南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (12): 2690-2698.doi: 10.12122/j.issn.1673-4254.2025.12.16
• • 上一篇
秦萌1,2( ), 孙思宇2,3, 刘佳琪2, 高玉娇1,2, 汪昊2,3, 王友坤2, 孙奥2, 严加纯2, 汪金宝2, 于影1,2(
), 孙思宇2,3, 刘佳琪2, 高玉娇1,2, 汪昊2,3, 王友坤2, 孙奥2, 严加纯2, 汪金宝2, 于影1,2( )
)
收稿日期:2025-04-14
出版日期:2025-12-20
发布日期:2025-12-22
通讯作者:
于影
E-mail:13017518983@163.com;yuying2011@126.com
作者简介:秦 萌,在读硕士研究生,E-mail:13017518983@163.com
基金资助:
Meng QIN1,2( ), Siyu SUN2,3, Jiaqi LIU2, Yujiao GAO1,2, Hao WANG2,3, Youkun WANG2, Ao SUN2, Jiachun YAN2, Jinbao WANG2, Ying YU1,2(
), Siyu SUN2,3, Jiaqi LIU2, Yujiao GAO1,2, Hao WANG2,3, Youkun WANG2, Ao SUN2, Jiachun YAN2, Jinbao WANG2, Ying YU1,2( )
)
Received:2025-04-14
Online:2025-12-20
Published:2025-12-22
Contact:
Ying YU
E-mail:13017518983@163.com;yuying2011@126.com
摘要:
目的 观察白藜芦醇(RES)对PM2.5诱导的脑缺血再灌注损伤小鼠血脑屏障的影响,并探讨线粒体分裂与融合在内皮屏障中的作用。 方法 将小鼠脑微血管内皮细胞分为4组:对照组(CON组)、模型组(OGD/R组)、实验组(OGD/R+PM2.5组)、RES组(OGD/R+PM2.5+RES组)。OGD/R+PM2.5组和RES组在OGD/R前先进行PM2.5(100 μg/mL)预处理24 h,RES组在复氧时更换含RES(40 mg/mL)的正常培养基培养18 h。对照组不给予任何处理。CCK-8检测细胞活性;跨内皮电阻(TEER)和FITC-Dextran评估细胞通透性;测定MDA含量和SOD活性;荧光探针检测细胞内及线粒体ROS水平;Mito-Tracker Red CMXRos检测线粒体形态;Western blotting检测细胞紧密连接蛋白(ZO-1、Occludin、Claudin-5)以及线粒体动力学相关蛋白(Drp1、Fis1、Mfn2、OPA1)表达水平。 结果 与对照组相比,OGD/R组及OGD/R+PM2.5组细胞TEER值降低、通透性增加,氧化应激水平升高,ROS荧光表达增强(P<0.05)。线粒体形态破碎不规则,紧密连接蛋白及线粒体融合蛋白表达降低,线粒体分裂蛋白表达升高(P<0.05)。RES干预后,可明显降低细胞膜通透性及ROS表达水平;改善线粒体形态,增加紧密连接蛋白与线粒体融合蛋白表达,降低分裂蛋白表达(P<0.05)。 结论 RES可通过调节线粒体动力学平衡,减轻PM2.5诱导的脑缺血再灌注血脑屏障的损伤,其机制可能与促进线粒体融合、抑制线粒体分裂有关。
秦萌, 孙思宇, 刘佳琪, 高玉娇, 汪昊, 王友坤, 孙奥, 严加纯, 汪金宝, 于影. 白藜芦醇改善PM2.5诱导的脑缺血再灌注损伤小鼠血脑屏障及维持线粒体动力学平衡[J]. 南方医科大学学报, 2025, 45(12): 2690-2698.
Meng QIN, Siyu SUN, Jiaqi LIU, Yujiao GAO, Hao WANG, Youkun WANG, Ao SUN, Jiachun YAN, Jinbao WANG, Ying YU. Resveratrol protects barrier function of mouse brain microvascular endothelial cell monolayers with oxygen/glucose deprivation and PM2.5 exposure by maintaining mitochondrial dynamics balance[J]. Journal of Southern Medical University, 2025, 45(12): 2690-2698.
