Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (5): 801-809.doi: 10.12122/j.issn.1673-4254.2024.05.01
• Basic Research • Next Articles
Tong YUAN( ), Yuying GUO, Junling ZHANG(
), Yuying GUO, Junling ZHANG( ), Saijun FAN(
), Saijun FAN( )
)
Received:2023-11-03
Accepted:2024-01-05
Online:2024-05-20
Published:2024-06-06
Contact:
Junling ZHANG, Saijun FAN
E-mail:yuantong1233@163.com;zhangjunling@irm-cams.ac.cn;fansaijun@irm-cams.ac.cn
Supported by:Tong YUAN, Yuying GUO, Junling ZHANG, Saijun FAN. Normal mouse serum alleviates radiation pneumonitis in mice by inhibiting the focal adhesion signaling pathway[J]. Journal of Southern Medical University, 2024, 44(5): 801-809.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.05.01
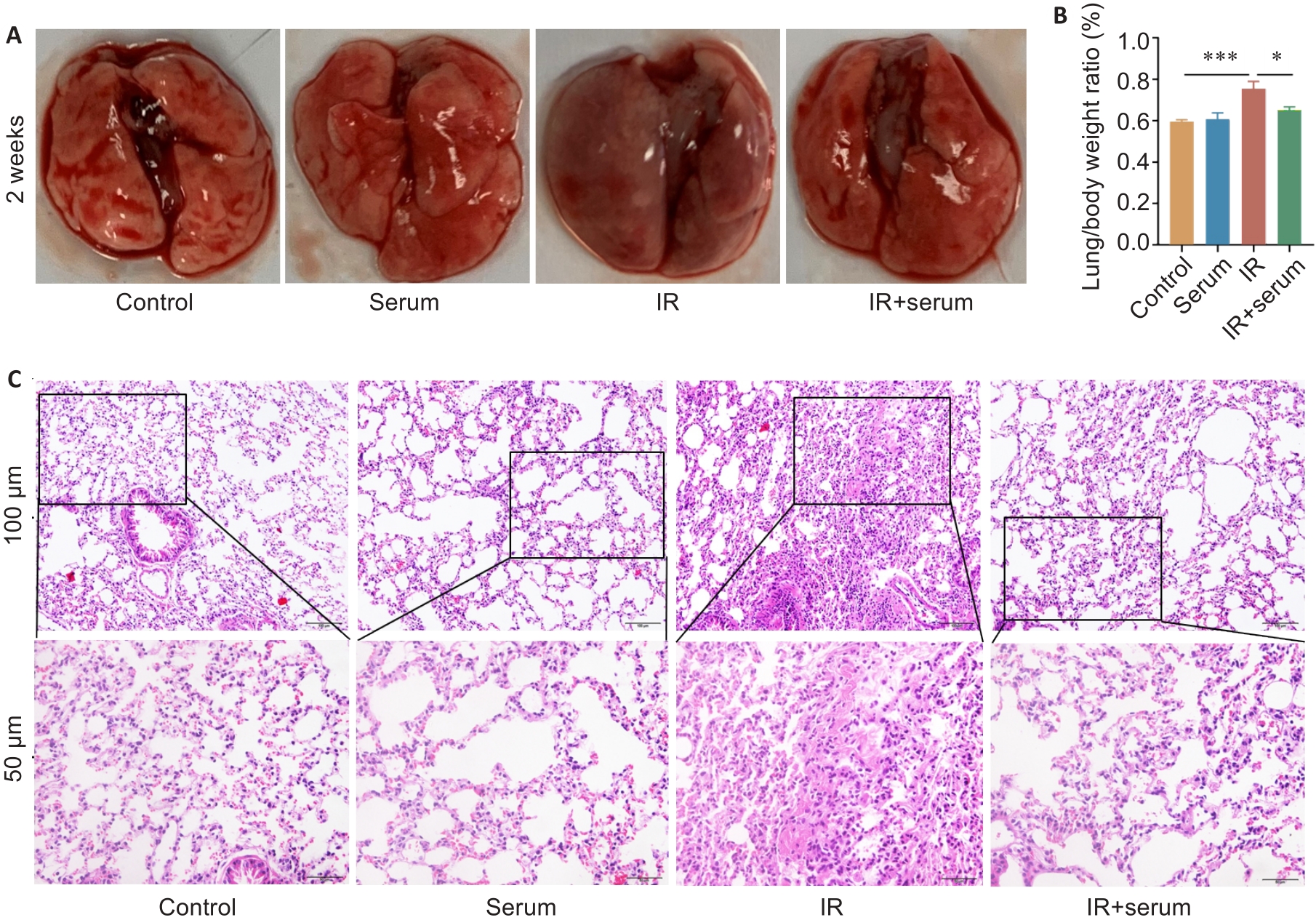
Fig.1 Normal mouse serum effectively protects normal histological structure of the lung tissue of irradiated mice. A: Gross observation of mouse lungs. B: Comparison of lung organ coefficients among the 4 groups. C: HE staining of the lung tissues in the 4 groups. Data are presented as Mean±SD (n=5). *P<0.05, ***P<0.001.

