Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (6): 1149-1158.doi: 10.12122/j.issn.1673-4254.2024.06.16
Xinrong HE( ), Sili XIONG(
), Sili XIONG( ), Zhenru ZHU, Jingyuan SUN, Chuanhui CAO(
), Zhenru ZHU, Jingyuan SUN, Chuanhui CAO( ), Hui WANG(
), Hui WANG( )
)
Received:2024-03-07
Online:2024-06-20
Published:2024-07-01
Contact:
Chuanhui CAO, Hui WANG
E-mail:theahexr@163.com;179980987@qq.com;huichuancao@163.com;635137884@qq.com
Supported by:Xinrong HE, Sili XIONG, Zhenru ZHU, Jingyuan SUN, Chuanhui CAO, Hui WANG. Overexpression of ubiquitin-conjugating enzyme 2T induces radiotherapy resistance in hepatocellular carcinoma by enriching regulatory T cells in the tumor microenvironment[J]. Journal of Southern Medical University, 2024, 44(6): 1149-1158.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.06.16
| Primer | Sequence (5'→3') |
|---|---|
Il-10-F Il-10-R | GTCCTTTCACTTGCCCTCATC CAAACYGGTCACAGCTTTCGA |
Tab.1 Primers used for RT-qPCR
| Primer | Sequence (5'→3') |
|---|---|
Il-10-F Il-10-R | GTCCTTTCACTTGCCCTCATC CAAACYGGTCACAGCTTTCGA |
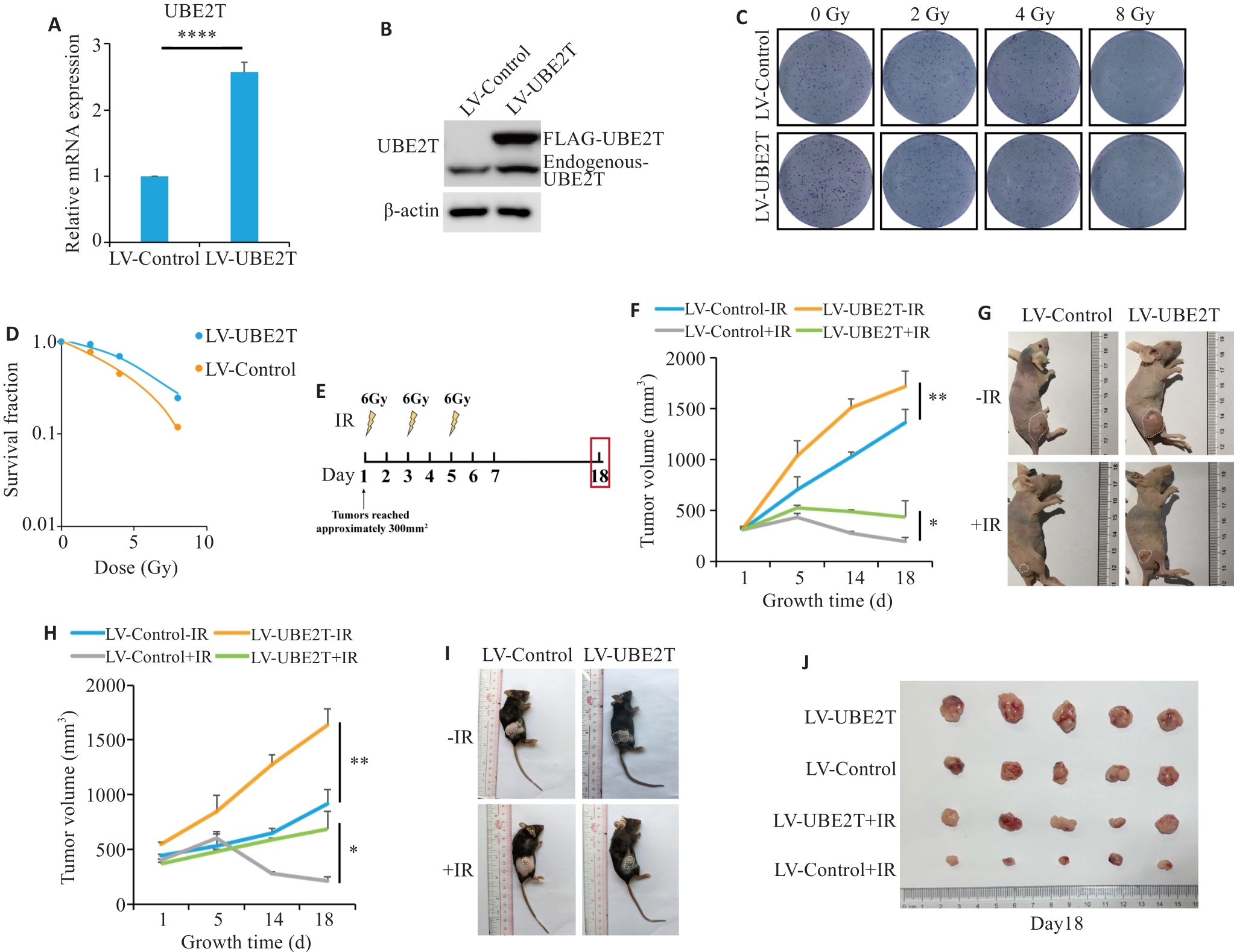
Fig.1 Overexpression of UBE2T results in radiotherapy resistance in HCC. A: Relative mRNA level of Ube2t adjusted to Actin. B: Western blotting of UBE2T protein in LV-Control and LV-UBE2T cells. C, D: Colony formation assays of Hepa 1-6 cells with stable UBE2T overexpression. E: Treatment schedules