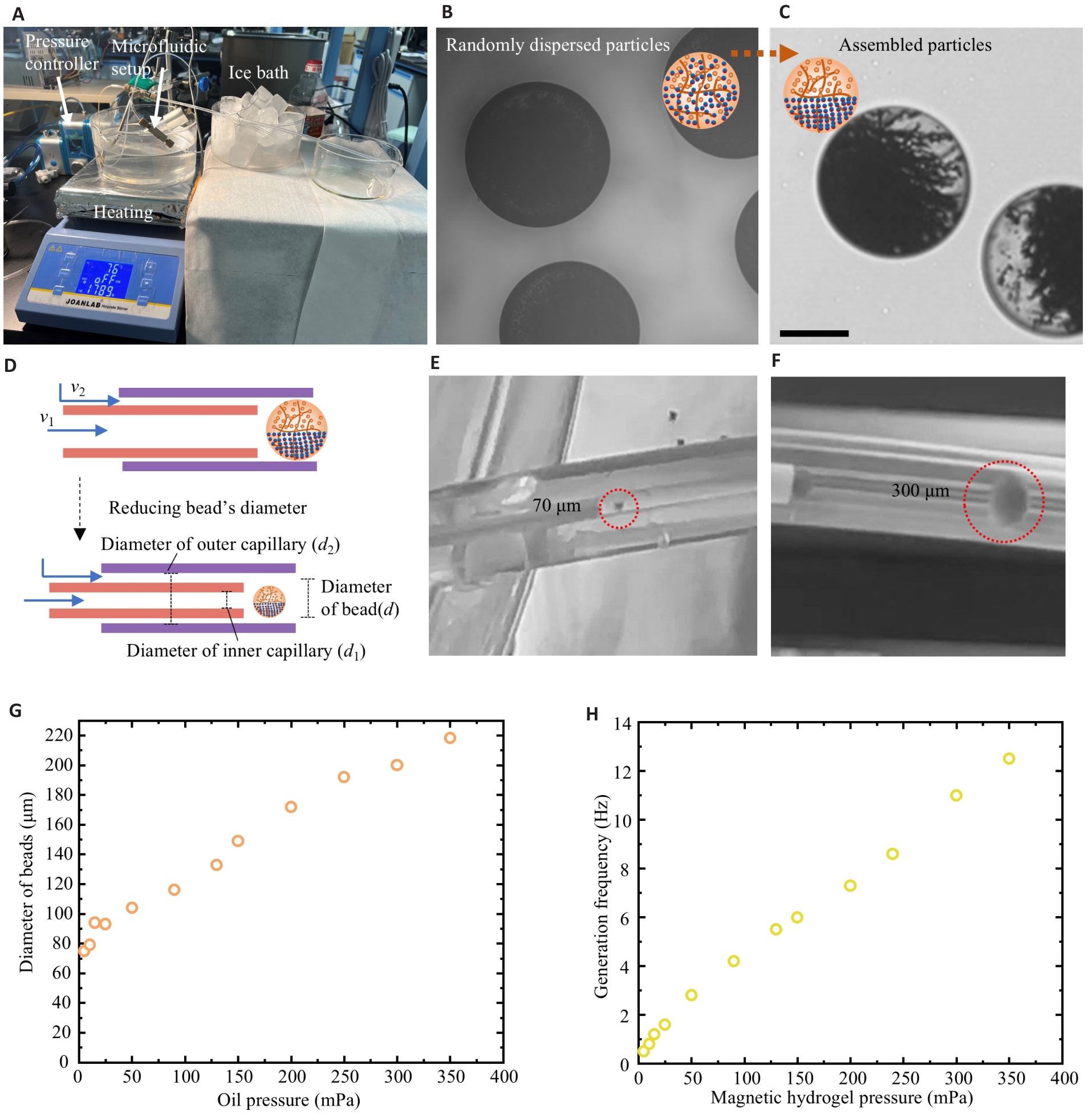
Fig.3 Synthesis of the MMMR. A: Synthesis device of MMMR. B: Randomly dispersed particles in magnetic hydrogel beads. C: Assembled particles in magnetic hydrogel beads (Scale bar=150 μm). D: Size control principle of the hydrogel beads. E: Magnetic hydrogel beads with a diameter of 70 μm. F: Magnetic hydrogel beads with a diameter of 500 μm. G: Relationship between oil pressure and the diameter of beads. H: Relationship between magnetic hydrogel pressure and the generation frequency of beads.
