Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (8): 1706-1717.doi: 10.12122/j.issn.1673-4254.2025.08.15
Shangping FANG1,2, Jiameng LIU1,2, Xingchen YUE1,2, Huan LI1,2, Wanning Li1,2, Xiaoyu TANG1,2, Pengju BAO3( )
)
Received:2025-03-27
Online:2025-08-20
Published:2025-09-05
Contact:
Pengju BAO
E-mail:273574156@qq.com
Shangping FANG, Jiameng LIU, Xingchen YUE, Huan LI, Wanning Li, Xiaoyu TANG, Pengju BAO. Racial differences in treatment and prognosis of gastric signet ring cell carcinoma: analysis based on SEER and TCGA databases[J]. Journal of Southern Medical University, 2025, 45(8): 1706-1717.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.08.15
| Characteristic | Cohort | P | ||
|---|---|---|---|---|
| Overall (2057) | Training cohort (1440) | Internal test cohort (617) | ||
| Gender | 0.718 | |||
| Female | 1026 (49.9%) | 722 (50.1%) | 304 (49.3%) | |
| Male | 1031 (50.1%) | 718 (49.9%) | 313 (50.7%) | |
| Race recode | 0.964 | |||
| American indian/alaska native | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Asian or pacific islander | 343 (16.7%) | 236 (16.4%) | 107 (17.3%) | |
| Black | 179 (8.7%) | 128 (8.9%) | 51 (8.3%) | |
| Unknown | 22 (1.1%) | 15 (1.0%) | 7 (1.1%) | |
| White | 1492 (72.5%) | 1047 (72.7%) | 445 (72.1%) | |
| Age recode with <1 year | ||||
| 20-24 years | 15 (0.7%) | 11 (0.8%) | 4 (0.6%) | |
| 25-29 years | 21 (1.0%) | 18 (1.3%) | 3 (0.5%) | |
| 30-34 years | 50 (2.4%) | 36 (2.5%) | 14 (2.3%) | |
| 35-39 years | 85 (4.1%) | 58 (4.0%) | 27 (4.4%) | |
| 40-44 years | 106 (5.2%) | 69 (4.8%) | 37 (6.0%) | |
| 45-49 years | 159 (7.7%) | 114 (7.9%) | 45 (7.3%) | |
| 50-54 years | 181 (8.8%) | 134 (9.3%) | 47 (7.6%) | |
| 55-59 years | 232 (11.3%) | 162 (11.3%) | 70 (11.3%) | |
| 60-64 years | 260 (12.6%) | 172 (11.9%) | 88 (14.3%) | |
| 65-69 years | 254 (12.3%) | 176 (12.2%) | 78 (12.6%) | |
| 70-74 years | 224 (10.9%) | 159 (11.0%) | 65 (10.5%) | |
| 75-79 years | 176 (8.6%) | 123 (8.5%) | 53 (8.6%) | |
| 80-84 years | 151 (7.3%) | 106 (7.4%) | 45 (7.3%) | |
| 85+ years | 143 (7.0%) | 102 (7.1%) | 41 (6.6%) | |
| Year of diagnosis | 0.683 | |||
| 2016 | 1036 (50.4%) | 721 (50.1%) | 315 (51.1%) | |
| 2017 | 1021 (49.6%) | 719 (49.9%) | 302 (48.9%) | |
| Primary site (Mean±SD) | 164.28±3.29 | 164.23±3.26 | 164.39±3.35 | 0.315 |
| Grade Recode (thru 2017) | 0.899 | |||
| Moderately differentiated; Grade II | 35 (1.7%) | 25 (1.7%) | 10 (1.6%) | |
| Poorly differentiated; Grade III | 1594 (77.5%) | 1116 (77.5%) | 478 (77.5%) | |