of ionizing radiation (IR) and endpoint for recording. F, G: Tumor growth curves of the nude mice (F, n=5 or 6) and the dissected xenografts from the mice (G). in each group. H-J: Tumor growth curves of C57BL/6 mice (H, n=5 or 6), the tumor-bearing mice (I), and the dissected xenografts (J). In (F) and (H), statistical analyses were performed using a mixed-effects model followed by Tukey's multiple comparison test. In (A), student's t-test was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ****P<0.0001.
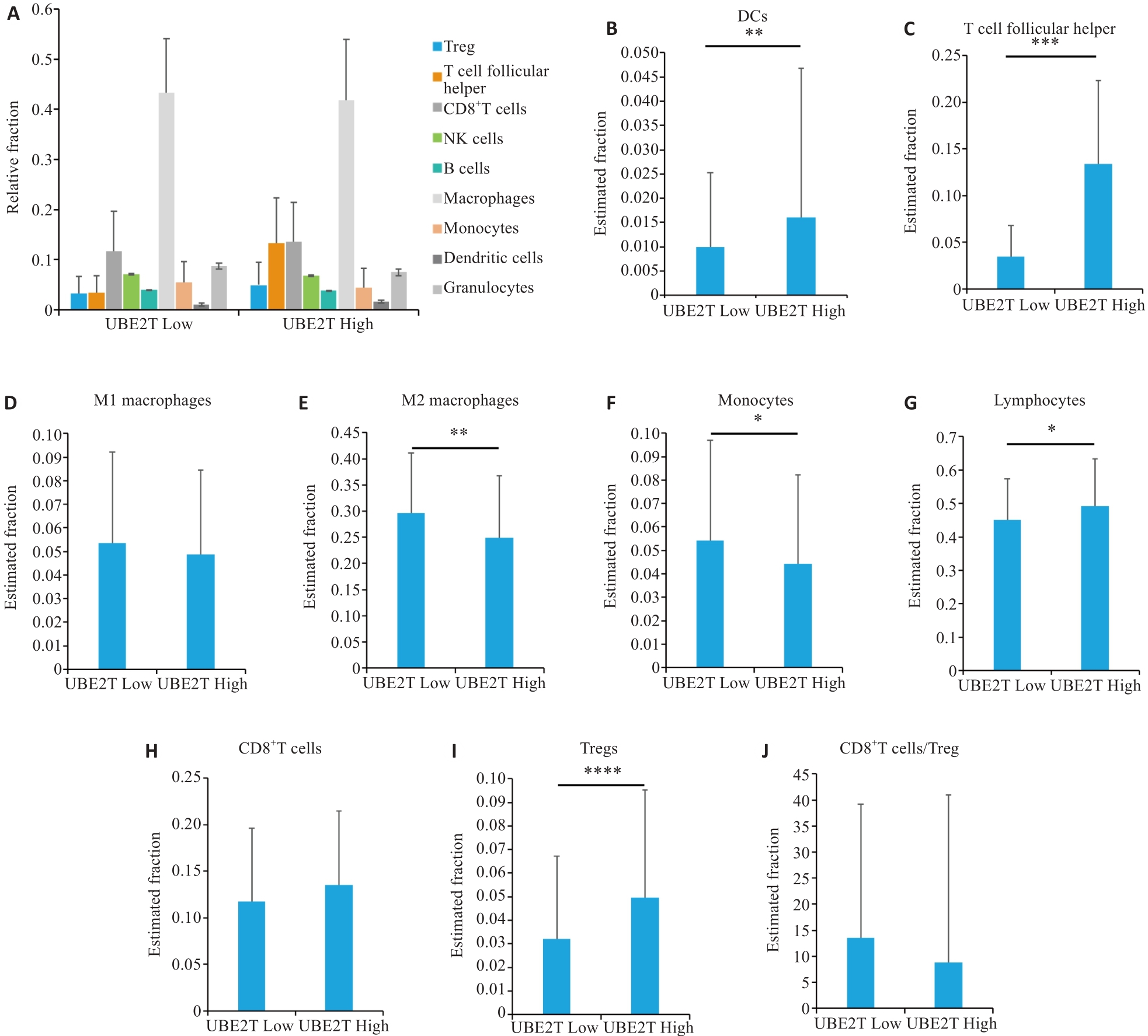
Fig.2 Immunological microenvironmental analysis of HCC by CIBERSORT. A: Percentages of different cell types in the two groups. B-J: Estimated fractions