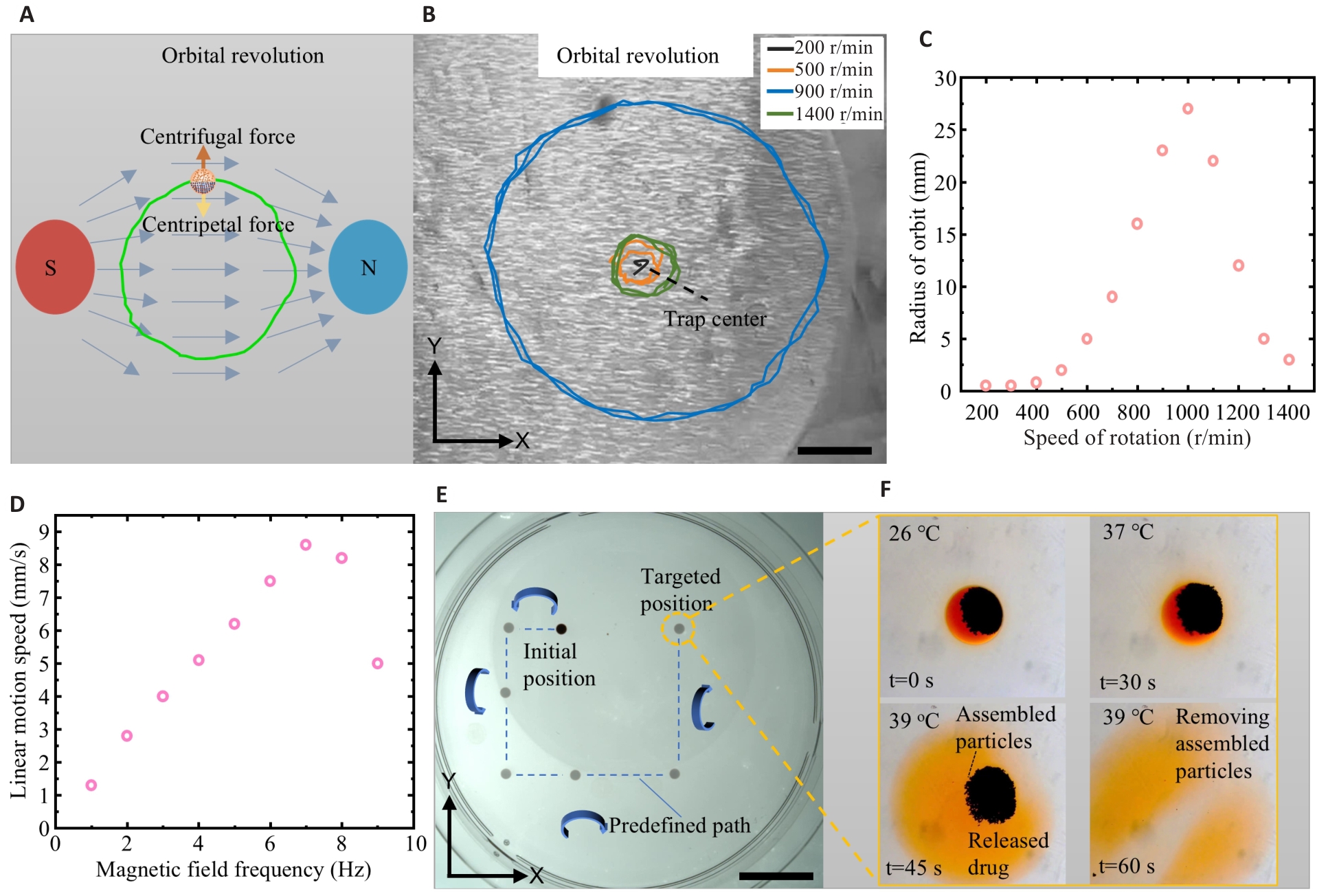
Fig.4 Local circular motion and targeted drug delivery experiments with MMMR. A: Local circular motion principle. B: Movement of the MMMR under a rotating gradient magnetic field (Scale bar=10 mm). C: Relationship between speed of rotation and the radius of orbit. D: Relationship between magnetic field frequency and the linear motion speed of the MMMR. E: Test of the MMMR for targeted drug delivery (Scale bar=5 mm). F: Test of the MMMR for drug release and magnetic particle removal.