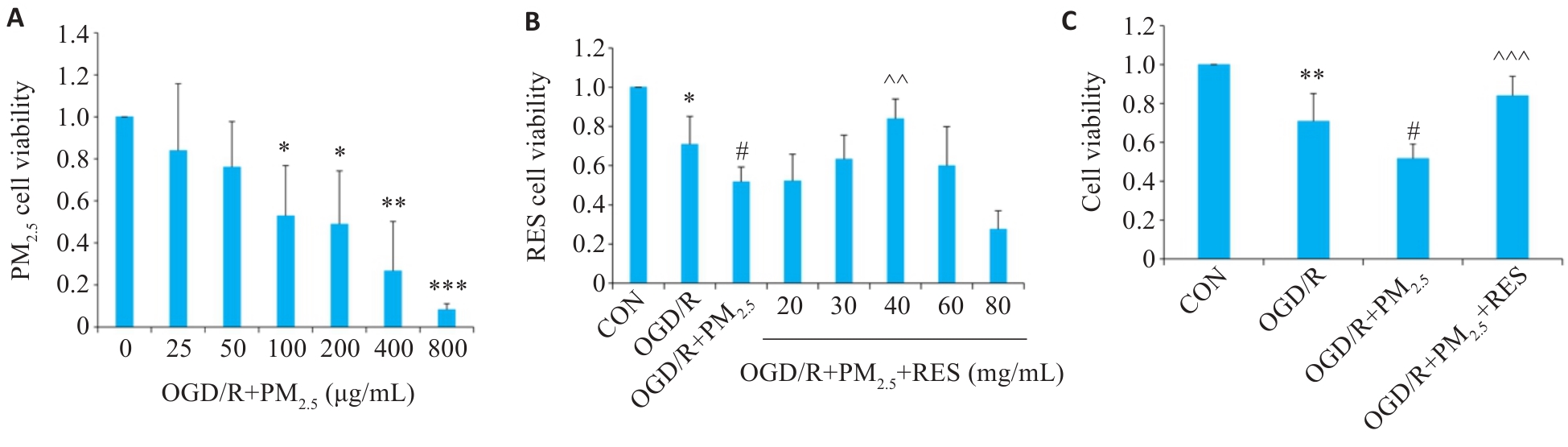
图1 各组脑微血管内皮细胞的细胞活性
Fig.1 Viability of brain microvascular endothelial cells exposed to oxygen/glucose deprivation/reoxygenation (OGD/R) and different concentrations of PM2.5 (A), pretreated with RES (B), and both (C) (Mean±SD, n=5). Data normalized to control (set as 1) and statistical comparisons are conducted on normalized data. *P<0.05, **P<0.01, ***P<0.001 vs CON group; #P<0.05 vs OGD/R group; ^^P<0.01, ^^^P<0.001 vs OGD/R+PM2.5 group.
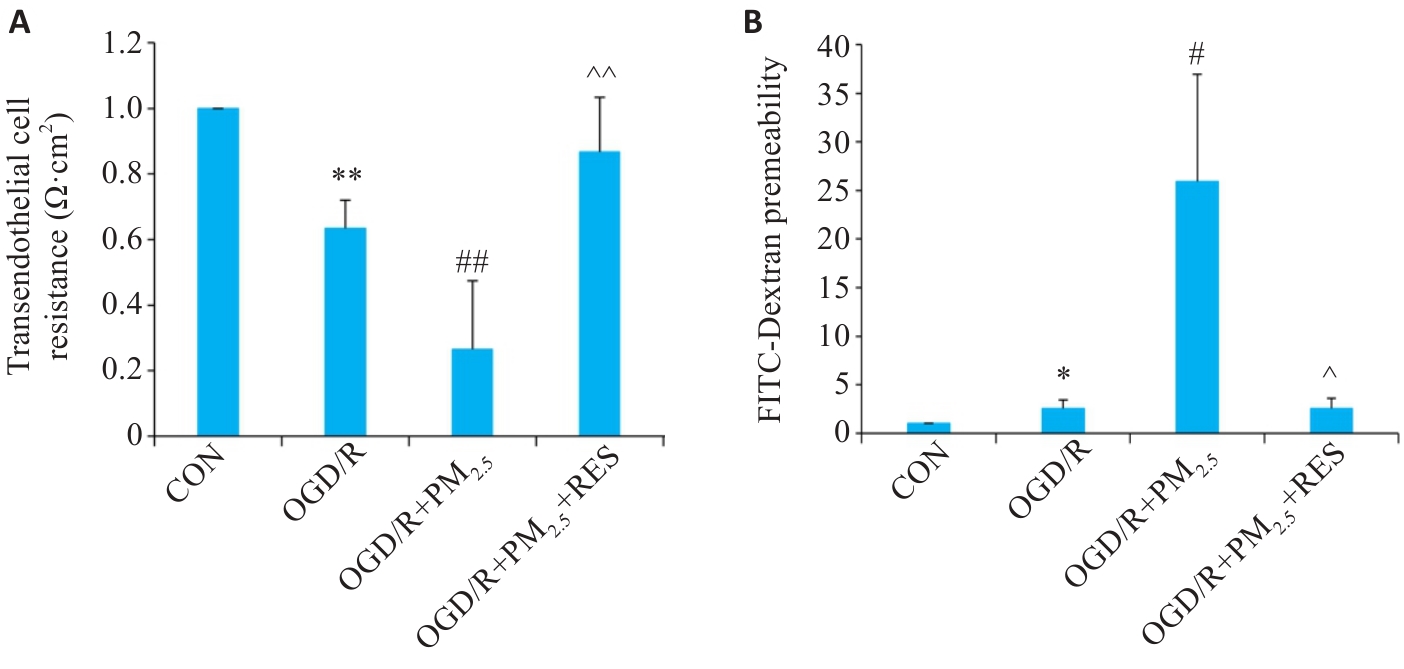
图2 PM2.5对OGD/R诱导脑微血管内皮细胞通透性的影响
Fig.2 Effect of PM2.5 on TEER (A) and FITC-dextran permeability (B) of mouse brain microvascular endothelial cells with OGD/R (Mean±SD, n=5). Data normalized to control (set as 1) and statistical comparisons are conducted on normalized data. *P<0.05, **P<0.01 vs CON group; #P<0.05, ##P<0.01 vs OGD/R group; ^P<0.05, ^^P<0.01 vs OGD/R+PM2.5 group.