of DCs (B), T follicular helper cells (C), M1 macrophages (D), M2 macrophages (E), monocytes (F), lymphocytes (G), CD8+ T cells (H), Tregs (I), and CD8+T cells/Tregs (J) in the two groups. Student's t-test was used for comparisons. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. DCs: Dendritic cells, Tregs: Regulatory T cells.
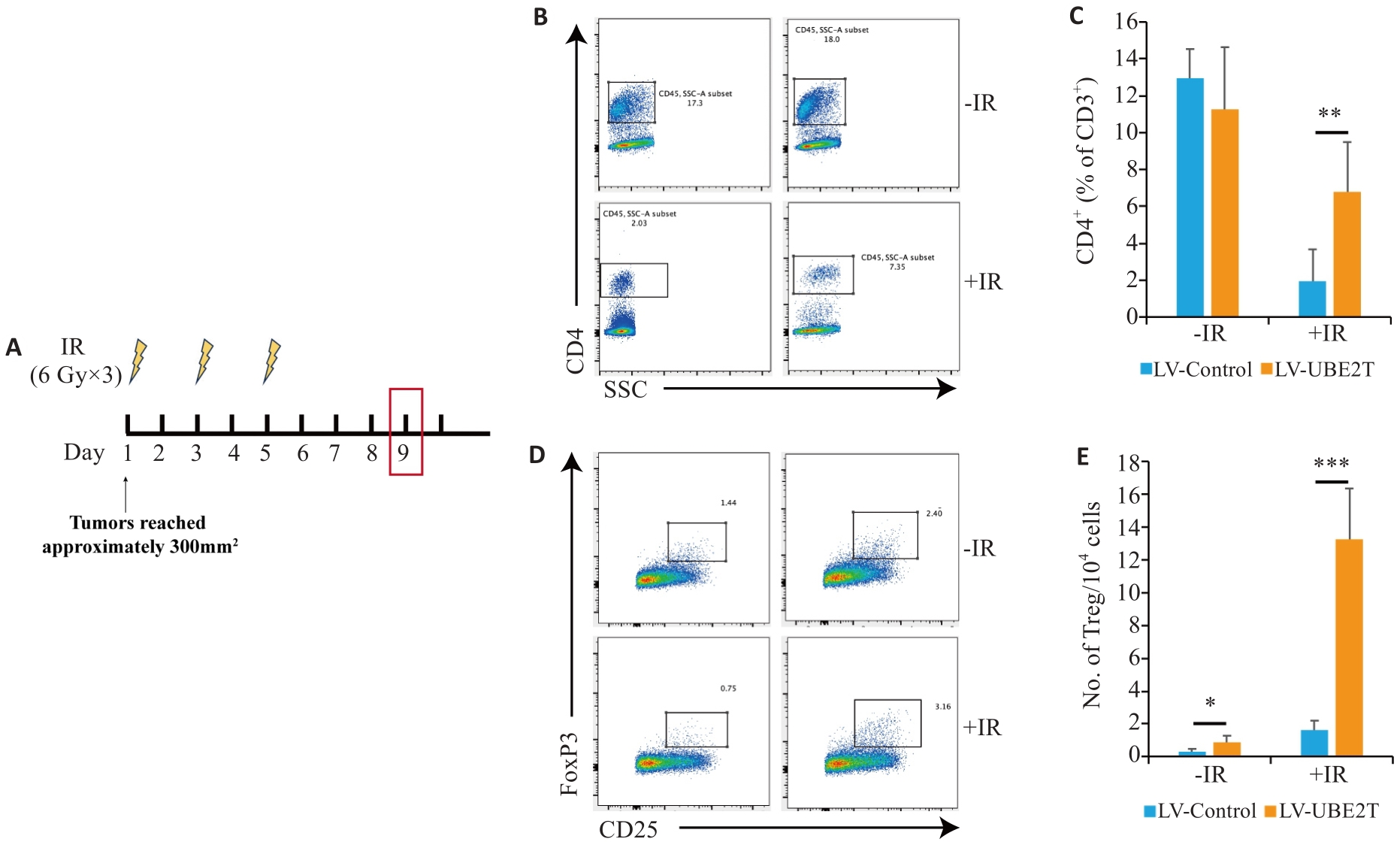
Fig.3 The number of Tregs is increased in HCC overexpressing UBE2T. A: Treatment schedules of IR and endpoint for analysis. B, C: Representative contour plots (B) and quantitation (C) of CD4+/CD3+ ratios (n=6-8). D, E: Representative contour plots (D) and quantitation (E) of the number of TIL Tregs (CD25+ Foxp3+) per 104 cells (n=6-8). Student's t-test was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001.