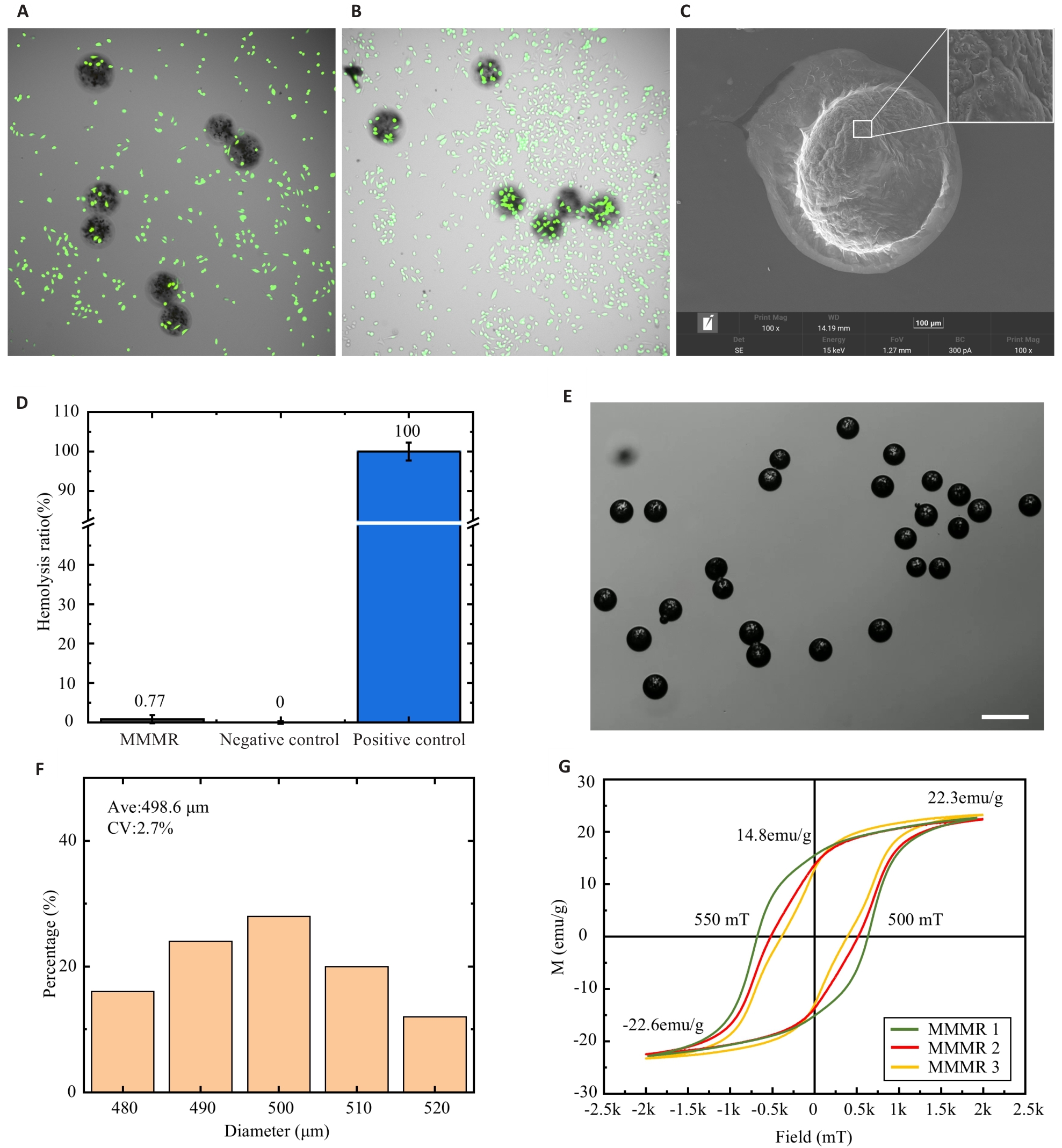
Fig.5 Synthesis quality and biocompatibility of the MMMR. A: Fluorescence images of the MMMR co-cultured with cells for 6 h. B: Fluorescence images of the MMMR co-cultured with cells for 12 h. C: Scanning electron microscopy of the MMMR. D: Blood compatibility test of the MMMR. E: MMMR images under microscope (Scale bar=1 mm). F: Coefficient of variation (CV) analysis of the MMMR. G: Hysteresis loop test of the 3 MMMRs.