| Undifferentiated; anaplastic; Grade IV | 48 (2.3%) | 31 (2.2%) | 17 (2.8%) | |
| Unknown | 379 (18.4%) | 267 (18.5%) | 112 (18.2%) | |
| Well differentiated; Grade I | 1 (0.0%) | 1 (0.1%) | 0 (0.0%) | |
| TNM/TCS v0204+Schema (thru 2017) | 0.963 | |||
| Esophagus/GEJunction | 408 (19.8%) | 286 (19.9%) | 122 (19.8%) | |
| Stomach | 1649 (80.2%) | 1154 (80.1%) | 495 (80.2%) | |
| Radiation recode | 0.823 | |||
| Beam radiation | 377 (18.3%) | 260 (18.1%) | 117 (19.0%) | |
| None/Unknown | 1626 (79.0%) | 1144 (79.4%) | 482 (78.1%) | |
| Radiation, NOS method or source not specified | 9 (0.4%) | 5 (0.3%) | 4 (0.6%) | |
| Recommended, unknown if administered | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Refused (1988+) | 24 (1.2%) | 17 (1.2%) | 7 (1.1%) | |
| Chemotherapy recode (yes, no/unk) | 0.621 | |||
| No/Unknown | 760 (36.9%) | 537 (37.3%) | 223 (36.1%) | |
| Yes | 1297 (63.1%) | 903 (62.7%) | 394 (63.9%) | |
| Surgery recode | 0.634 | |||
| Not recommended | 955 (57.8%) | 667 (57.7%) | 286 (57.5%) | |
| Surgery performed | 639 (38.7%) | 446 (38.6%) | 191 (38.5%) | |
| Recommended but not performed, patient refused | 56 (3.5%) | 42 (3.7%) | 18 (3.8%) | |
| Tumor size summary (2016+) (mm, Mean±SD) | 570±474 | 563±475 | 587±471 | 0.275 |
Tab.1 Baseline demographic characteristics of the patients included [n (%)]
| Characteristic | Cohort | P | ||
|---|---|---|---|---|
| Overall (2057) | Training cohort (1440) | Internal test cohort (617) | ||
| Gender | 0.718 | |||
| Female | 1026 (49.9%) | 722 (50.1%) | 304 (49.3%) | |
| Male | 1031 (50.1%) | 718 (49.9%) | 313 (50.7%) | |
| Race recode | 0.964 | |||
| American indian/alaska native | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Asian or pacific islander | 343 (16.7%) | 236 (16.4%) | 107 (17.3%) | |
| Black | 179 (8.7%) | 128 (8.9%) | 51 (8.3%) | |
| Unknown | 22 (1.1%) | 15 (1.0%) | 7 (1.1%) | |
| White | 1492 (72.5%) | 1047 (72.7%) | 445 (72.1%) | |
| Age recode with <1 year | ||||
| 20-24 years | 15 (0.7%) | 11 (0.8%) | 4 (0.6%) | |
| 25-29 years | 21 (1.0%) | 18 (1.3%) | 3 (0.5%) | |
| 30-34 years | 50 (2.4%) | 36 (2.5%) | 14 (2.3%) | |
| 35-39 years | 85 (4.1%) | 58 (4.0%) | 27 (4.4%) | |
| 40-44 years | 106 (5.2%) | 69 (4.8%) | 37 (6.0%) | |
| 45-49 years | 159 (7.7%) | 114 (7.9%) | 45 (7.3%) | |
| 50-54 years | 181 (8.8%) | 134 (9.3%) | 47 (7.6%) | |
| 55-59 years | 232 (11.3%) | 162 (11.3%) | 70 (11.3%) | |
| 60-64 years | 260 (12.6%) | 172 (11.9%) | 88 (14.3%) | |