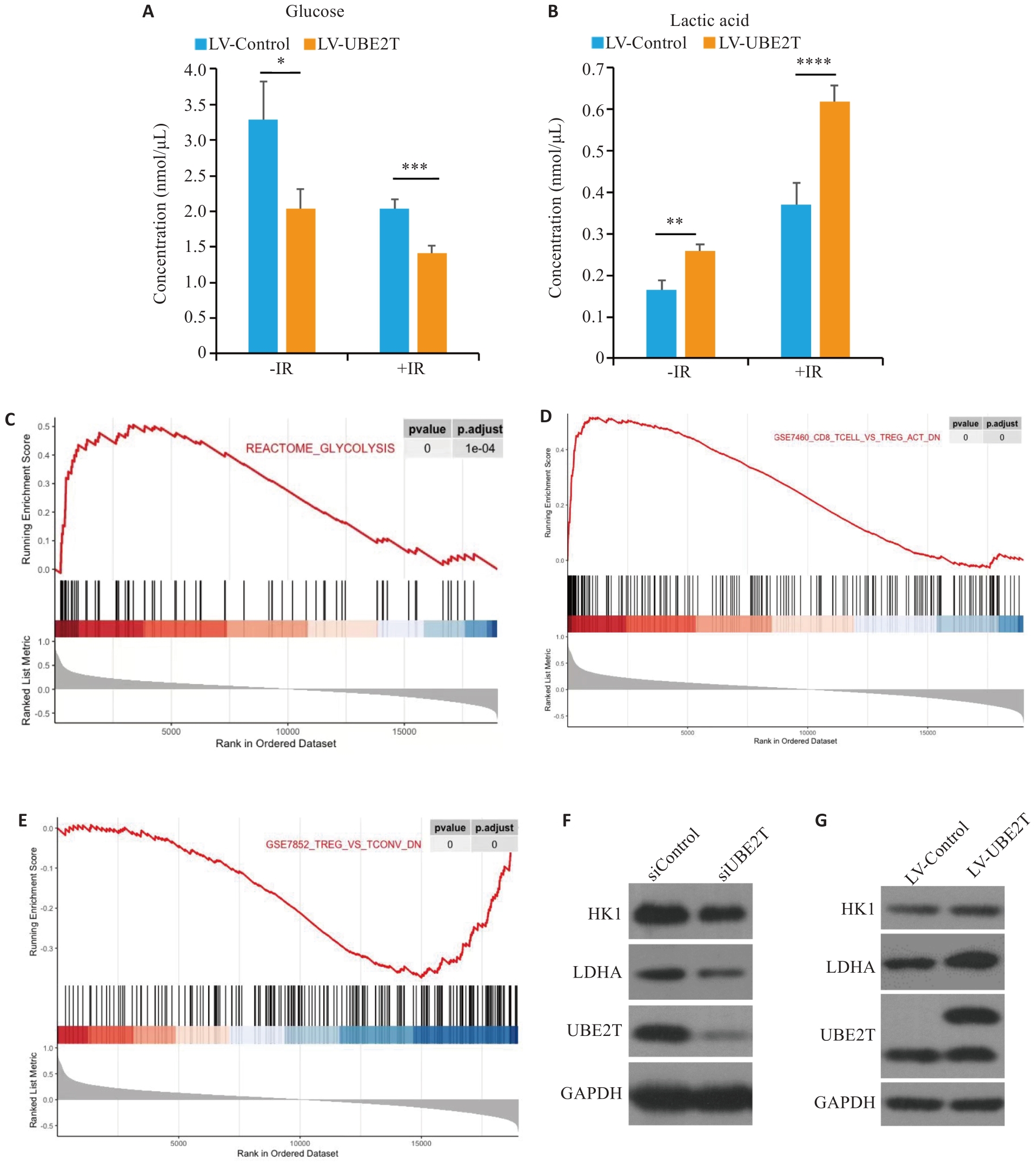
Fig.4 Effect of UBE2T expression on glycolysis levels in HCC and its regulatory effect on immune microenvironment. A, B: Concentration of glucose (A) and lactic acid (B) in the two groups with or without IR. C-E: Results of GSEA are plotted to visualize the correlation between the expression of UBE2T and the gene signatures of glycolysis and Tregs infiltration in the TCGA cohort. Student's t-test was used for comparisons. F, G: Western blotting of HK1, LDHA, and UBE2T protein in siControl, siUNE2T, LV-Control and LV-UBE2T cells. *P<0.05, **P<0.01, ***P<0.001.