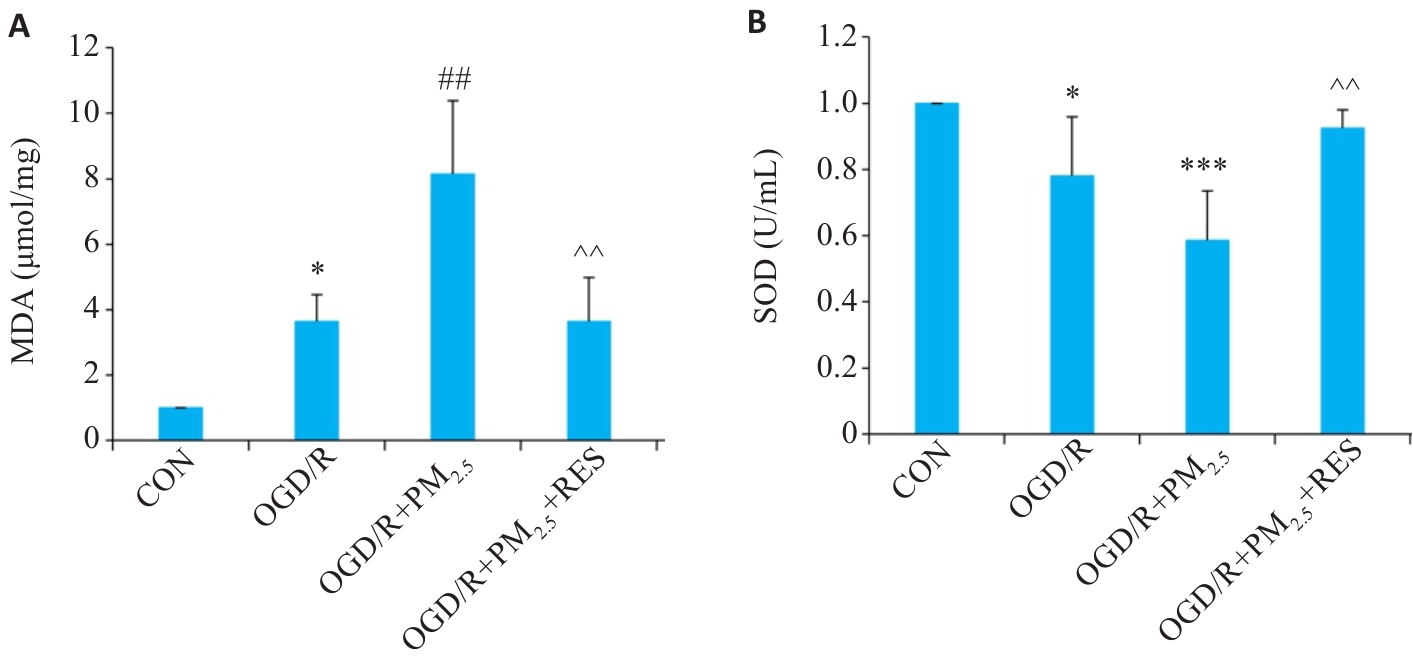
图3 各组细胞MDA、SOD水平比较
Fig.3 Comparison of MDA (A) and SOD (B) levels in the cells in different groups (Mean±SD, n=5). Data normalized to control (set as 1) and statistical comparisons are conducted on normalized data. *P<0.05, ***P<0.001 vs CON group; ##P<0.01 vs OGD/R group; ^^P<0.01 vs OGD/R+PM2.5 group.

图4 各组脑微血管内皮细胞ROS免疫荧光染色
Fig.4 Immunofluorescence staining of ROS in the cells in different groups. A: Immunofluorescence staining of ROS and DAPI (Scale bar=100 μm). B: Quantitative analysis of ROS fluorescence intensity (Mean±SD, n=5). ***P<0.001 vs CON group; ##P<0.01 vs OGD/R group; ^^P<0.01 vs OGD/R+PM2.5 group.