| 65-69 years | 254 (12.3%) | 176 (12.2%) | 78 (12.6%) | |
| 70-74 years | 224 (10.9%) | 159 (11.0%) | 65 (10.5%) | |
| 75-79 years | 176 (8.6%) | 123 (8.5%) | 53 (8.6%) | |
| 80-84 years | 151 (7.3%) | 106 (7.4%) | 45 (7.3%) | |
| 85+ years | 143 (7.0%) | 102 (7.1%) | 41 (6.6%) | |
| Year of diagnosis | 0.683 | |||
| 2016 | 1036 (50.4%) | 721 (50.1%) | 315 (51.1%) | |
| 2017 | 1021 (49.6%) | 719 (49.9%) | 302 (48.9%) | |
| Primary site (Mean±SD) | 164.28±3.29 | 164.23±3.26 | 164.39±3.35 | 0.315 |
| Grade Recode (thru 2017) | 0.899 | |||
| Moderately differentiated; Grade II | 35 (1.7%) | 25 (1.7%) | 10 (1.6%) | |
| Poorly differentiated; Grade III | 1594 (77.5%) | 1116 (77.5%) | 478 (77.5%) | |
| Undifferentiated; anaplastic; Grade IV | 48 (2.3%) | 31 (2.2%) | 17 (2.8%) | |
| Unknown | 379 (18.4%) | 267 (18.5%) | 112 (18.2%) | |
| Well differentiated; Grade I | 1 (0.0%) | 1 (0.1%) | 0 (0.0%) | |
| TNM/TCS v0204+Schema (thru 2017) | 0.963 | |||
| Esophagus/GEJunction | 408 (19.8%) | 286 (19.9%) | 122 (19.8%) | |
| Stomach | 1649 (80.2%) | 1154 (80.1%) | 495 (80.2%) | |
| Radiation recode | 0.823 | |||
| Beam radiation | 377 (18.3%) | 260 (18.1%) | 117 (19.0%) | |
| None/Unknown | 1626 (79.0%) | 1144 (79.4%) | 482 (78.1%) | |
| Radiation, NOS method or source not specified | 9 (0.4%) | 5 (0.3%) | 4 (0.6%) | |
| Recommended, unknown if administered | 21 (1.0%) | 14 (1.0%) | 7 (1.1%) | |
| Refused (1988+) | 24 (1.2%) | 17 (1.2%) | 7 (1.1%) | |
| Chemotherapy recode (yes, no/unk) | 0.621 | |||
| No/Unknown | 760 (36.9%) | 537 (37.3%) | 223 (36.1%) | |
| Yes | 1297 (63.1%) | 903 (62.7%) | 394 (63.9%) | |
| Surgery recode | 0.634 | |||
| Not recommended | 955 (57.8%) | 667 (57.7%) | 286 (57.5%) | |
| Surgery performed | 639 (38.7%) | 446 (38.6%) | 191 (38.5%) | |
| Recommended but not performed, patient refused | 56 (3.5%) | 42 (3.7%) | 18 (3.8%) | |
| Tumor size summary (2016+) (mm, Mean±SD) | 570±474 | 563±475 | 587±471 | 0.275 |
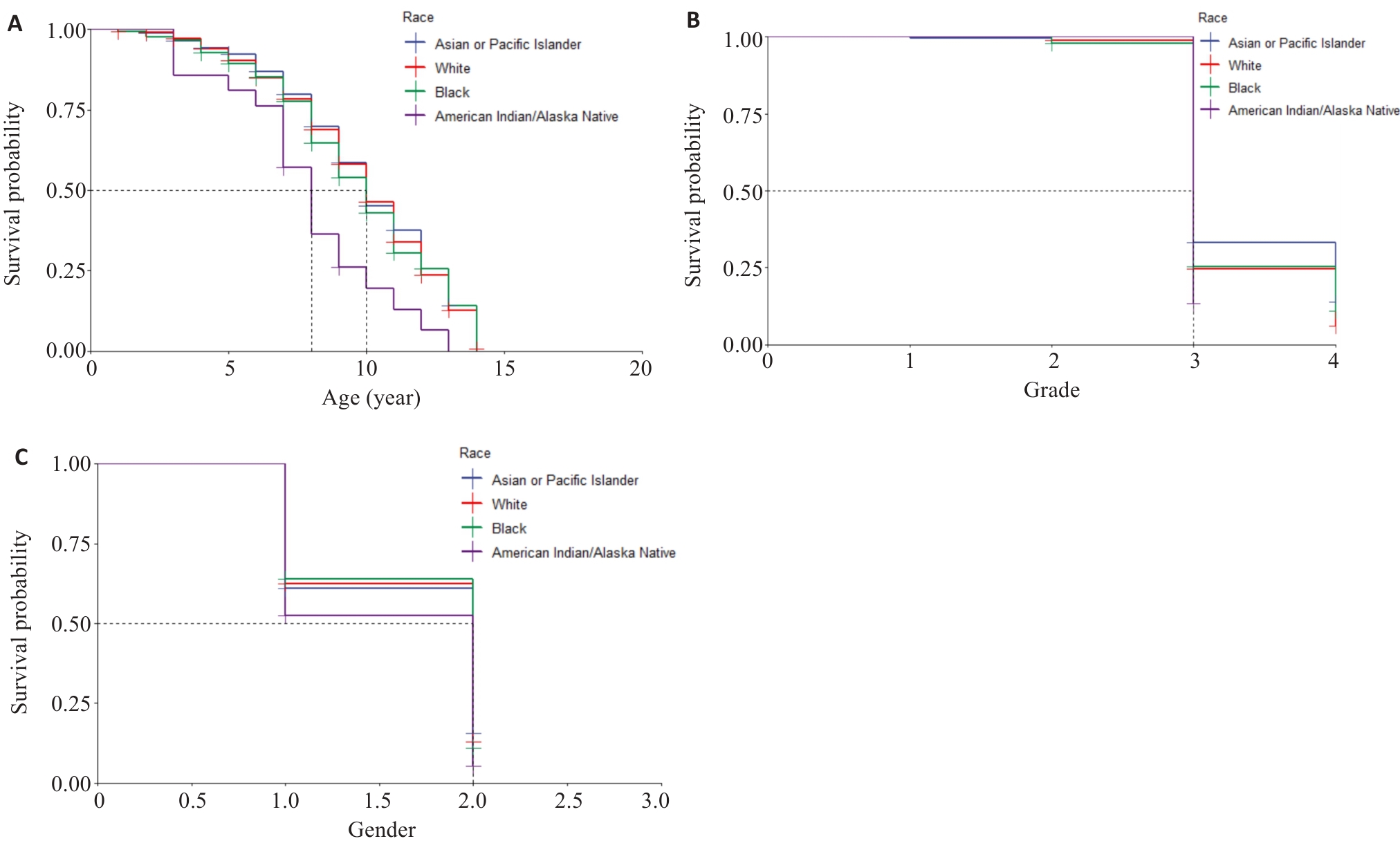
Fig.2 Kaplan-Meier survival curves. A: Survival rates of the patients of different ages in different races. B: Survival rates of patients with different tumor grades in different races. C: Survival rates of the patients of different genders in different races (1 and 2 represents male and female, respectively).
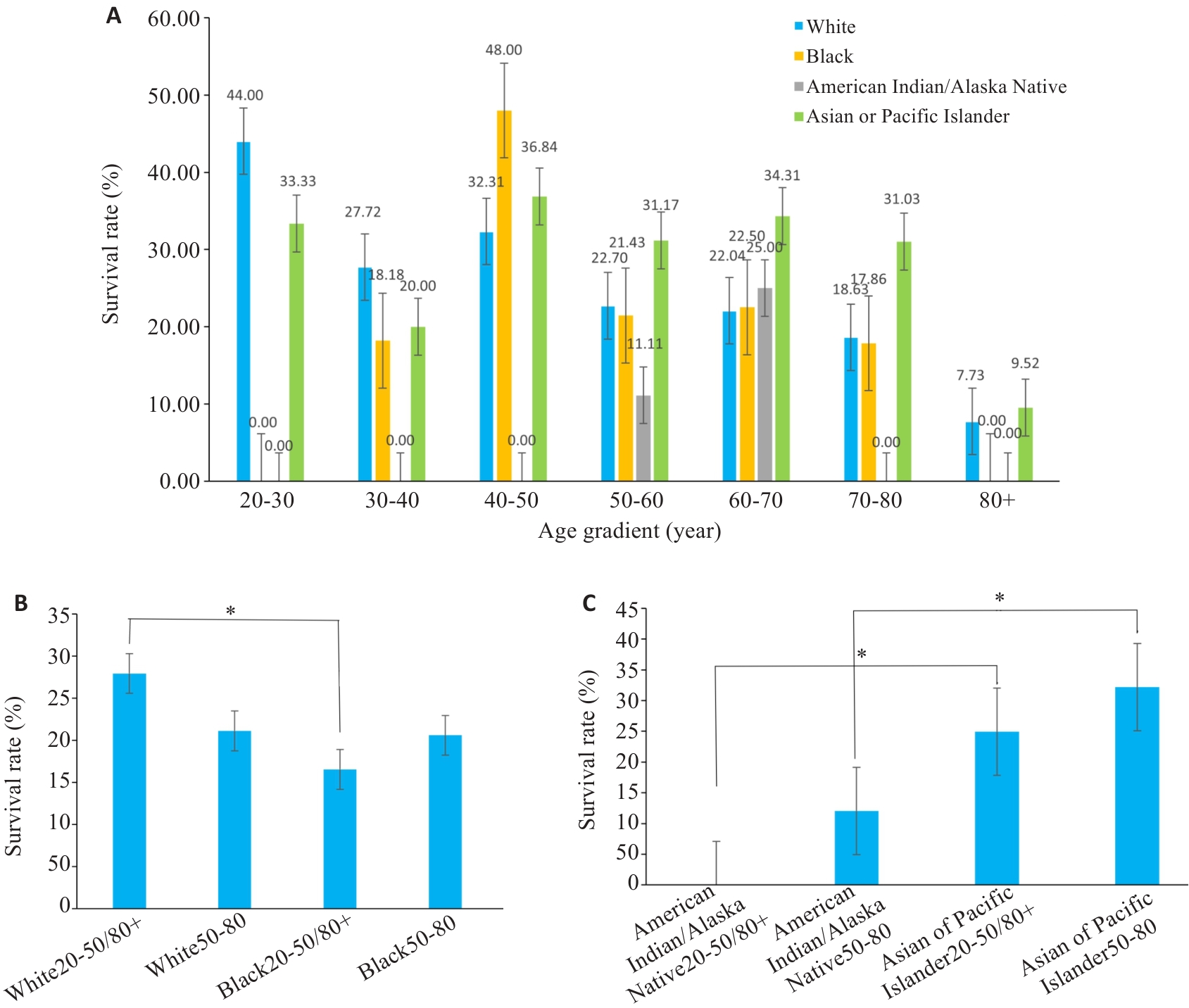
Fig.3 Bar charts of subgroup analysis. A: Survival rates for different races at different ages. B, C: Statistical analyses of the survival rates. *P<0.05.