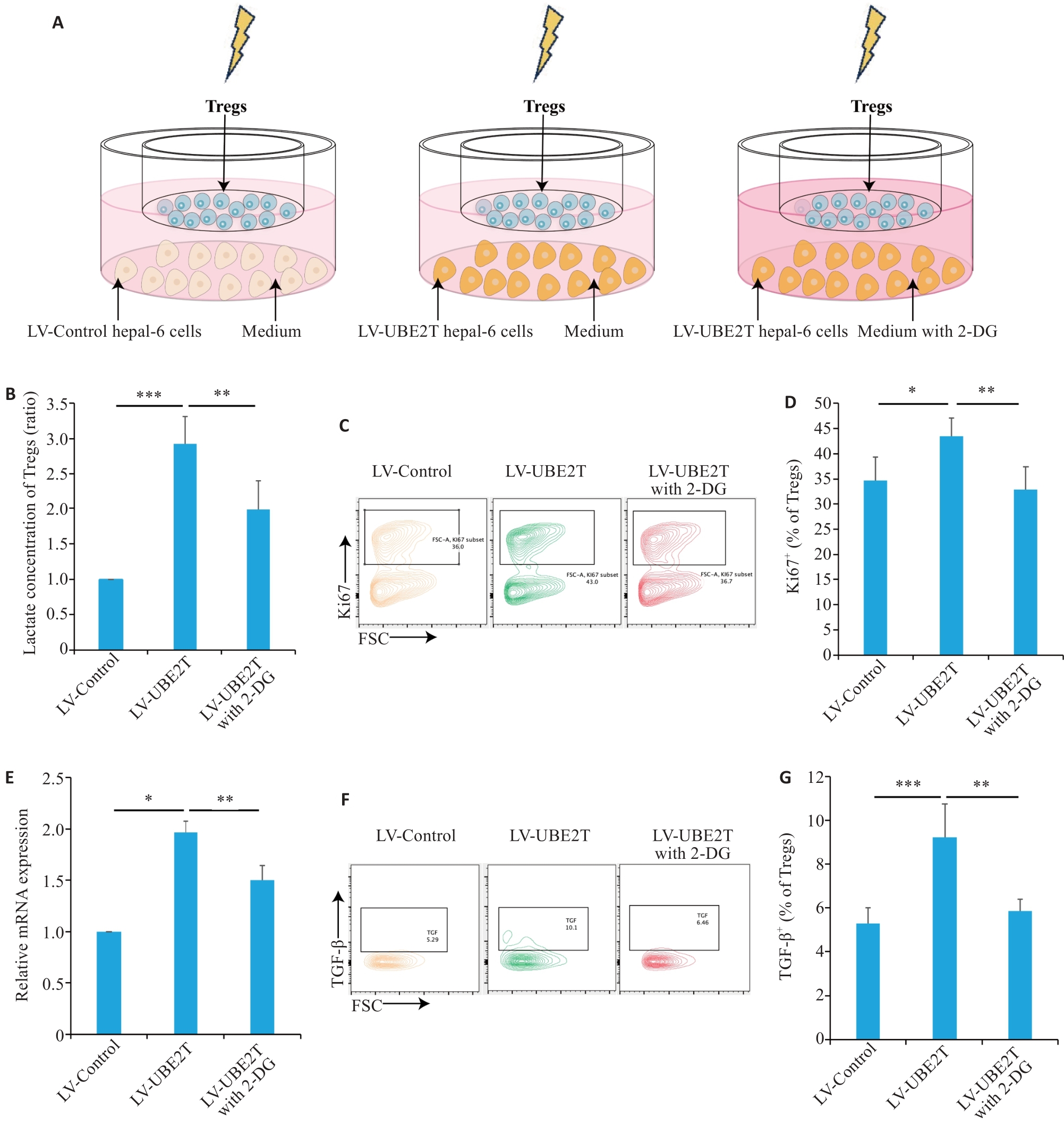
Fig.5 HCC overexpressing UBE2T promoted lactate secretion and activated Tregs.A: Schematic diagram of the co-culture model. B: Concentrations of Tregs in different co-culture groups (n=5). C, D: Representative contour plots (C) and quantitation (D) of Ki67+ ratios (n=6). E: Relative mRNA level of IL-10 adjusted to Actin in different co-culture groups (n=3). F, G: Representative contour plots (F) and quantitation (G) of the TGF‑β+ ratios (n=5). One-way ANOVA was used for comparisons. Data are presented as Mean±SD. *P<0.05, **P<0.01, ***P<0.001.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. |
| 2 | Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7: 6. |
| 3 | Chen LC, Lin HY, Hung SK, et al. Role of modern radiotherapy in managing patients with hepatocellular carcinoma[J]. World J Gastroenterol, 2021, 27(20): 2434-57. |
| 4 | Kim TH, Koh YH, Kim BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial[J]. J Hepatol, 2021, 74(3): 603-12. |
| 5 | Shi CY, Li Y, Geng L, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: a randomised controlled trial[J]. Eur J Cancer, 2022, 166: 176-84. |
| 6 | Byun HK, Kim HJ, Im YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma[J]. Radiother Oncol, 2019, 133: 1-8. |
| 7 | Bang A, Dawson LA. Radiotherapy for HCC: ready for prime time?[J]. JHEP Rep, 2019, 1(2): 131-7. |
| 8 | Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer[J]. Nat Rev Clin Oncol, 2015, 12(9): 527-40. |
| 9 | Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies[J]. Trends Cancer, 2022, 8(1): 9-20. |
| 10 | Koukourakis MI, Giatromanolaki A. Tumor draining lymph nodes, immune response, and radiotherapy: towards a revisal of therapeutic principles[J]. Biochim Biophys Acta Rev Cancer, 2022, 1877(3): 188704. |
| 11 | McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy[J]. Nat Rev Cancer, 2020, 20(4): 203-17. |
| 12 | Yum S, Li MH, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS[J]. Cell Res, 2020, 30(8): 639-48. |
| 13 | Li JY, Zhao Y, Gong S, et al. TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models[J]. Nat Commun, 2023, 14(1): 865. |
| 14 | Jiang XY, Ma Y, Wang T, et al. Targeting UBE2T potentiates gemcitabine efficacy in PancreaticCancer by regulating pyrimidine metabolism and replication stress[J]. Gastroenterology, 2023, 164(7): 1232-47. |
| 15 | Yu ZY, Jiang XY, Qin L, et al. A novel UBE2T inhibitor suppresses Wnt/β‑catenin signaling hyperactivation and gastric cancer progression by blocking RACK1 ubiquitination[J]. Oncogene, 2021, 40(5): 1027-42. |
| 16 | Zhu ZR, Cao CH, Zhang DY, et al. UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism[J]. Cell Death Dis, 2022, 13(2): 154. |