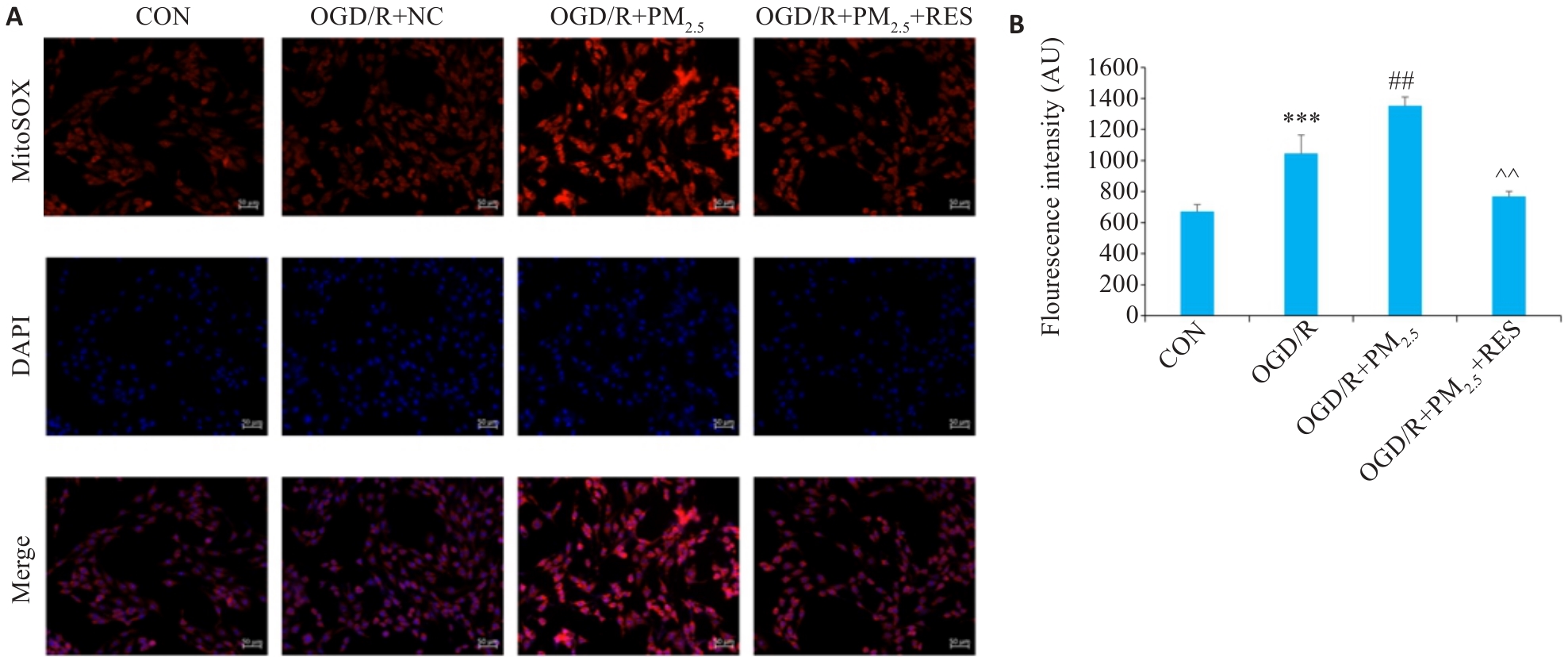
图5 各组脑微血管内皮线粒体ROS免疫荧光染色
Fig.5 Immunofluorescence staining for detecting mitochondrial ROS in different groups. A: Immunofluorescence staining of ROS and DAPI (Scale bar=100 μm). B: Quantitative analysis of ROS fluorescence intensity (Mean±SD, n=5). ***P<0.001 vs CON group; ##P<0.01 vs OGD/R group; ^^P<0.01 vs OGD/R+PM2.5 group.

图6 各组脑微血管内皮线粒体膜电位免疫荧光染色
Fig.6 Immunofluorescence staining for evaluating mitochondrial membrane potential in different groups. A: Immunofluorescence staining of aggregates (red) and monomers (green) (scale bar=20 μm). B, C: Quantitative analysis of membrane potential fluorescence intensity (Mean±SD, n=5). **P<0.01 vs CON group; #P<0.05 vs OGD/R group; ^P<0.05, ^^P<0.01 vs OGD/R+PM2.5 group.