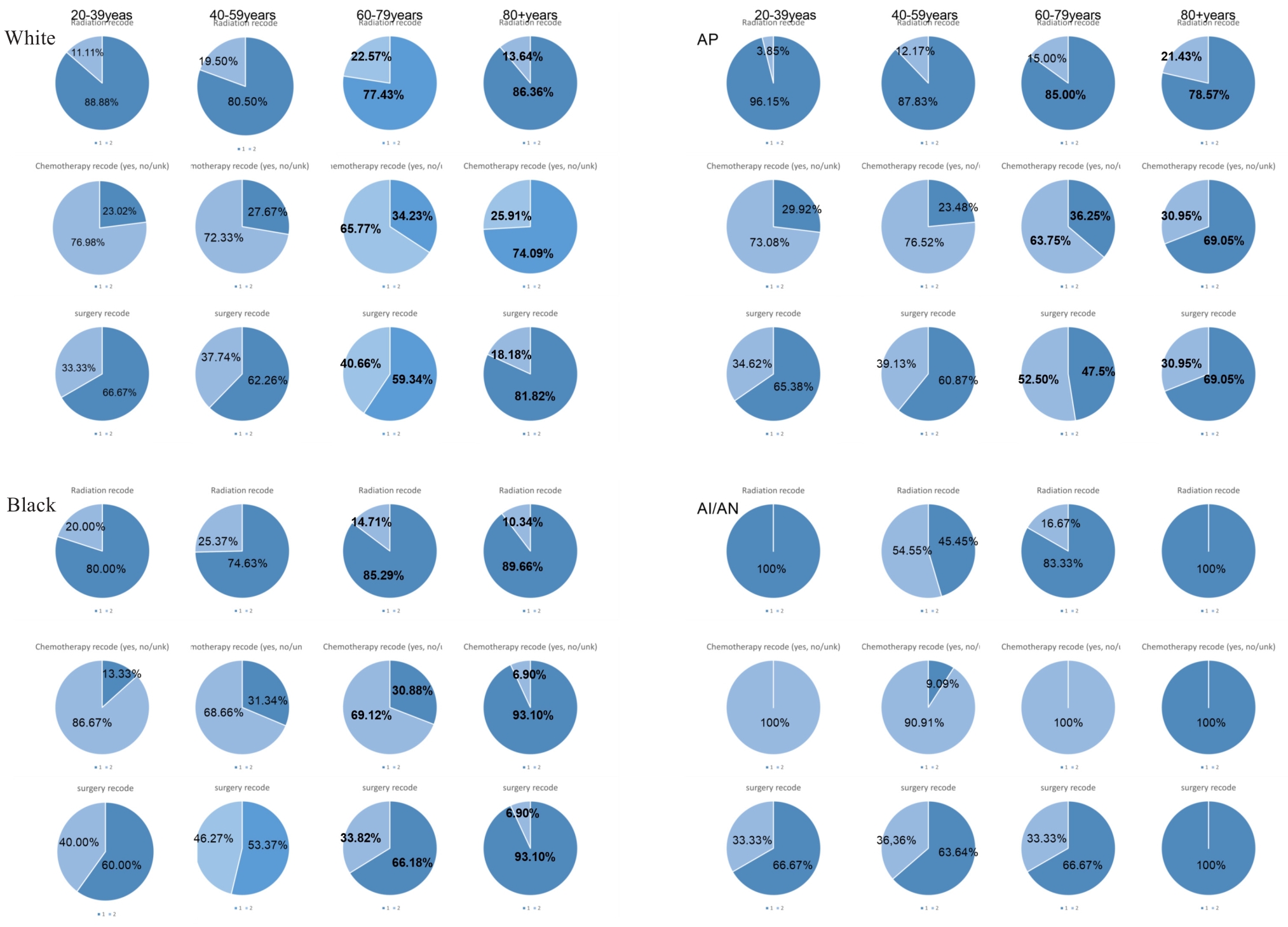
Fig.4 Pie charts of treatment modalities by age and ethnicity. AP: Asian or Pacific Islander; AI/AN: American Indian/Alaska Native. Superscripts Radiation recode is radiation, Chemotherapy recode is chemotherapy, surgery recode is surgery; subscripts 1 represents not receiving the treatment modality, 2 means received the treatment modality.