| 17 | Sun JY, Zhu ZR, Li WW, et al. UBE2T-regulated H2AX monoubiquitination induces hepatocellular carcinoma radioresistance by facilitating CHK1 activation[J]. J Exp Clin Cancer Res, 2020, 39(1): 222. |
| 18 | Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles[J]. Nat Methods, 2015, 12(5): 453-7. |
| 19 | Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77(6): 1598-606. |
| 20 | Qiao L, Dong C, Ma BL. UBE2T promotes proliferation, invasion and glycolysis of breast cancer cells by regualting the PI3K/AKT signaling pathway[J]. J Recept Signal Transduct Res, 2022, 42(2): 151-9. |
| 21 | Wang Y, Leng H, Chen H, et al. Knockdown of UBE2T inhibits osteosarcoma cell proliferation, migration, and invasion by suppressing the PI3K/akt signaling pathway[J]. Oncol Res, 2016, 24(5): 361-9. |
| 22 | Cao K, Ling XD, Jiang XY, et al. Pan-canceranalysis of UBE2T with a focus on prognostic and immunological roles in lung adenocarcinoma[J]. Respir Res, 2022, 23(1): 306. |
| 23 | Huang W, Huang HY, Xiao YZ, et al. UBE2T is upregulated, predicts poor prognosis, and promotes cell proliferation and invasion by promoting epithelial-mesenchymal transition via inhibiting autophagy in an AKT/mTOR dependent manner in ovarian cancer[J]. Cell Cycle, 2022, 21(8): 780-91. |
| 24 | Allen C, Her S, Jaffray DA. Radiotherapy for cancer: present and future[J]. Adv Drug Deliv Rev, 2017, 109: 1-2. |
| 25 | Citrin DE. Recent developments in radiotherapy[J]. N Engl J Med, 2017, 377(22): 2200-1. |
| 26 | Ahmad SS, Duke S, Jena R, et al. Advances in radiotherapy[J]. BMJ, 2012, 345(dec04 1): e7765. |
| 27 | Ohri N, Dawson LA, Krishnan S, et al. Radiotherapy for hepatocellular carcinoma: new indications and directions for future study[J]. J Natl Cancer Inst, 2016, 108(9): djw133. |
| 28 | Fang Y, Zhan YZ, Xie YW, et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC[J]. Hepatology, 2022, 75(6): 1386-401. |
| 29 | Hong WF, Zhang Y, Wang SW, et al. RECQL4 inhibits radiation-induced tumor immune awakening via suppressing the cGAS-STING pathway in hepatocellular carcinoma[J]. Adv Sci, 2024, 11(16): e2308009. |
| 30 | Yin H, Wang XY, Zhang X, et al. UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation[J]. Cancer Lett, 2020, 494: 121-31. |
| 31 | Zhang YH, Hu JY, Ji K, et al. CD39 inhibition and VISTA blockade may overcome radiotherapy resistance by targeting exhausted CD8+ T cells and immunosuppressive myeloid cells[J]. Cell Rep Med, 2023, 4(8): 101151. |
| 32 | Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment[J]. Nat Immunol, 2013, 14(10): 1014-22. |
