Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (5): 876-884.doi: 10.12122/j.issn.1673-4254.2024.05.09
• Basic Research • Previous Articles Next Articles
Feixia WANG1,2,3( ), Zheng ZHANG2,3, Yan SUN2,3, Liujing YANG1,2,3, Tongtong GUO1,2,3, Yeting PAN1,2,3, Songtao DING1, Lin JIANG1, Handeng LIU1,2,3(
), Zheng ZHANG2,3, Yan SUN2,3, Liujing YANG1,2,3, Tongtong GUO1,2,3, Yeting PAN1,2,3, Songtao DING1, Lin JIANG1, Handeng LIU1,2,3( )
)
Received:2023-12-15
Online:2024-05-20
Published:2024-06-06
Contact:
Handeng LIU
E-mail:fiexia@stu.cqmu.edu.cn;hdliu@cqmu.edu.cn
Feixia WANG, Zheng ZHANG, Yan SUN, Liujing YANG, Tongtong GUO, Yeting PAN, Songtao DING, Lin JIANG, Handeng LIU. Bmal1 mediates the neuroprotective effect of sodium butyrate in a mouse model of Parkinson's disease[J]. Journal of Southern Medical University, 2024, 44(5): 876-884.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.05.09
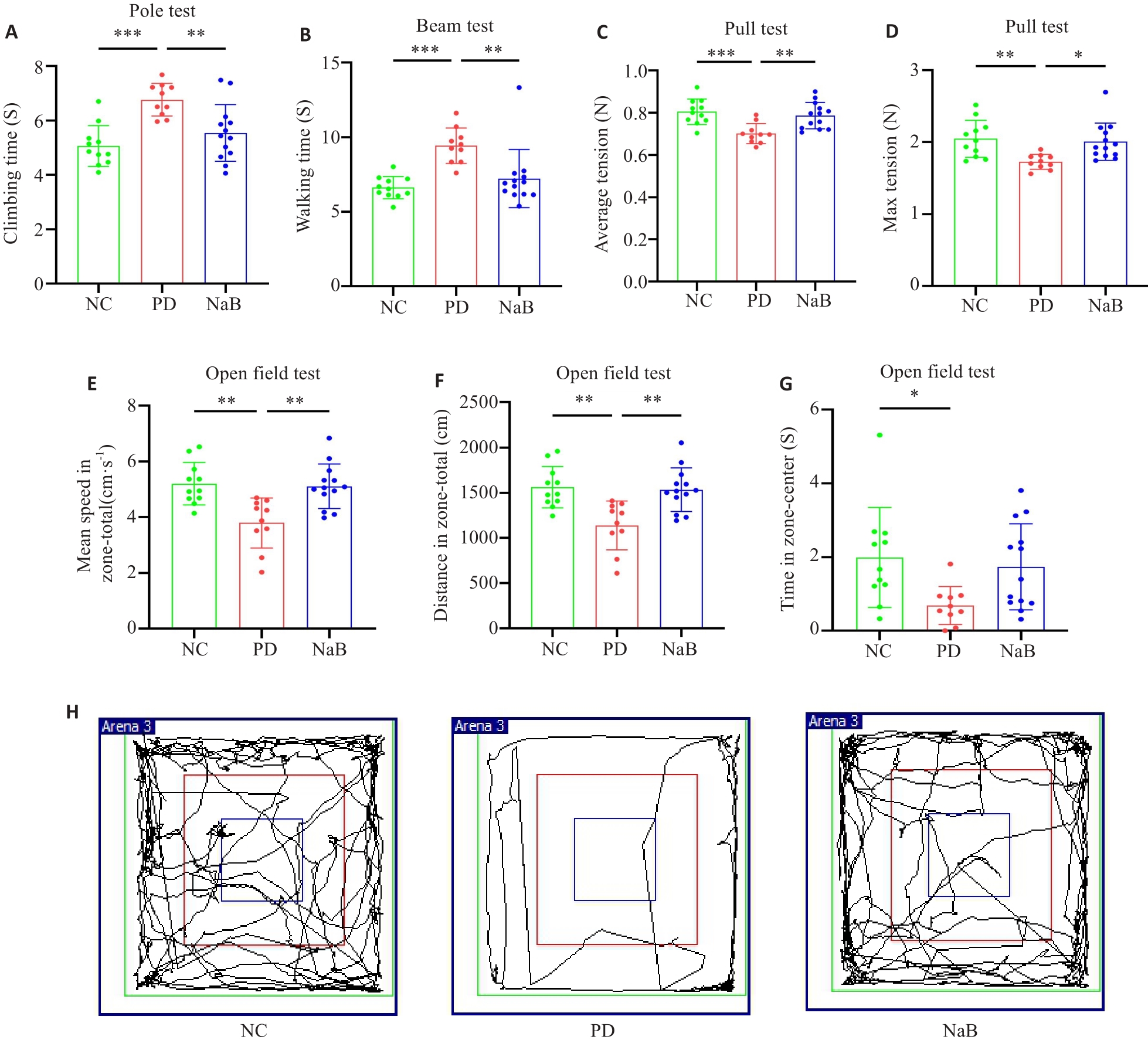
Fig.1 NaB improves motor dysfunction and relieves anxiety symptoms in PD mice. A: Pole test. B: Beam test. C: Average tension of pull test. D: The maximum tension in pull test. E: Mean speed in open field test. F: Total moving distance in open field test. G: Time in the central area in open field test. H: Representative movement tracks in open field test. n=11 in NC group, n=10 in PD group, n=13 in NaB group. *P<0.05, **P<0.01, ***P<0.001.
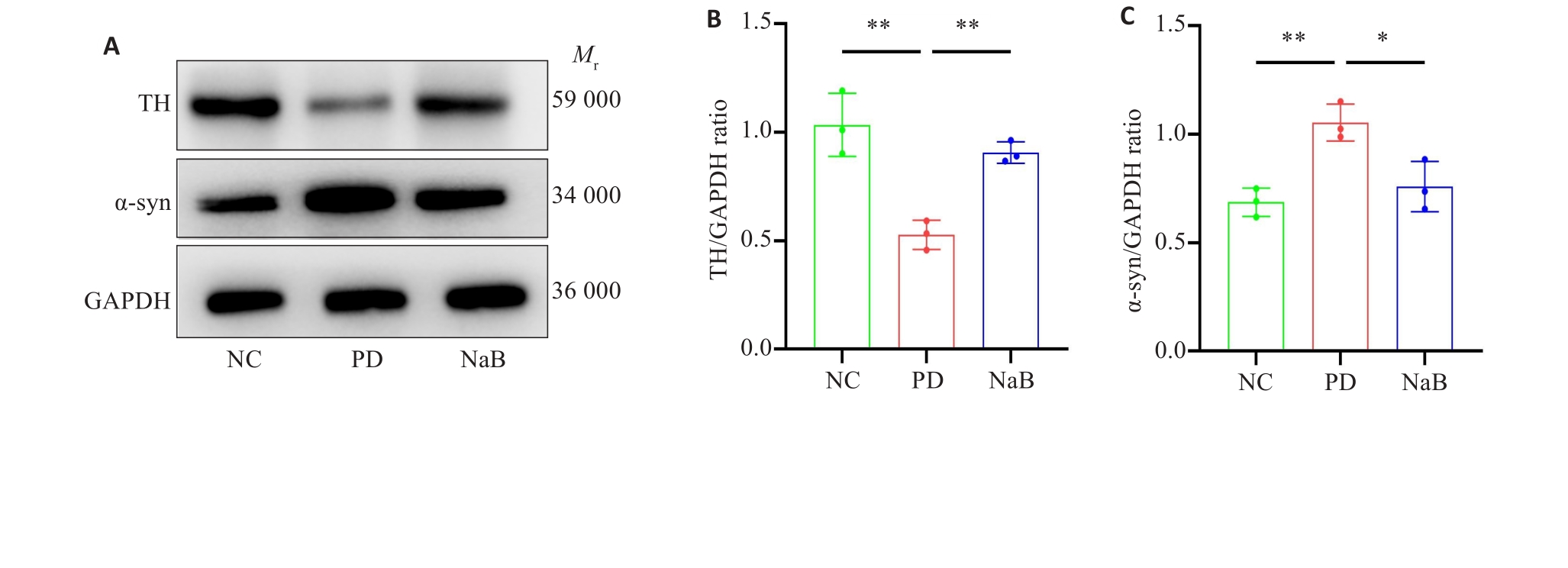
Fig.2 NaB reverses upregulation of α‑syn and downregulation of TH protein expression in the striatum of PD mice. A: Western blots of TH and α-syn proteins. B, C: Quantitative analysis of protein expression levels pf TH and α-syn (n=3). *P<0.05, **P<0.01.