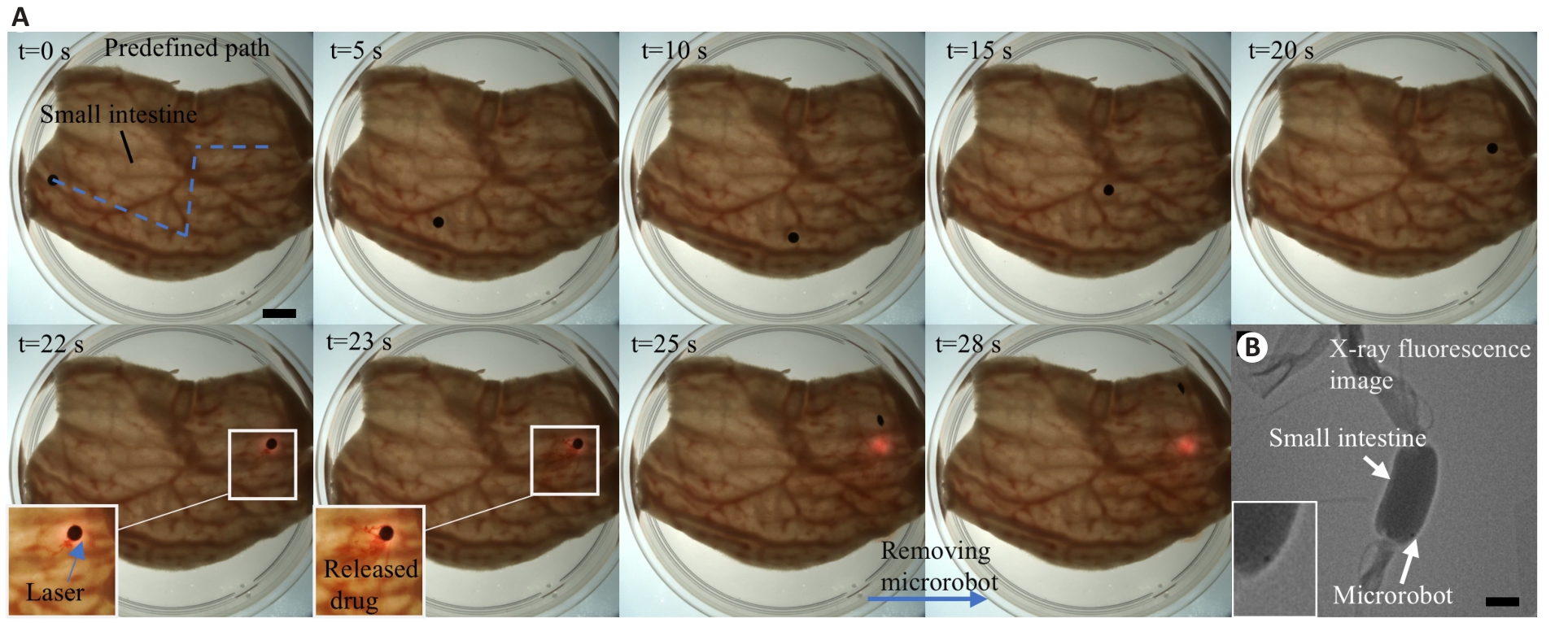
Fig.6 Experiments of the MMMR for drug delivery in isolated organs. A: Test of the MMMR for targeted drug delivery and laser heating release on the surface of the small intestine (Scale bar=5 mm). B: X-ray image of the MMMR (Scale bar=5 mm).