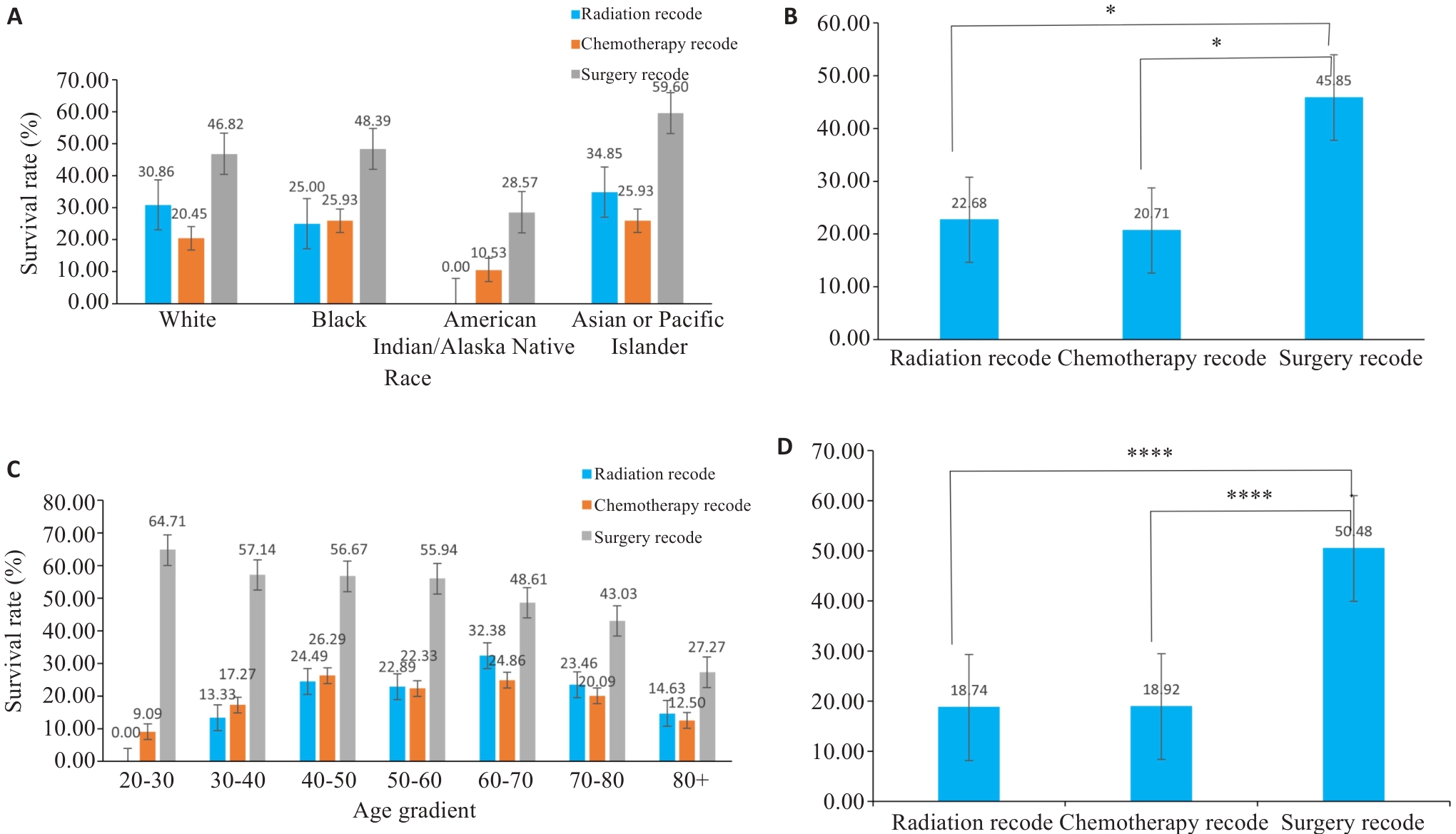
Fig.5 Bar graphs of subgroup analysis. A, C: Survival rates of patients of different races using various treatment methods. B, D: Corresponding statistical analyses for A and C. *P<0.05, ****P<0.0001.
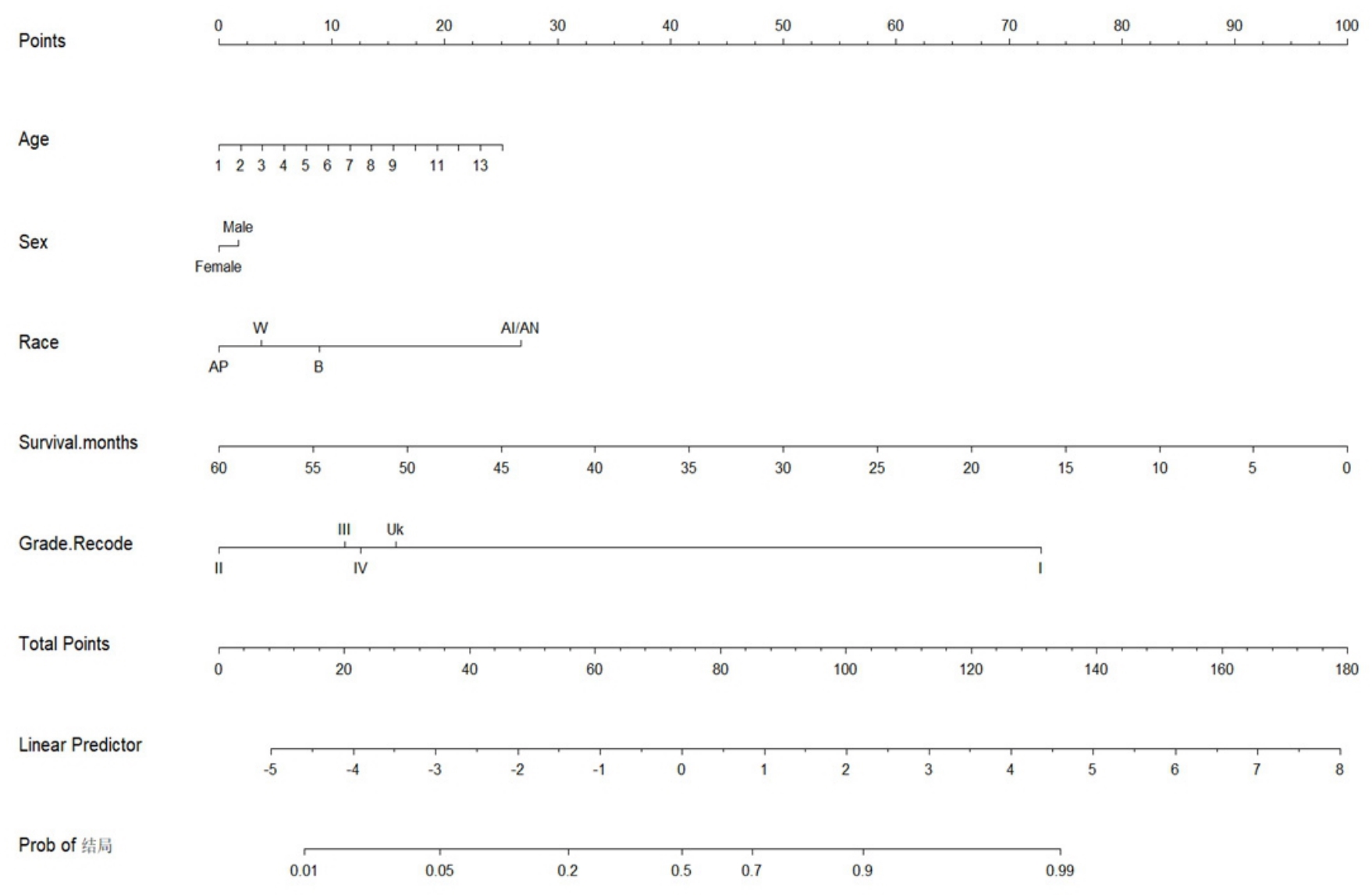
Fig.6 Nomogram analysis. In the row of Age, the number 1 represents patients aged 20-24 years, 2 represents patients aged 25-29 years, and so forth; the number 14 represents patients above 85 years. In the row of Race, W represents Caucasians, B represents Blacks, AI/AN represents American Indians/Alaskan Natives, and Ap represents Asians and Pacific Islanders.