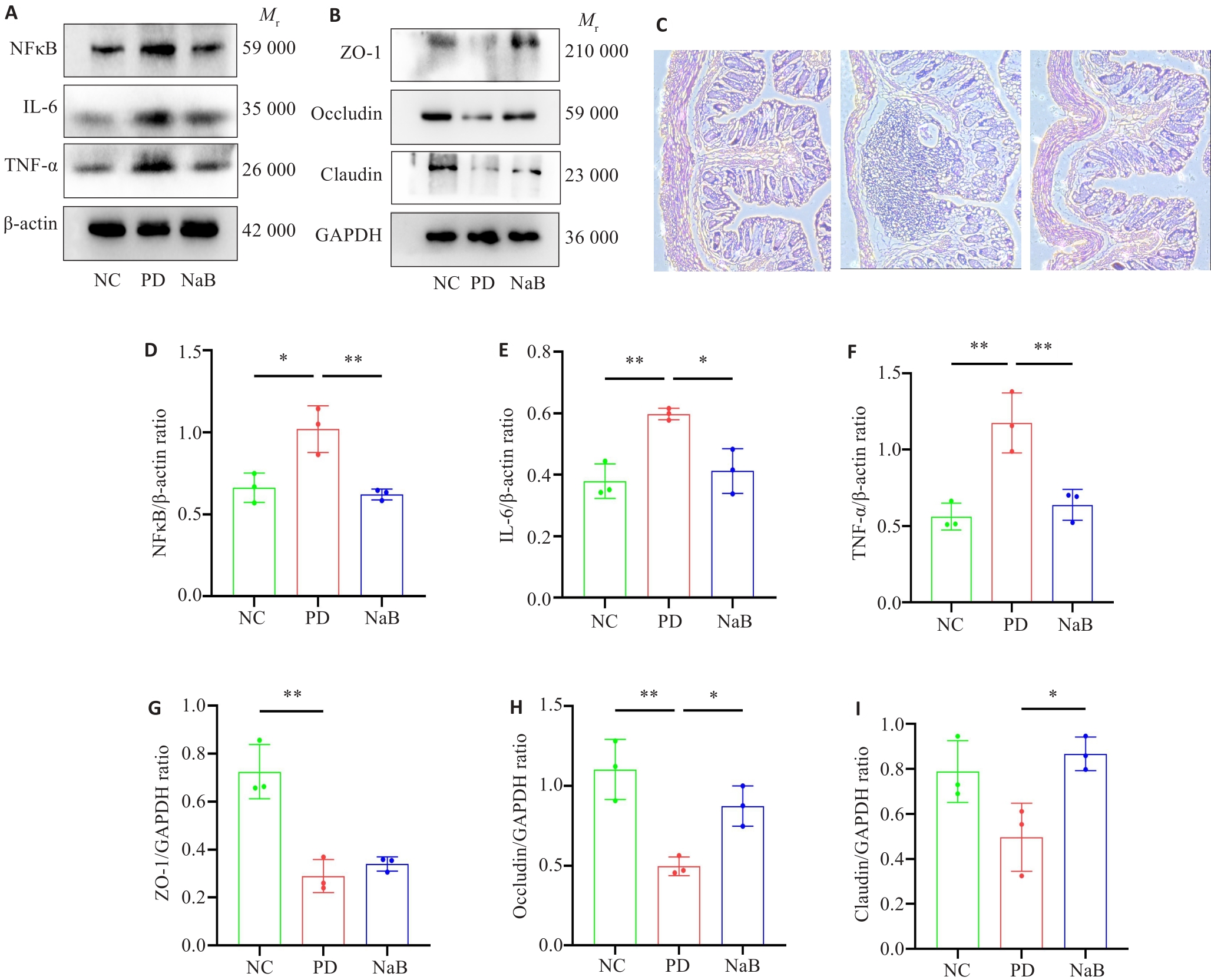
Fig.3 NaB treatment reduces colonic inflammatory factor levels and increases colonic tight junction protein expressions in PD mice. A: Western blots of NF‑κB, IL-6, and TNF‑α in mouse colon. B: Western blots of ZO-1, Occludin, and Claudin proteins. C: HE staining of mouse colon tissues (Original magnification: ×100). D-I: Quantitative analysis of the protein expression levels of NF‑κB, IL-6, TNF‑α, ZO-1, Occludin and Claudin (n=3). *P<0.05, **P<0.01.