Fig.2 Intravenous injections of normal mouse serum down-regulate the levels of inflammatory factors in the lung tissue and serum of irradiated mice. A-D: Levels of TGF-β, TNF-α, IL-1α, and IL-6 in the lung tissue, respectively. E-H: Serum levels of TGF-β, TNF-α, IL-1α, and IL-6 in the serum, respectively. Data are presented as Mean±SD (n=8 in panel A and panel E, and n=5 in the rest panels). *P<0.05, **P<0.01, ***P<0.001.

Fig.3 NMS reduces lymphocyte infiltration in the lung tissue of irradiated mice A-D: Results of fluorescence-activated cell sorting showing gating for CD45+, CD4+ and Treg cells and their percentages in total cells. Data are presented as Mean±SD (n=5). *P<0.05, **P<0.01.

Fig.5 Number of immune-related differential genes and their expression at mRNA and protein levels in irradiated and NMS-treated mice. A: Classification of KEGG pathways. B, C: Relative mRNA expressions of Gsk3b, Cul3, Tiam1, Prkci, Egfr and Pik3cd in the lung tissue. D, E: Western blots of EGFR and P110δ proteins and their relative expression levels. Data are presented as Mean±SD (n=5 in panel B and panel C, and n=3 in panel D and panel E). *P<0.05, **P<0.01, ***P<0.001.

Fig.6 Normal mouse serum significantly down-regulates the expression of Focal adhesion pathway-related proteins in irradiated mice. A: Enrichment bubble chart of KEGG pathway. B-D: Western blotting of the key proteins in the focal adhesion signaling pathway and their relative expression levels in the lung tissues. Data are presented as Mean±SD (n=3). *P<0.05, **P<0.01, ***P<0.001.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. DOI: 10.3322/caac.21660 |
| 2 | Arora A, Bhuria V, Singh S, et al. Amifostine analog, DRDE-30, alleviates radiation induced lung damage by attenuating inflammation and fibrosis[J]. Life Sci, 2022, 298: 120518. DOI: 10.1016/j.lfs.2022.120518 |
| 3 | Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-induced lung injury: assessment and management[J]. Chest, 2019, 156(1): 150-62. DOI: 10.1016/j.chest.2019.03.033 |
| 4 | Giridhar P, Mallick S, Rath GK, et al. Radiation induced lung injury: prediction, assessment and management[J]. Asian Pac J Cancer Prev, 2015, 16(7): 2613-7. DOI: 10.7314/apjcp.2015.16.7.2613 |
| 5 | Citrin DE, Shankavaram U, Horton JA, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis[J]. J Natl Cancer Inst, 2013, 105(19): 1474-84. DOI: 10.1093/jnci/djt212 |
| 6 | Abratt RP, Morgan GW, Silvestri G, et al. Pulmonary complications of radiation therapy[J]. Clin Chest Med, 2004, 25(1): 167-77. DOI: 10.1016/s0272-5231(03)00126-6 |
| 7 | Gao J, Peng S, Shan XN, et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis[J]. Cell Death Dis, 2019, 10(12): 957. DOI: 10.1038/s41419-019-2195-8 |
| 8 | Zhang JL, Han XD, Zhao Y, et al. Mouse serum protects against total body irradiation-induced hematopoietic system injury by improving the systemic environment after radiation[J]. Free Radic Biol Med, 2019, 131: 382-92. DOI: 10.1016/j.freeradbiomed.2018.12.021 |
| 9 | He CJ, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine[J]. Theranostics, 2018, 8(1): 237-55. DOI: 10.7150/thno.21945 |
| 10 | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. DOI: 10.1126/science.aau6977 |
| 11 | Gurunathan S, Kang MH, Jeyaraj M, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes[J]. Cells, 2019, 8(4): 307. DOI: 10.3390/cells8040307 |
| 12 | Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis[J]. Cancer Res, 2011, 71(11): 3792-801. DOI: 10.1158/0008-5472.can-10-4455 |
| 13 | Zhang L, Yu DH. Exosomes in cancer development, metastasis, and immunity[J]. Biochim Biophys Acta Rev Cancer, 2019, 1871(2): 455-68. DOI: 10.1016/j.bbcan.2019.04.004 |