| Variable | OR | 95% CI (profile likelihood) | P |
|---|---|---|---|
| Age[50-54] | 0.2301 | 0.06367 to 0.7122 | 0.0159 |
| Age[80-84] | 0.4881 | 0.1247 to 1.695 | 0.2762 |
| Age[55-59] | 0.2203 | 0.06110 to 0.6788 | 0.0128 |
| Age[60-64] | 0.2239 | 0.06378 to 0.6660 | 0.0114 |
| Age[75-79] | 0.2472 | 0.06738 to 0.7818 | 0.0239 |
| Age[70-74] | 0.2988 | 0.08325 to 0.9184 | 0.0461 |
| Age[65-69] | 0.2962 | 0.08392 to 0.8893 | 0.041 |
| Age[ | 0.1224 | 0.03227 to 0.4025 | 0.001 |
| Age[ | 0.1179 | 0.03002 to 0.4066 | 0.0012 |
| Age[ | 0.03687 | 0.006592 to 0.2072 | 0.0002 |
| Age[ | 0.1643 | 0.04528 to 0.5101 | 0.0031 |
| Age[ | 0.208 | 0.04599 to 0.8911 | 0.0359 |
| Age[ | 0.09731 | 0.01383 to 0.8520 | 0.0254 |
| Gender | 0.8876 | 0.6470 to 1.217 | 0.4589 |
| Race | 0.825 | 0.6946 to 0.9818 | 0.0291 |
| Grade | 1.089 | 0.8700 to 1.370 | 0.4621 |
| Survival months | 0.8865 | 0.8768 to 0.8957 | <0.0001 |
| Tumor size summary (2016+) | 1 | 0.0755 |
Tab.3 Logistic regression analysis
| Variable | OR | 95% CI (profile likelihood) | P |
|---|---|---|---|
| Age[50-54] | 0.2301 | 0.06367 to 0.7122 | 0.0159 |
| Age[80-84] | 0.4881 | 0.1247 to 1.695 | 0.2762 |
| Age[55-59] | 0.2203 | 0.06110 to 0.6788 | 0.0128 |
| Age[60-64] | 0.2239 | 0.06378 to 0.6660 | 0.0114 |
| Age[75-79] | 0.2472 | 0.06738 to 0.7818 | 0.0239 |
| Age[70-74] | 0.2988 | 0.08325 to 0.9184 | 0.0461 |
| Age[65-69] | 0.2962 | 0.08392 to 0.8893 | 0.041 |
| Age[ | 0.1224 | 0.03227 to 0.4025 | 0.001 |
| Age[ | 0.1179 | 0.03002 to 0.4066 | 0.0012 |
| Age[ | 0.03687 | 0.006592 to 0.2072 | 0.0002 |
| Age[ | 0.1643 | 0.04528 to 0.5101 | 0.0031 |
| Age[ | 0.208 | 0.04599 to 0.8911 | 0.0359 |
| Age[ | 0.09731 | 0.01383 to 0.8520 | 0.0254 |
| Gender | 0.8876 | 0.6470 to 1.217 | 0.4589 |
| Race | 0.825 | 0.6946 to 0.9818 | 0.0291 |
| Grade | 1.089 | 0.8700 to 1.370 | 0.4621 |
| Survival months | 0.8865 | 0.8768 to 0.8957 | <0.0001 |
| Tumor size summary (2016+) | 1 | 0.0755 |
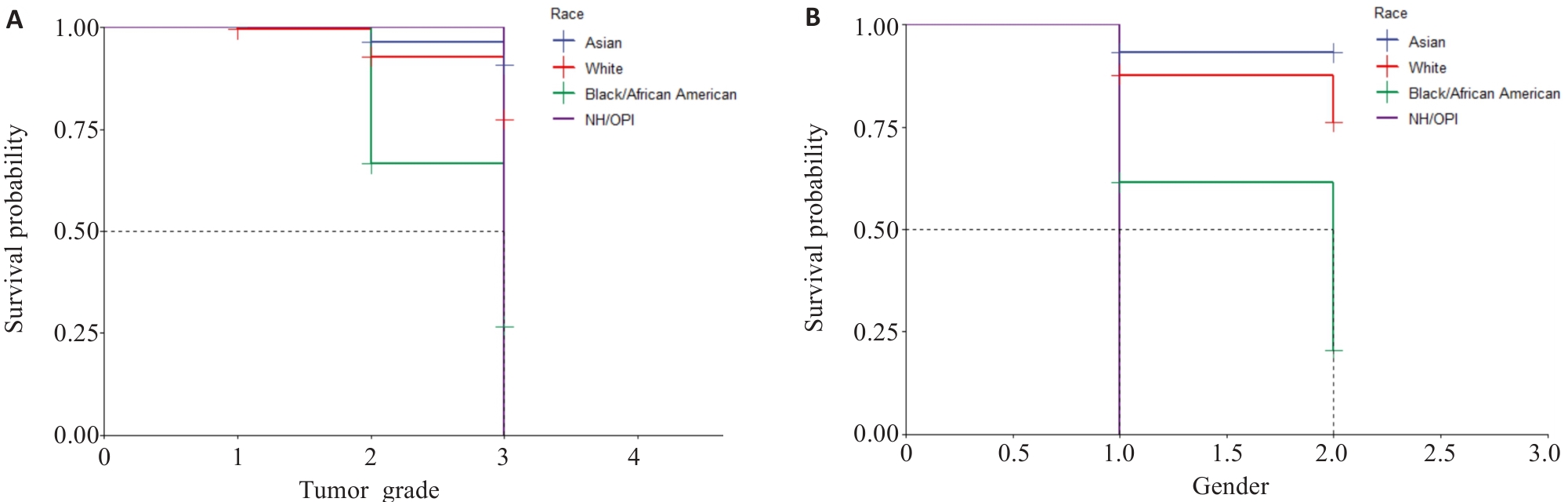
Fig.9 Kaplan-Meier survival curves. A: Survival of patients with different tumor grades in different races (1, 2, and 3 represent tumor grades I, II, and III, respectively). B: Survival curves of patients of different genders in different races (1 and 2 represents male and female, respectively. BOAA: Black or African American; NHOOPI: Native Hawallan or other pacific islanders).