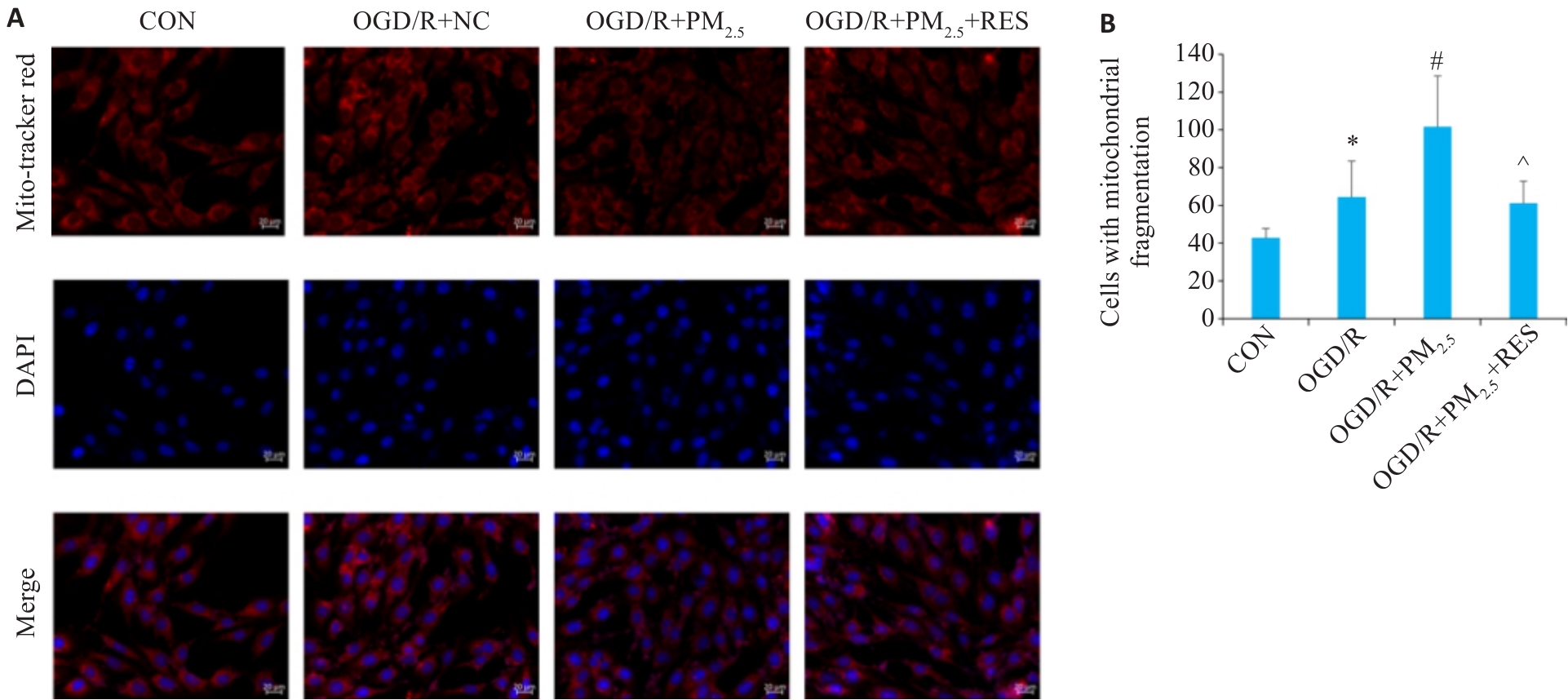
图7 各组脑微血管内皮线粒体形态免疫荧光染色
Fig.7 Immunofluorescence staining for observing mitochondrial morphology in different groups. A: Immunofluorescence staining with Mito-Tracker Red and DAPI (scale bar=20 μm). B: Comparison of mitochondrial fragmentation in each group (Mean±SD, n=5). *P<0.05 vs CON group; #P<0.05 vs OGD/R group; ^P<0.05 vs OGD/R+PM2.5 group.
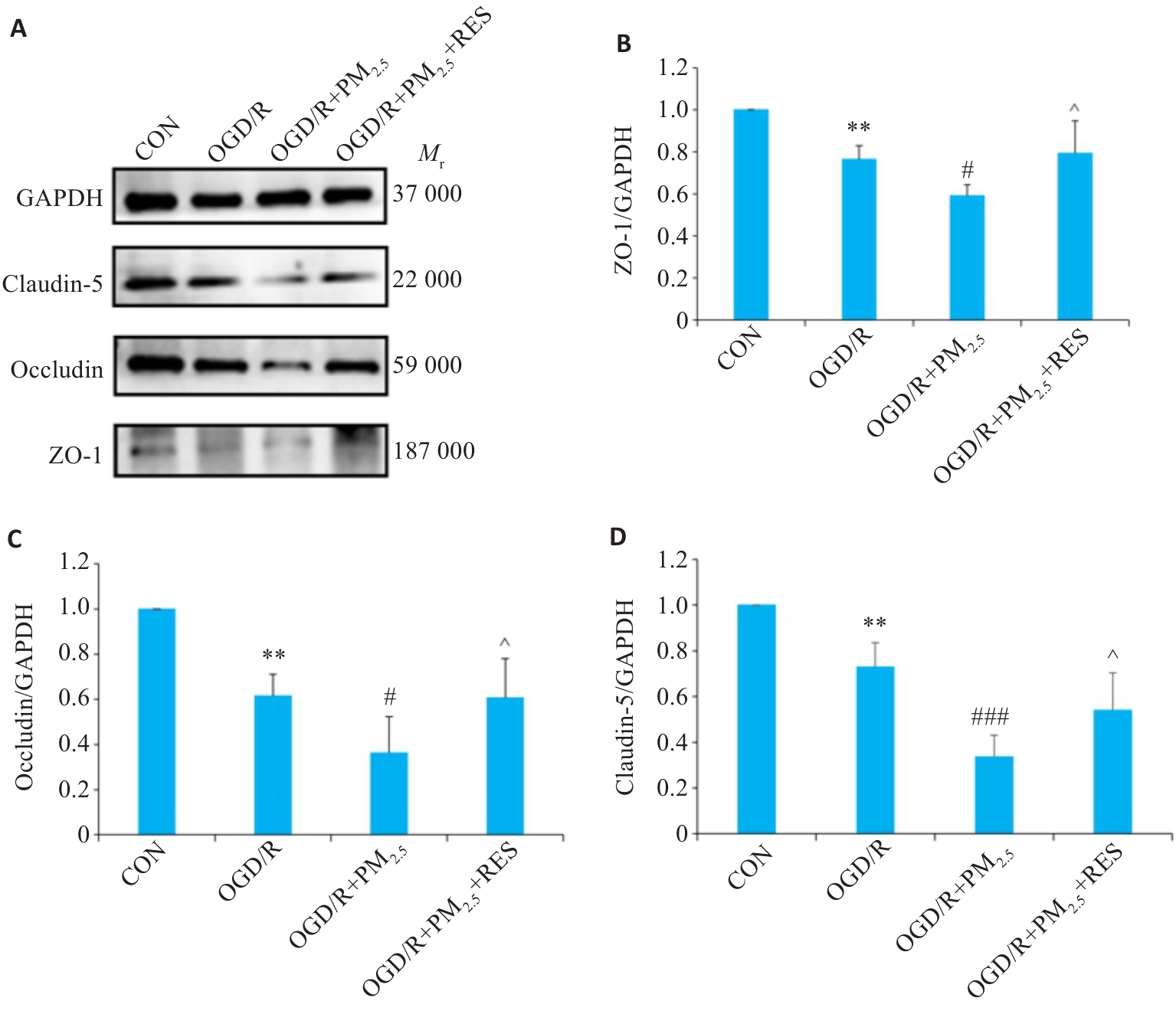
图8 各组ZO-1、Occludin、Claudin-5蛋白表达情况
Fig.8 Expression of ZO-1, occludin, claudin-5 proteins in each group detected by Western blotting. A: Western blots of ZO-1, occludin, claudin-5 and GAPDH. B-D: Expression levels of ZO-1 (B), occludin (C), and claudin-5 (D) proteins normalized by GAPDH levels (Mean±SD, n=5). Data normalized to control (set as 1) and statistical comparisons are conducted on normalized data.**P<0.01 vs CON group; #P<0.05, ###P<0.001 vs OGD/R group; ^P<0.05 vs OGD/R+PM2.5 group.
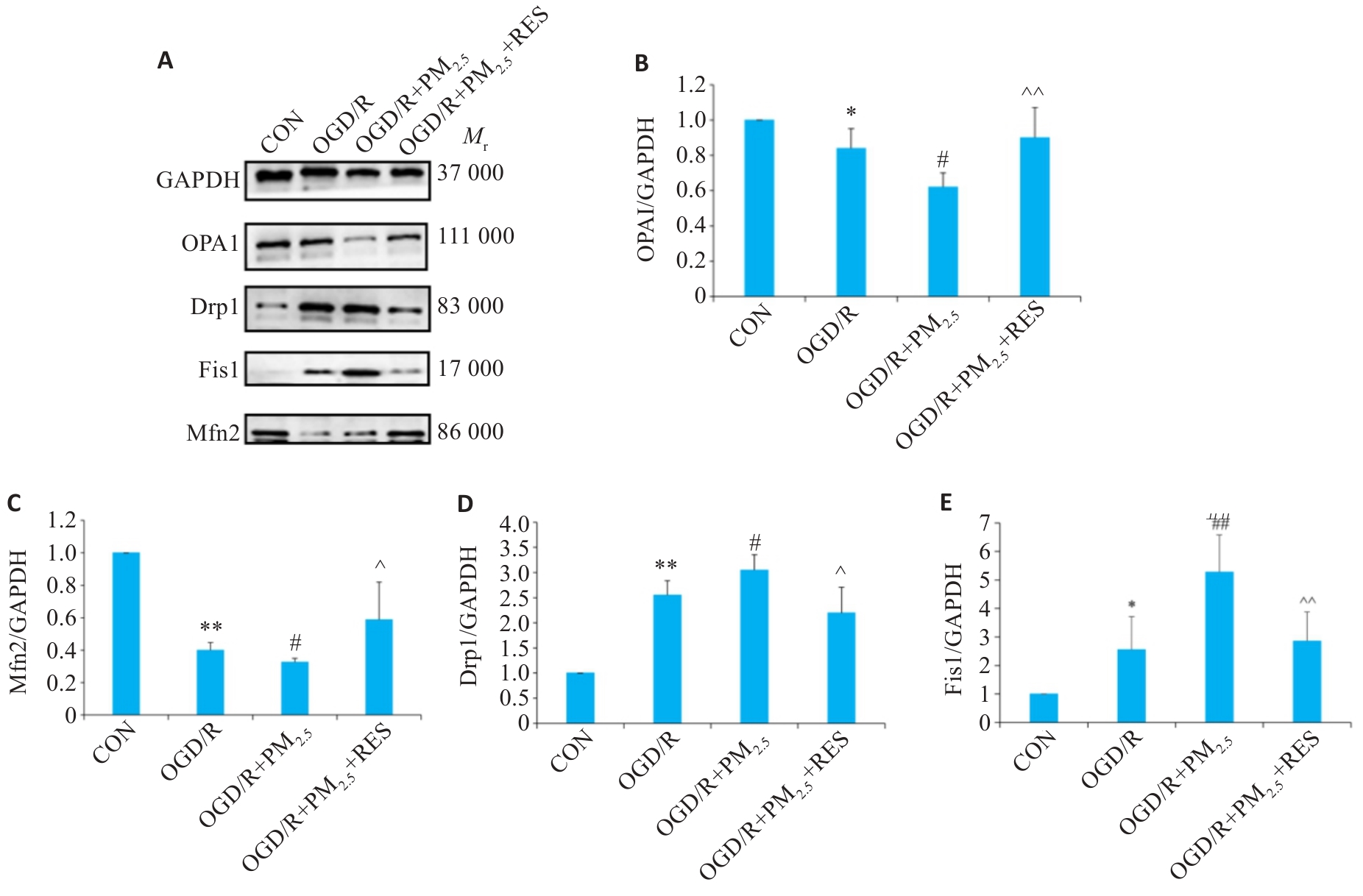
图9 各组OPA1、Mfn2、Drp1和Fis1蛋白表达情况
Fig.9 Expressions of OPA1, Mfn2, Drp1and Fis1 proteins in each group detected by Western blotting. A: Western blots of OPA1, Mfn2, Drp1, Fis1 and GAPDH. B-E: Expression levels of OPA1, Mfn2, Drp1 and Fis1 proteins normalized by GAPDH levels (Mean±SD, n=5). Data normalized to control (set as 1) and statistical comparisons are conducted on normalized data. *P<0.05, **P<0.01 vs CON group; #P<0.05, ##P<0.01 vs OGD/R group; ^P<0.05, ^^P<0.01 vs OGD/R+PM2.5 group.
| [1] | Hendriks S, Ranson JM, Peetoom K, et al. Risk factors for young-onset dementia in the UK biobank[J]. JAMA Neurol, 2024, 81(2): 134-42. doi:10.1001/jamaneurol.2023.4929 |
| [2] | Bennett EE, Song ZW, Lynch KM, et al. The association of long-term exposure to criteria air pollutants, fine particulate matter components, and airborne trace metals with late-life brain amyloid burden in the Atherosclerosis Risk in Communities (ARIC) study[J]. Environ Int, 2023, 180: 108200. doi:10.1016/j.envint.2023.108200 |