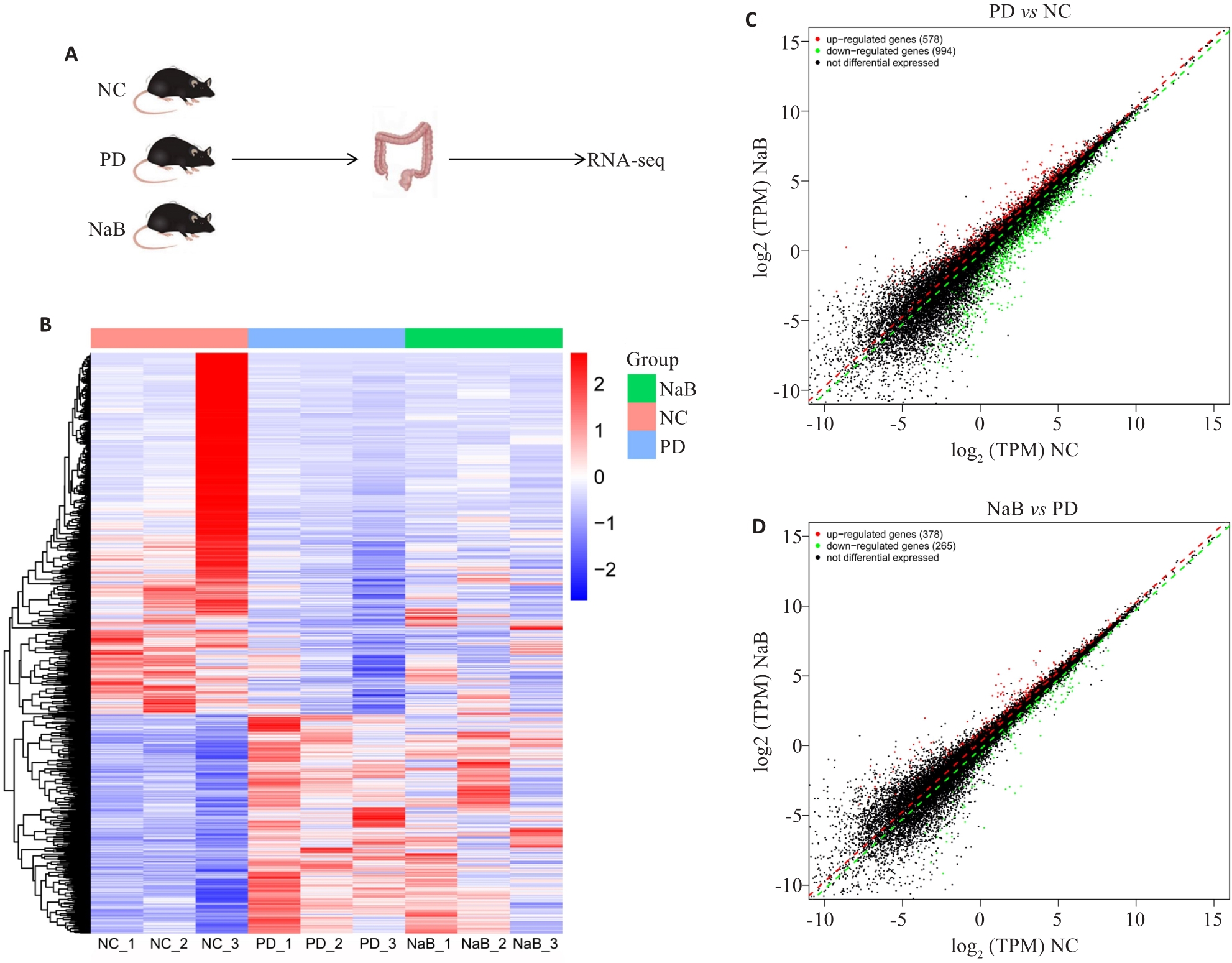
Fig.4 Differentially expressed genes (DEGs) in PD mice with NaB treatment. A: Schematic diagram of RNA sequencing of mouse colon tissues. B: Heat map of DEGs. C: Scatter plot of DEGs between PD and NC groups. D: Scatter plot of DEGs between NaB and PD groups. FC>1.2, P<0.05.