| [1] | Costa TM, Alves F, Miranda H, et al. Pure signet ring cell carcinoma of the breast: a rare entity[J]. BMJ Case Rep, 2024, 17(11): e252263. doi:10.1136/bcr-2022-252263 |
| [2] | Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classi-fication of tumours of the digestive system[J]. Histopathology, 2020, 76(2): 182-8. doi:10.1111/his.13975 |
| [3] | Machlowska J, Pucułek M, Sitarz M, et al. State of the art for gastric signet ring cell carcinoma: from classification, prognosis, and genomic characteristics to specified treatments[J]. Cancer Manag Res, 2019, 11: 2151-61. doi:10.2147/cmar.s188622 |
| [4] | Kumar S, Seshadri RA, Trivadi SG, et al. Perioperative versus postoperative chemotherapy for gastric cancer: a propensity score matched analysis[J]. Asia Pac J Clin Oncol, 2020, 16(5): e252-6. doi:10.1111/ajco.13401 |
| [5] | Yang MG, Yang YL, Chen J, et al. A case report of primary signet ring cell carcinoma of the lung: imaging study and literature review[J]. Transl Lung Cancer Res, 2021, 10(9): 3840-9. doi:10.21037/tlcr-21-654 |
| [6] | Han YH, Yang WM, Ma Q, et al. Case Report: Systemic treatment for breast and vulvar metastases from resected rectal signet ring cell carcinoma[J]. Front Oncol, 2023, 13: 1213888. doi:10.3389/fonc.2023.1213888 |
| [7] | An Y, Zhou JL, Lin GL, et al. Clinicopathological and molecular characteristics of colorectal signet ring cell carcinoma: a review[J]. Pathol Oncol Res, 2021, 27: 1609859. doi:10.3389/pore.2021.1609859 |
| [8] | Chen L, Liu X, Gao LG, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: a 10-year retrospective study in China[J]. PLoS One, 2017, 12(5): e0176637. doi:10.1371/journal.pone.0176637 |
| [9] | Zhang L, Wu LY, Li JY, et al. Gastrointestinal metastatic signet ring cell breast cancer in young females: a case report[J]. Gland Surg, 2022, 11(5): 943-52. doi:10.21037/gs-22-242 |
| [10] | Kasapoğlu E, Kandil B, Gökyer A, et al. Primary signet ring cell carcinoma of the breast: a case report and literature review[J]. J Cancer Res Ther, 2024, 20(5): 1615-7. doi:10.4103/jcrt.jcrt_1963_22 |
| [11] | Luo S, Tian XX, Xu T, et al. Primary signet-ring cell carcinoma of the prostate involving the pelvis: a case report[J]. Front Oncol, 2024, 14: 1444541. doi:10.3389/fonc.2024.1444541 |
| [12] | Gupta M, Budhwar A, Prasad N, et al. Primary signet ring cell carcinoma of prostate: a rare case report and review of literature[J]. J Cancer Res Ther, 2023, 19(5): 1075-8. doi:10.4103/jcrt.jcrt_827_21 |
| [13] | Tan Y, Huang YH, Xue JW, et al. Clinicopathological features and prognostic significance of pulmonary adenocarcinoma with signet ring cell components: meta-analysis and SEER analysis[J]. Clin Exp Med, 2023, 23(8): 4341-54. doi:10.1007/s10238-023-01200-3 |
| [14] | 田华开. 基于SEER数据库比较研究胃印戒细胞癌与不同分化程度胃腺癌临床病理特点及预后[D]. 南昌: 南昌大学, 2021. |
| [15] | 陈碧钰. 基于SEER数据库的回顾性研究: 放疗联合手术治疗进展期胃印戒细胞癌的疗效分析[D]. 福州: 福建医科大学, 2021. |
| [16] | 王 震. 基于SEER数据库的胃印戒细胞癌临床病理特征及预后分析[D]. 郑州: 郑州大学, 2020. |
| [17] | 赵金匣, 康慧慧, 张 垚, 等. 胃癌患者根治性切除术前血脂水平与预后的相关性[J]. 临床荟萃, 2024, 39(10): 889-95. doi:10.3969/j.issn.1004-583X.2024.10.004 |
| [18] | 邢智远, 张凤娟, 谭晓杰, 等. 脐上或脐下观察孔在腹腔镜远端胃癌根治性切除术中的选择[J]. 腹腔镜外科杂志, 2024, 29(11): 825-9. |
| [19] | 常 宁. 胃印戒细胞癌手术方式及预后的临床研究[D]. 郑州: 郑州大学, 2018. doi:10.19347/j.cnki.2096-1413.201818004 |
| [20] | Gaba AG, Cao L, Renfrew RJ, et al. Impact of racial disparities on treatment of early triple negative breast cancer among American indians/Alaska natives and non-hispanic whites[J]. Clin Breast Cancer, 2025, 25(6): 534-43. doi:10.1016/j.clbc.2025.04.002 |
| [21] | Krishnamurthy S, Jazowski SA, Roberson ML, et al. Racial and ethnic disparities in receipt of ERBB2-targeted therapy for breast cancer, 2010-2020[J]. JAMA Netw Open, 2025, 8(5): e258086. doi:10.1001/jamanetworkopen.2025.8086 |
| [22] | Tsai MH, Shahsavari D, Chen J, et al. Racial/ethnic disparities in early-onset colorectal cancer outcomes[J]. J Racial Ethn Health Disparities. 2025, [Online ahead of print]. doi:10.1007/s40615-025-02450-5 |