| [3] | Stinear CM, Lang CE, Zeiler S, et al. Advances and challenges in stroke rehabilitation[J]. Lancet Neurol, 2020, 19(4): 348-60. doi:10.1016/s1474-4422(19)30415-6 |
| [4] | Li C, van Donkelaar A, Hammer MS, et al. Reversal of trends in global fine particulate matter air pollution[J]. Nat Commun, 2023, 14(1): 5349. doi:10.1038/s41467-023-41086-z |
| [5] | Huang KY, Liang FC, Yang XL, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project[J]. BMJ, 2019, 367: l6720. doi:10.1136/bmj.l6720 |
| [6] | Cao JJ, Shen ZX, Chow JC, et al. Winter and summer PM2.5 chemical compositions in fourteen Chinese cities[J]. J Air Waste Manag Assoc, 2012, 62(10): 1214-26. doi:10.1080/10962247.2012.701193 |
| [7] | Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies[J]. Exp Neurol, 2021, 335: 113518. doi:10.1016/j.expneurol.2020.113518 |
| [8] | Koton S, Pike JR, Johansen M, et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the athero-sclerosis risk in communities cohort study[J]. JAMA Neurol, 2022, 79(3): 271-80. doi:10.1001/jamaneurol.2021.5080 |
| [9] | GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019[J]. Lancet, 2020, 396(10258): 1223-49. |
| [10] | Chen ZZ, Liu PL, Xia XS, et al. The underlying mechanism of PM2.5-induced ischemic stroke[J]. Environ Pollut, 2022, 310: 119827. doi:10.1016/j.envpol.2022.119827 |
| [11] | Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options[J]. Curr Neuropharmacol, 2018, 16(9): 1396-415. doi:10.2174/1570159x16666180302115544 |
| [12] | Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease[J]. Trends Neurosci, 2009, 32(9): 506-16. doi:10.1016/j.tins.2009.05.009 |
| [13] | MohanKumar SMJ, Campbell A, Block M, et al. Particulate matter, oxidative stress and neurotoxicity[J]. Neurotoxicology, 2008, 29(3): 479-88. doi:10.1016/j.neuro.2007.12.004 |
| [14] | Rovira-Llopis S, Bañuls C, Diaz-Morales N, et al. Mitochondrial dynamics in type 2 diabetes: pathophysiological implications[J]. Redox Biol, 2017, 11: 637-45. doi:10.1016/j.redox.2017.01.013 |