| 14 | Bobrie A, Colombo M, Raposo G, et al. Exosome secretion: molecular mechanisms and roles in immune responses[J]. Traffic, 2011, 12(12): 1659-68. DOI: 10.1111/j.1600-0854.2011.01225.x |
| 15 | Gregory CD, Pound JD. Microenvironmental influences of apoptosis in vivo and in vitro [J]. Apoptosis, 2010, 15(9): 1029-49. DOI: 10.1007/s10495-010-0485-9 |
| 16 | Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases[J]. J Biol Chem, 2016, 291(52): 26589-97. DOI: 10.1074/jbc.r116.757955 |
| 17 | Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future[J]. J Cell Biol, 2013, 200(4): 367-71. DOI: 10.1083/jcb.201212113 |
| 18 | Kang H, Kim SC, Oh Y. Fucoxanthin abrogates ionizing radiation-induced inflammatory responses by modulating sirtuin 1 in macrophages[J]. Mar Drugs, 2023, 21(12): 635. DOI: 10.3390/md21120635 |
| 19 | Téoule R. Radiation-induced DNA damage and its repair[J]. Int J Radiat Biol Relat Stud Phys Chem Med, 1987, 51(4): 573-89. DOI: 10.1080/09553008414552111 |
| 20 | Zheng Y, Li H, Bo XC, et al. Ionizing radiation damage and repair from 3D-genomic perspective[J]. Trends Genet, 2023, 39(1): 1-4. DOI: 10.1016/j.tig.2022.07.004 |
| 21 | Zhang JX, Wang XL, Vikash V, et al. ROS and ROS-mediated cellular signaling[J]. Oxid Med Cell Longev, 2016, 2016: 4350965. DOI: 10.1155/2016/4350965 |
| 22 | Xu TK, Zhang YY, Chang PY, et al. Mesenchymal stem cell-based therapy for radiation-induced lung injury[J]. Stem Cell Res Ther, 2018, 9(1): 18. DOI: 10.1186/s13287-018-0776-6 |
| 23 | Milane L, Singh A, Mattheolabakis G, et al. Exosome mediated communication within the tumor microenvironment[J]. J Control Release, 2015, 219: 278-94. DOI: 10.1016/j.jconrel.2015.06.029 |
| 24 | McCall CE, El Gazzar M, Liu TF, et al. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation[J]. J Leukoc Biol, 2011, 90(3): 439-46. DOI: 10.1189/jlb.0211075 |
| 25 | Wang CL, Xu MC, Fan QZ, et al. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases[J]. Asian J Pharm Sci, 2023, 18(1): 100772. DOI: 10.1016/j.ajps.2022.100772 |
| 26 | Zheng X, Zhao D, Liu Y, et al. Regeneration and anti-inflammatory effects of stem cells and their extracellular vesicles in gynecological diseases[J]. Biomed Pharmacother, 2023, 168: 115739. DOI: 10.1016/j.biopha.2023.115739 |
| 27 | Liu C, Xiao K, Xie LX. Advances in the use of exosomes for the treatment of ALI/ARDS[J]. Front Immunol, 2022, 13: 971189. DOI: 10.3389/fimmu.2022.971189 |
| 28 | Xu CJ, Zhao J, Li QY, et al. Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model[J]. Stem Cell Res Ther, 2020, 11(1): 503. DOI: 10.1186/s13287-020-02023-9 |
| 29 | Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses[J]. Theranostics, 2021, 11(9): 4436-51. DOI: 10.7150/thno.54004 |
| 30 | Sun ZQ, Yang SX, Zhou QB, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment[J]. Mol Cancer, 2018, 17(1): 82. DOI: 10.1186/s12943-018-0831-z |
| 31 | Li H, Zhao SY, Jiang M, et al. Biomodified extracellular vesicles remodel the intestinal microenvironment to overcome radiation enteritis[J]. ACS Nano, 2023, 17(14): 14079-98. DOI: 10.1021/acsnano.3c04578 |
| 32 | Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling[J]. Nat Med, 2011, 18(1): 148-52. DOI: 10.1038/nm.2574 |
| 33 | Chen WC, Yu WK, Su VYF, et al. NLRP3 inflammasome activates endothelial-to-mesenchymal transition via focal adhesion kinase pathway in bleomycin-induced pulmonary fibrosis[J]. Int J Mol Sci, 2023, 24(21): 15813. DOI: 10.3390/ijms242115813 |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||