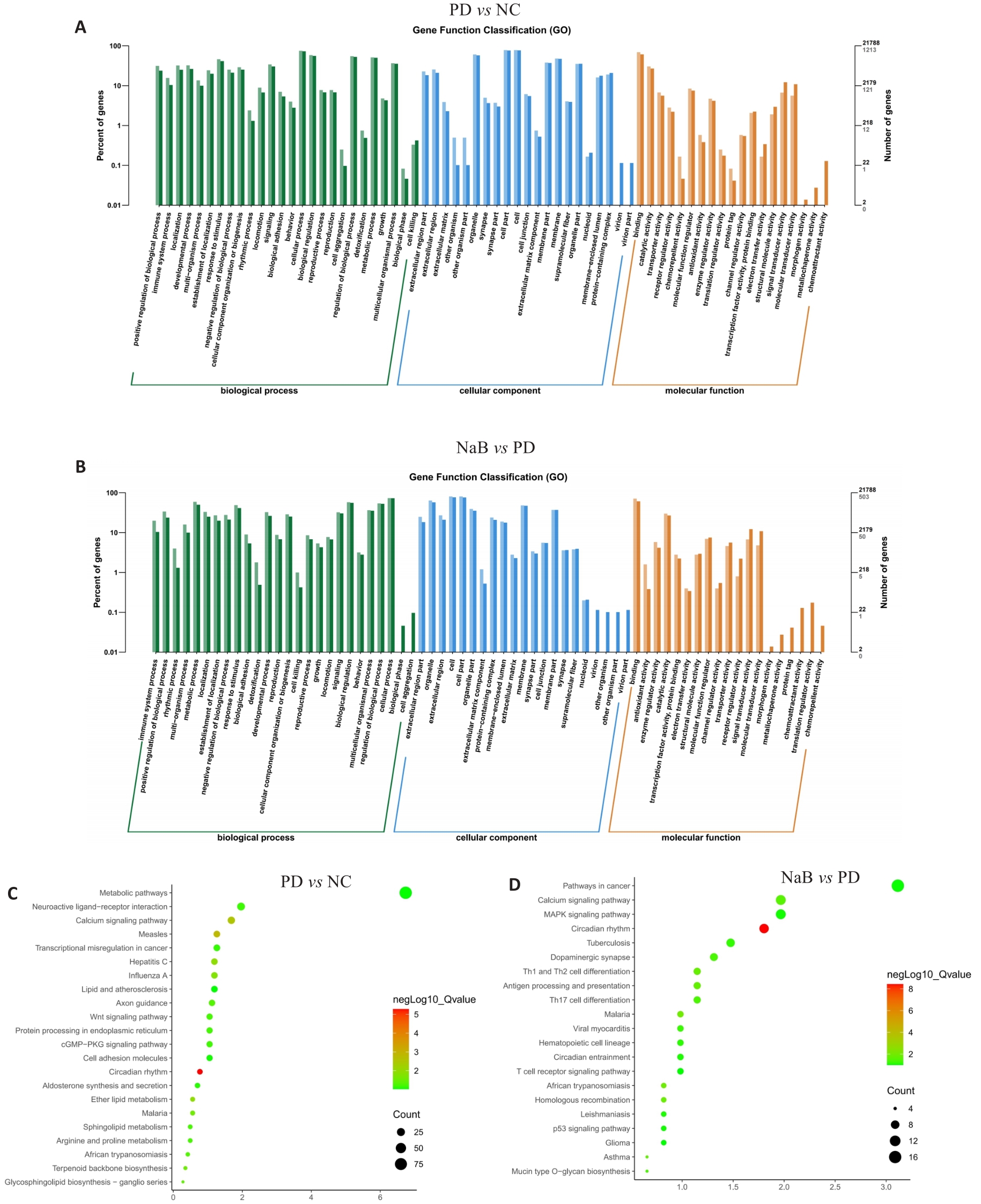
Fig.5 GO and KEGG analysis of the DEGs in PD mice with NaB treatment. A: GO analysis of DEGs between PD and NC group. B: GO analysis of DEGs between NaB and PD groups. C: KEGG analysis of DEGs between PD and NC groups. D: KEGG analysis of DEGs between NaB and PD group. FC>1.2, P<0.05.