| [1] | Erkoc P, Yasa IC, Ceylan H, et al. Mobile microrobots for active therapeutic delivery[J]. Adv Ther, 2019, 2(1): 1800064. doi:10.1002/adtp.201800064 |
| [2] | Luo M, Feng YZ, Wang TW, et al. Micro-/ nanorobots at work in active drug delivery[J]. Adv Funct Mater, 2018, 28(25): 1706100. doi:10.1002/adfm.201706100 |
| [3] | Liu Y, Huang J, Liu C, et al. Soft millirobot capable of switching motion modes on the fly for targeted drug delivery in the oviduct[J]. ACS Nano, 2024, 18(12): 8694-705. doi:10.1021/acsnano.3c09753 |
| [4] | Xu HF, Medina-Sánchez M, Magdanz V, et al. Sperm-hybrid micromotor for targeted drug delivery[J]. ACS Nano, 2018, 12(1): 327-37. doi:10.1021/acsnano.7b06398 |
| [5] | Cai YP, Chen YH, Hong XY, et al. Porous microsphere and its applications[J]. Int J Nanomedicine, 2013, 8: 1111-20. doi:10.2147/ijn.s41271 |
| [6] | Yawalkar AN, Pawar MA, Vavia PR. Microspheres for targeted drug delivery- A review on recent applications[J]. J Drug Deliv Sci Technol, 2022, 75: 103659. doi:10.1016/j.jddst.2022.103659 |
| [7] | Sharifi-Azad M, Fathi M, Cho WC, et al. Recent advances in targeted drug delivery systems for resistant colorectal cancer[J]. Cancer Cell Int, 2022, 22(1): 196. doi:10.1186/s12935-022-02605-y |
| [8] | Alapan Y, Bozuyuk U, Erkoc P, et al. Multifunctional surface microrollers for targeted cargo delivery in physiological blood flow[J]. Sci Robot, 2020, 5(42): eaba5726. doi:10.1126/scirobotics.aba5726 |
| [9] | Xin C, Yang L, Li JW, et al. Conical hollow microhelices with superior swimming capabilities for targeted cargo delivery[J]. Adv Mater, 2019, 31(25): e1808226. doi:10.1002/adma.201808226 |
| [10] | Kubota T, Kurashina Y, Zhao JY, et al. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer[J]. Mater Des, 2021, 203: 109580. doi:10.1016/j.matdes.2021.109580 |
| [11] | Yuan LC, Xu XX, Song XT, et al. Effect of bone-shaped nanotube-hydrogel drug delivery system for enhanced osseointegration[J]. Biomater Adv, 2022, 137: 212853. doi:10.1016/j.bioadv.2022.212853 |
| [12] | Li JY, Mooney DJ. Designing hydrogels for controlled drug delivery[J]. Nat Rev Mater, 2016, 1: 16071. doi:10.1038/natrevmats.2016.71 |
| [13] | Dave PN, Macwan PM, Kamaliya B. Drug release and thermal properties of magnetic cobalt ferrite (CoFe2O4) nanocomposite hydrogels based on poly(acrylic acid-g-N-isopropyl acrylamide) grafted onto gum ghatti[J]. Int J Biol Macromol, 2023, 224: 358-69. doi:10.1016/j.ijbiomac.2022.10.129 |
| [14] | Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture[J]. Nat Methods, 2016, 13(5): 405-14. doi:10.1038/nmeth.3839 |
| [15] | Prince E, Kumacheva E. Design and applications of man-made biomimetic fibrillar hydrogels[J]. Nat Rev Mater, 2019, 4(2): 99-115. doi:10.1038/s41578-018-0077-9 |
| [16] | Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics[J]. Nat Rev Mater, 2016, 1: 15012. doi:10.1038/natrevmats.2015.12 |
| [17] | Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering: a review[J]. Carbohydr Polym, 2013, 92(2): 1262-79. doi:10.1016/j.carbpol.2012.10.028 |
| [18] | Dumont CM, Carlson MA, Munsell MK, et al. Aligned hydrogel tubes guide regeneration following spinal cord injury[J]. Acta Biomater, 2019, 86: 312-22. doi:10.1016/j.actbio.2018.12.052 |