| [15] | Li XL, Klaus JA, Zhang J, et al. Contributions of poly(ADP-ribose) polymerase-1 and-2 to nuclear translocation of apoptosis-inducing factor and injury from focal cerebral ischemia[J]. J Neurochem, 2010, 113(4): 1012-22. doi:10.1111/j.1471-4159.2010.06667.x |
| [16] | Owjfard M, Rahimian Z, Karimi F, et al. A comprehensive review on the neuroprotective potential of resveratrol in ischemic stroke[J]. Heliyon, 2024, 10(14): e34121. doi:10.1016/j.heliyon.2024.e34121 |
| [17] | Wang J, Zhu Q, Wang Y, et al. Irisin protects against sepsis-associated encephalopathy by suppressing ferroptosis via activation of the Nrf2/GPX4 signal axis[J]. Free Radic Biol Med, 2022, 187: 171-84. doi:10.1016/j.freeradbiomed.2022.11.024 |
| [18] | Yadav E, Yadav P, Khan MMU, et al. Resveratrol: a potential therapeutic natural polyphenol for neurodegenerative diseases associated with mitochondrial dysfunction[J]. Front Pharmacol, 2022, 13: 922232. doi:10.3389/fphar.2022.922232 |
| [19] | Wang N, Luo ZW, Jin M, et al. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina[J]. Aging (Albany NY), 2019, 11(10): 3117-37. doi:10.18632/aging.101966 |
| [20] | Yin Y, Lv GG, Zhang W, et al. Resveratrol glycoside mediates microglial endoplasmic reticulum stress to mitigate LPS-induced sepsis-associated cognitive dysfunction[J]. Behav Brain Res, 2023, 443: 114326. doi:10.1016/j.bbr.2023.114326 |
| [21] | Çetin R, Bahadir S, Basar İ, et al. Neuroprotective effects of the combined treatment of resveratrol and urapidil in experimental cerebral ischemia-reperfusion injury in rats[J]. Acta Cir Bras, 2024, 39: e395329. doi:10.1590/acb395329 |
| [22] | Sonneville R, Verdonk F, Rauturier C, et al. Understanding brain dysfunction in sepsis[J]. Ann Intensive Care, 2013, 3(1): 15. doi:10.1186/2110-5820-3-15 |
| [23] | Xiao MJ, Xiao ZJ, Yang BB, et al. Blood-brain barrier: more contributor to disruption of central nervous system homeostasis than victim in neurological disorders[J]. Front Neurosci, 2020, 14: 764. doi:10.3389/fnins.2020.00764 |
| [24] | RONALDSON P T, DAVIS T P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity [J]. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2020, 40(): S6-s24. doi:10.1177/0271678x20951995 |