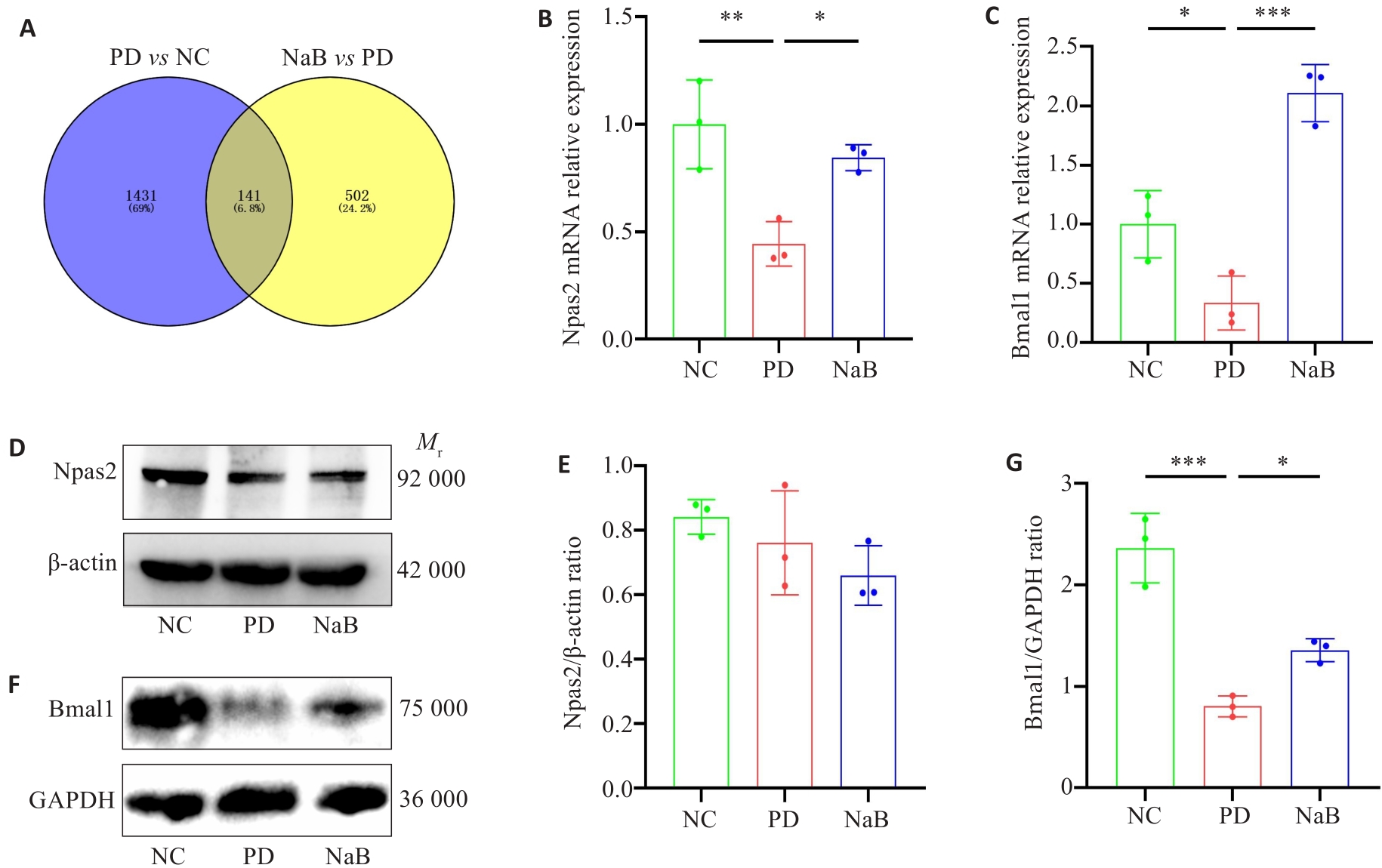
Fig.6 Validation of RNA sequencing results of Bmal1. A: Venn diagram. B, C: qRT-PCR detection of relative expression of Npas2 and Bmal1 mRNA in mouse colonic tissues. D, F: Western blots of Npas2 and Bmal1 proteins. E, G: Quantitative analysis of the protein expression levels of Npas2 and Bmal1 (n=3). *P<0.05, **P<0.01, ***P<0.001.