| [19] | Shen HL, Cai SX, Wang Z, et al. Magnetically driven microrobots: Recent progress and future development[J]. Mater Des, 2023, 227: 111735. doi:10.1016/j.matdes.2023.111735 |
| [20] | Zhou HJ, Mayorga-Martinez CC, Pané S, et al. Magnetically driven micro and nanorobots[J]. Chem Rev, 2021, 121(8): 4999-5041. doi:10.1021/acs.chemrev.0c01234 |
| [21] | Liu Y, Lin GG, Bao GC, et al. Stratified disk microrobots with dynamic maneuverability and proton-activatable luminescence for in vivo imaging[J]. ACS Nano, 2021, 15(12): 19924-37. doi:10.1021/acsnano.1c07431 |
| [22] | Huang J, Liu Y, Wu JD, et al. An extracellular matrix-mimicking magnetic microrobot for targeted elimination of circulating cancer cells[J]. Nanoscale, 2024, 16(2): 624-34. doi:10.1039/d3nr03799a |
| [23] | Qiao SS, Ouyang HK, Zheng XG, et al. Magnetically actuated hydrogel-based capsule microrobots for intravascular targeted drug delivery[J]. J Mater Chem B, 2023, 11(26): 6095-105. doi:10.1039/d3tb00852e |
| [24] | Lin FC, Zheng JJ, Guo WH, et al. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release[J]. Cellulose, 2019, 26(11): 6861-77. doi:10.1007/s10570-019-02572-0 |
| [25] | Kim DI, Lee H, Kwon SH, et al. Magnetic nano-particles retrievable biodegradable hydrogel microrobot[J]. Sens Actuat B Chem, 2019, 289: 65-77. doi:10.1016/j.snb.2019.03.030 |
| [26] | Xie MH, Zhang W, Fan CY, et al. Bioinspired soft microrobots with precise magneto-collective control for microvascular thrombolysis[J]. Adv Mater, 2020, 32(26): e2000366. doi:10.1002/adma.202000366 |
| [27] | Bielas R, Hornowski T, Paulovičová K, et al. The effect of magnetic particles covering the droplets on the heating rate of Pickering emulsions in the AC magnetic field[J]. J Mol Liq, 2020, 320: 114388. doi:10.1016/j.molliq.2020.114388 |
| [28] | Pinelli F, Perale G, Rossi F. Coating and functionalization strategies for nanogels and nanoparticles for selective drug delivery[J]. Gels, 2020, 6(1): 6. doi:10.3390/gels6010006 |
| [29] | Wu B, Li Y, Li YY, et al. Pickering emulsions-chitosan hydrogel beads carrier system for loading of resveratrol: Formulation approach and characterization studies[J]. React Funct Polym, 2021, 169: 105074. doi:10.1016/j.reactfunctpolym.2021.105074 |
| [30] | Liu Y, Lin GG, Chen YH, et al. Coding and decoding stray magnetic fields for multiplexing kinetic bioassay platform[J]. Lab Chip, 2020, 20(24): 4561-71. doi:10.1039/d0lc00848f |
| [31] | Mun SJ, Ko D, Kim HU, et al. Photopolymerization-based synthesis of uniform magnetic hydrogels and colorimetric glucose detection[J]. Materials (Basel), 2020, 13(19): 4401. doi:10.3390/ma13194401 |
| [32] | Won S, Kim S, Park JE, et al. On-demand orbital maneuver of multiple soft robots via hierarchical magnetomotility[J]. Nat Commun, 2019, 10: 4751. doi:10.1038/s41467-019-12679-4 |
| [33] | Bozuyuk U, Aghakhani A, Alapan Y, et al. Reduced rotational flows enable the translation of surface-rolling microrobots in confined spaces[J]. Nat Commun, 2022, 13(1): 6289. doi:10.1038/s41467-022-34023-z |