| 33 | Song M, Yeku OO, Rafiq S, et al. Tumor derived UBR5 promotes ovarian cancer growth and metastasis through inducing immunosuppressive macrophages[J]. Nat Commun, 2020, 11(1): 6298. |
| 34 | Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma[J]. Cancer Discov, 2019, 9(8): 1124-41. |
| 35 | Dikiy S, Rudensky AY. Principles of regulatory Tcell function[J]. Immunity, 2023, 56(2): 240-55. |
| 36 | Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development[J]. Annu Rev Immunol, 2020, 38: 421-53. |
| 37 | Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer[J]. Immunity, 2019, 50(2): 302-16. |
| 38 | Kang JH, Zappasodi R. Modulating Treg stability to improve cancer immunotherapy[J]. Trends Cancer, 2023, 9(11): 911-27. |
| 39 | Shan F, Somasundaram A, Bruno TC, et al. Therapeutic targeting of regulatory T cells in cancer[J]. Trends Cancer, 2022, 8(11): 944-61. |
| 40 | Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression-implications for anticancer therapy[J]. Nat Rev Clin Oncol, 2019, 16(6): 356-71. |
| 41 | Persa E, Balogh A, Sáfrány G, et al. The effect of ionizing radiation on regulatory T cells in health and disease[J]. Cancer Lett, 2015, 368(2): 252-61. |
| 42 | 郭 康, 刘晓敏, 戴 聪, 等. UBE2T基因在乳腺癌中的差异表达及其与免疫细胞浸润之间的相关性研究: 基于生物信息学分析的结果[J]. 临床医学研究与实践, 2020, 5(34): 5-7, 15. DOI: 10.19347/j.cnki.2096-1413.202034002 |
| 43 | 于运亮, 李 婷, 王莉莉, 等. UBE2C和UBE2T与胰腺癌患者预后及免疫细胞浸润的关系[J]. 山东医药, 2021, 61(28): 13-8. DOI: 10.3969/j.issn.1002-266X.2021.28.004 |
| 44 | Pouysségur J, Marchiq I, Parks SK, et al. 'Warburg effect' controls tumor growth, bacterial, viral infections and immunity-Genetic deconstruction and therapeutic perspectives[J]. Semin Cancer Biol, 2022, 86(Pt 2): 334-46. |
| 45 | Kang H, Kim B, Park J, et al. The Warburg effect on radioresistance: survival beyond growth[J]. Biochim Biophys Acta Rev Cancer, 2023, 1878(6): 188988. |
| 46 | Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells[J]. Mol Aspects Med, 2010, 31(1): 60-74. |
| 47 | Apostolova P, Pearce EL. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment[J]. Trends Immunol, 2022, 43(12): 969-77. |
| 48 | Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment[J]. Science, 2015, 348(6230): 74-80. |
| 49 | Park J, Hsueh PC, Li ZY, et al. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity[J]. Immunity, 2023, 56(1): 32-42. |
| 50 | Watson MJ, Vignali PDA, Mullett SJ, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid[J]. Nature, 2021, 591(7851): 645-51. |
| 51 | Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments[J]. Cell Metab, 2017, 25(6): 1282-93.e7. |
| 52 | Li N, Kang YQ, Wang LL, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment[J]. Proc Natl Acad Sci U S A, 2020, 117(33): 20159-70. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||