| 1 | Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, et al. The epidemiology of Parkinson's disease[J]. Lancet, 2024, 403(10423): 283-92. DOI: 10.1016/s0140-6736(23)01419-8 |
| 2 | Elfil M, Bayoumi A, Sayed A, et al. Stroke in Parkinson's disease: a review of epidemiological studies and potential pathophysiological mechanisms[J]. Acta Neurol Belg, 2023, 123(3): 773-83. DOI: 10.1007/s13760-023-02202-4 |
| 3 | Yemula N, Dietrich C, Dostal V, et al. Parkinson's disease and the gut: symptoms, nutrition, and microbiota[J]. J Parkinsons Dis, 2021, 11(4): 1491-505. DOI: 10.3233/jpd-212707 |
| 4 | Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease[J]. Cell, 2016, 167(6): 1469-80. e12. DOI: 10.1016/j.cell.2016.11.018 |
| 5 | Cheng QC, Wang JW, Li M, et al. CircSV2b participates in oxidative stress regulation through miR-5107-5p-Foxk1-Akt1 axis in Parkinson's disease[J]. Redox Biol, 2022, 56: 102430. DOI: 10.1016/j.redox.2022.102430 |
| 6 | González-Rodríguez P, Zampese E, Stout KA, et al. Disruption of mitochondrial complex I induces progressive Parkinsonism[J]. Nature, 2021, 599(7886): 650-6. DOI: 10.1038/s41586-021-04059-0 |
| 7 | Patil RS, Tupe RS. Communal interaction of glycation and gut microbes in diabetes mellitus, Alzheimer's disease, and Parkinson's disease pathogenesis[J]. Med Res Rev, 2024, 44(1): 365-405. DOI: 10.1002/med.21987 |
| 8 | Esteves AR, Munoz-Pinto MF, Nunes-Costa D, et al. Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria[J]. Gut, 2023, 72(1): 73-89. DOI: 10.1136/gutjnl-2021-326023 |
| 9 | Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders[J]. Acta Neuropathol, 2010, 119(6): 689-702. DOI: 10.1007/s00401-010-0664-3 |
| 10 | Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis[J]. Neuropathol Appl Neurobiol, 2007, 33(6): 599-614. DOI: 10.1111/j.1365-2990.2007.00874.x |
| 11 | Kim S, Kwon SH, Kam TI, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson's disease[J]. Neuron, 2019, 103(4): 627-41.e7. DOI: 10.1016/j.neuron.2019.05.035 |
| 12 | Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease[J]. Ann Neurol, 2015, 78(4): 522-9. DOI: 10.1002/ana.24448 |
| 13 | Mayer EA, Nance K, Chen S. The gut-brain axis[J]. Annu Rev Med, 2022, 73: 439-53. DOI: 10.1146/annurev-med-042320-014032 |
| 14 | Wang Q, Luo YQ, Ray Chaudhuri K, et al. The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options[J]. Brain, 2021, 144(9): 2571-93. DOI: 10.1093/brain/awab156 |
| 15 | LeWitt PA. Levodopa therapy for Parkinson's disease: Pharmacokinetics and pharmacodynamics[J]. Mov Disord, 2015, 30(1): 64-72. DOI: 10.1002/mds.26082 |
| 16 | Reich SG, Savitt JM. Parkinson's disease[J]. Med Clin N Am, 2019, 103(2): 337-50. DOI: 10.1016/j.mcna.2018.10.014 |
| 17 | Wei HL, Yu CY, Zhang C, et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis[J]. Biomed Pharmacother, 2023, 160: 114308. DOI: 10.1016/j.biopha.2023.114308 |
| 18 | Szacawa E, Dudek K, Bednarek D, et al. A pilot study on the effect of a novel feed additive containing exogenous enzymes, acidifiers, sodium butyrate and silicon dioxide nanoparticles on selected cellular immune indices and body weight gains of calves[J]. J Vet Res, 2021, 65(4): 497-504. DOI: 10.2478/jvetres-2021-000068 |