| [34] | Liu Y, Cao QL, Xu HF, et al. Flow tweezing of anisotropic magnetic microrobots in a dynamic magnetic trap for active retention and localized flow sensing[J]. Lab Chip, 2024, 24(18): 4242-52. doi:10.1039/d4lc00474d |
| [35] | Daly AC, Riley L, Segura T, et al. Hydrogel microparticles for biomedical applications[J]. Nat Rev Mater, 2020, 5(1): 20-43. doi:10.1038/s41578-019-0148-6 |
| [36] | Huang Y, Yin S, Li HW, et al. One-step fabrication of moon-shaped microrobots through in situ solidification of magnetic Janus droplets in microchannels[J]. Droplet, 2023, 2(2): e56. doi:10.1002/dro2.56 |
| [37] | Su L, Jin DD, Wang YQ, et al. Modularized microrobot with lock-and-detachable modules for targeted cell delivery in bile duct[J]. Sci Adv, 2023, 9(50): eadj0883. doi:10.1126/sciadv.adj0883 |
| [38] | Dong Y, Wang L, Zhang ZF, et al. Endoscope-assisted magnetic helical micromachine delivery for biofilm eradication in tympanostomy tube[J]. Sci Adv, 2022, 8(40): eabq8573. doi:10.1126/sciadv.abq8573 |
| [39] | Nguyen KT, Go G, Jin Z, et al. A magnetically guided self-rolled microrobot for targeted drug delivery, real-time X-ray imaging, and microrobot retrieval[J]. Adv Healthc Mater, 2021, 10(6): 2001681. doi:10.1002/adhm.202001681 |
| [40] | Aziz A, Pane S, Iacovacci V, et al. Medical imaging of microrobots: toward in vivo applications[J]. ACS Nano, 2020, 14(9): 10865-93. doi:10.1021/acsnano.0c05530 |
| [1] | FAN Yiping, LUO Menglin, HUANG Dongzong, LIU Lin, FU Bo, WANG Xiaoyu, GUAN Miaosheng, LI Hongbo. A sericin hydrogel scaffold for sustained dexamethasone release modulates macrophage polarization to promote mandibular bone defect repair in rats [J]. Journal of Southern Medical University, 2024, 44(3): 533-540. |
| [2] | WANG Ting, YANG Ling, XIE Yuhan, CHENG Siyu, XIONG Min, LUO Xiaoming. An injectable hydrogel/staple fiber composite for sustained release of CA4P and doxorubicin for combined chemotherapy of xenografted breast tumor in mice [J]. Journal of Southern Medical University, 2022, 42(5): 625-632. |
| [3] | . Cytotoxic effect of photodynamic liposome gel combined with trastuzumab on drug-resistant breast cancer cells in vitro [J]. Journal of Southern Medical University, 2021, 41(2): 164-172. |
| [4] | . Application of self-propelled micro-/nanomotors in active targeted drug delivery [J]. Journal of Southern Medical University, 2020, 40(03): 445-452. |
| [5] | . Anti-CD206 antibody- conjugated Fe3O4-based PLGA nanoparticles selectively promotes M1 polarization of tumor-associated macrophages in mice [J]. Journal of Southern Medical University, 2020, 40(02): 246-254. |
| [6] | WANG Yong-feng1, JIN An-min1, WEI Kun2, WANG Xu-dong2, TANG Shan-hua1, MIN Shao-xiong1 1Department of Othopedics, Zhujiang Hospital, Southern Medical University, Guangzhou 510282, China; 2Institute of Material Science and Engineering, South China University of Technology, Guangzhou 510641, China. Development of an anti-infection nano-hydroxypatite drug delivery microsphere and its drug-release in vitro [J]. Journal of Southern Medical University, 2006, 26(06): 754-756. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||