| [23] | Thakkar Z, Khan MA, Wu Y, et al. Disaggregated colorectal cancer mortality among asian american subgroups between 2005-2020[J]. Cancer Epidemiol Biomarkers Prev, 2025, 34(7):1134-40. doi:10.1158/1055-9965.epi-24-1688 |
| [24] | Qiu HB, Cao SM, Xu RH. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020[J]. Cancer Commun (Lond), 2021, 41(10): 1037-48. doi:10.1002/cac2.12197 |
| [25] | Aitchison A, Hakkaart C, Whitehead M, et al. CDH1 gene mutation in early-onset, colorectal signet-ring cell carcinoma[J]. Pathol Res Pract, 2020, 216(5): 152912. doi:10.1016/j.prp.2020.152912 |
| [26] | 杨易成, 曹 佳. 早期胃印戒细胞癌研究现状与进展[J]. 临床军医杂志, 2023, 51(9): 896-900. |
| [27] | Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma[J]. Ann Surg Oncol, 2014, 21(5): 1678-85. doi:10.1245/s10434-013-3466-8 |
| [28] | Sargent C, Larchanché S. The construction of “cultural difference” and its therapeutic significance in immigrant mental health services in France[J]. Cult Med Psychiatry, 2009, 33(1): 2-20. doi:10.1007/s11013-008-9115-1 |
| [29] | 田 帆. 区域均衡发展视角下中国县域医疗卫生资源的空间配置及其优化研究[D]. 成都: 四川大学, 2023. |
| [30] | 于宏博, 何晓丹, 张书芸, 等. 基层医疗卫生机构优质卫生资源配置区域差异与公平性分析[J]. 中国卫生经济, 2025, 44(2): 59-63. |
| [31] | 孙 瑜, 吴 爽, 曹志辉. 我国优质医疗资源配置公平性的区域差异及时空演进研究[J]. 中国医院, 2024, 28(12): 29-35. doi:10.19660/j.issn.1671-0592.2024.12.06 |
| [32] | Saunders PA, Clark L, Matthews T, et al. Exploring empathy and patient-centered communication behaviors of third-year medical students during a clinical skills examination[J]. Patient Educ Couns, 2025, 137: 108786. doi:10.1016/j.pec.2025.108786 |
| [33] | Norberg BL, Austad B, Kristiansen E, et al. The dynamics of doctor-patient communication during remote consultations: qualitative study among Norwegian contract general practitioners[J]. J Med Internet Res, 2025, 27: e57679. doi:10.2196/57679 |
| [34] | 陈 剑. 论健康公平[J]. 中国农村卫生事业管理, 2022, 42(1): 2-9. |
| [35] | Prue G, Czamanski-Cohen J, Kassianos AP, et al. Models of care and associated targeted implementation strategies for cancer survivorship support in Europe: a scoping review protocol[J]. BMJ Open, 2025, 15(2): e085456. doi:10.1136/bmjopen-2024-085456 |
| [36] | El-Deiry WS, Bresson C, Wunder F, et al. Worldwide innovative network (WIN) consortium in personalized cancer medicine: bringing next-generation precision oncology to patients[J]. Oncotarget, 2025, 16: 140-62. |
| [37] | Srivastava R. Advancing precision oncology with AI-powered genomic analysis[J]. Front Pharmacol, 2025, 16: 1591696. doi:10.3389/fphar.2025.1591696 |
| [38] | Roman Y. Bridging the United States population diversity gaps in clinical research: roadmap to precision health and reducing health disparities[J]. Pers Med, 2025,[Online ahead of print]. doi:10.1080/17410541.2025.2504329 |
| [39] | Bhatte S, Frederick J, Serrano S, et al. Bridging the vitamin A and deworming coverage gap among underserved populations in India through government and civil society organization partnerships[J]. Arch Public Health, 2024, 82(1): 75. doi:10.1186/s13690-024-01302-8 |
| [40] | 孙梅华, 刁青花, 王秀珍. 胃癌患者实施人文关怀护理的探讨: 评《癌症·瘀毒论》[J]. 中国实验方剂学杂志, 2024, 30(17): 202. |
| [41] | 苏喜凰. 将人文关怀融入手术患者的各阶段[N]. 甘肃科技报, 2024-07-19(008). |
| [42] | Che WQ, Li YJ, Tsang CK, et al. How to use the surveillance, epidemiology, and end results (SEER) data: research design and methodology[J]. Mil Med Res, 2023, 10(1): 50. doi:10.1186/s40779-023-00488-2 |
| [43] | Fung BM, Patel M, Patel N, et al. Signet ring cell gastric carcinoma: clinical epidemiology and outcomes in a predominantly Latino County hospital population[J]. Dig Dis Sci, 2021, 66(4): 1240-8. doi:10.1007/s10620-020-06341-z |
| [44] | 陈鑫明, 黄 坤, 赵平武, 等. 手术治疗对胃印戒细胞癌预后的影响: 基于SEER数据库分析[J]. 中国普外基础与临床杂志, 2023, 30(12): 1472-7. |
| [45] | 齐忆虹. 医疗卫生服务公平中的责任机制探讨[J]. 健康教育与健康促进, 2023, 18(1): 98-102. |
| [46] | 芦鹏飞, 李 响, 王丽娜, 等. 基于空间可达性的特定医疗与综合医疗资源分配公平差异研究[J]. 测绘科学技术学报, 2024, 41(4): 418-24. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||