| 19 | Li CY, Chen JL, Zhao M, et al. Effect of sodium butyrate on slaughter performance, serum indexes and intestinal barrier of rabbits[J]. J Anim Physiol Anim Nutr, 2022, 106(1): 156-66. DOI: 10.1111/jpn.13571 |
| 20 | Lan RX, Zhao ZH, Li SQ, et al. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions[J]. Poult Sci, 2020, 99(11): 5491-500. DOI: 10.1016/j.psj.2020.06.042 |
| 21 | Zhou TT, Xu HW, Cheng X, et al. Sodium butyrate attenuates diabetic kidney disease partially via histone butyrylation modification[J]. Mediators Inflamm, 2022, 2022: 7643322. DOI: 10.1155/2022/7643322 |
| 22 | Zhou ZH, Xu NB, Matei N, et al. Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats[J]. J Cereb Blood Flow Metab, 2021, 41(2): 267-81. DOI: 10.1177/0271678x20910533 |
| 23 | Chen SJ, Chen CC, Liao HY, et al. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with parkinson disease[J]. Neurology, 2022, 98(8): e848-58. DOI: 10.1212/wnl.0000000000013225 |
| 24 | Guo TT, Zhang Z, Sun Y, et al. Neuroprotective effects of sodium butyrate by restoring gut microbiota and inhibiting TLR4 signaling in mice with MPTP-induced Parkinson's disease[J]. Nutrients, 2023, 15(4): 930. DOI: 10.3390/nu15040930 |
| 25 | Dodiya HB, Forsyth CB, Voigt RM, et al. Chronic stress-induced gut dysfunction exacerbates Parkinson's disease phenotype and pathology in a rotenone-induced mouse model of Parkinson's disease[J]. Neurobiol Dis, 2020, 135: 104352. DOI: 10.1016/j.nbd.2018.12.012 |
| 26 | Lee HS, Lobbestael E, Vermeire S, et al. Inflammatory bowel disease and Parkinson's disease: common pathophysiological links[J]. Gut, 2021, 70(2): 408-17. |
| 27 | Facchin S, Vitulo N, Calgaro M, et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease[J]. Neurogastroenterol Motil, 2020, 32(10): e13914. DOI: 10.1111/nmo.13914 |
| 28 | Zheng YJ, Pan LY, Wang FX, et al. Neural function of Bmal1: an overview[J]. Cell Biosci, 2023, 13(1): 1. DOI: 10.1186/s13578-022-00947-8 |
| 29 | Early JO, Menon D, Wyse CA, et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2[J]. Proc Natl Acad Sci USA, 2018, 115(36): E8460-8. DOI: 10.1073/pnas.1800431115 |
| 30 | Breen DP, Vuono R, Nawarathna U, et al. Sleep and circadian rhythm regulation in early Parkinson disease[J]. JAMA Neurol, 2014, 71(5): 589-95. DOI: 10.1001/jamaneurol.2014.65 |
| 31 | Liu WW, Wei SZ, Huang GD, et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson's disease mouse model[J]. FASEB J, 2020, 34(5): 6570-81. DOI: 10.1096/fj.201901565rr |
| [1] | GUI Jianjun, SUN Xiaodong, WEN Shu, LIU Xin, QIN Bingqing, SANG Ming. Resveratrol protects dopaminergic neurons in a mouse model of Parkinson's disease by regulating the gut-brain axis via inhibiting the TLR4 signaling pathway [J]. Journal of Southern Medical University, 2024, 44(2): 270-279. |
| [2] | FAN Jianing, SUN Yingjie, LIANG Bing, ZHANG Xiaoyan, XIAO Cheng, HUANG Zeqing. Role of gut microbiota in perioperative neurocognitive disorders after cardiopulmonary bypass surgery in rats with humanized gut flora [J]. Journal of Southern Medical University, 2023, 43(6): 964-969. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||