| [25] | Haupt M, Zechmeister B, Bosche B, et al. Lithium enhances post-stroke blood-brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice[J]. Neuropharmacology, 2020, 181: 108357. doi:10.1016/j.neuropharm.2020.108357 |
| [26] | Ni HZ, Li JX, Zheng JY, et al. Cardamonin attenuates cerebral ischemia/reperfusion injury by activating the HIF-1α/VEGFA pathway[J]. Phytother Res, 2022, 36(4): 1736-47. doi:10.1002/ptr.7409 |
| [27] | Wu Q, Liu J, Mao ZG, et al. Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK[J]. Phytomedicine, 2022, 95: 153884. doi:10.1016/j.phymed.2021.153884 |
| [28] | Murata D, Arai K, Iijima M, et al. Mitochondrial division, fusion and degradation[J]. J Biochem, 2020, 167(3): 233-41. doi:10.1093/jb/mvz106 |
| [29] | Zou GP, Yu CX, Shi SL, et al. Mitochondrial dynamics mediated by DRP1 and MFN2 contributes to cisplatin chemoresistance in human ovarian cancer SKOV3 cells[J]. J Cancer, 2021, 12(24): 7358-73. doi:10.7150/jca.61379 |
| [30] | Fu PF, Epshtein Y, Ramchandran R, et al. Essential role for paxillin tyrosine phosphorylation in LPS-induced mitochondrial fission, ROS generation and lung endothelial barrier loss[J]. Sci Rep, 2021, 11(1): 17546. doi:10.1038/s41598-021-97006-y |
| [31] | Lai YX, Lin PQ, Chen ML, et al. Retraction notice to “Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function” [Redox Biol. 34 (2020) 101503[J]. Redox Biol, 2024, 75: 103271. doi:10.1016/j.redox.2024.103271 |
| [32] | Fan LF, He PY, Peng YC, et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis[J]. Free Radic Biol Med, 2017, 112: 336-49. doi:10.1016/j.freeradbiomed.2017.08.003 |
| [33] | Wang SS, Liu ZY, Li R, et al. Acetaldehyde dehydrogenase 2 attenuates lipopolysaccharide-induced endothelial barrier damage by inhibiting mitochondrial fission in sepsis-associated encephalo-pathy[J]. Eur J Pharmacol, 2025, 997: 177468. doi:10.1016/j.ejphar.2025.177468 |
| [34] | Chen CH, Budas GR, Churchill EN, et al. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart[J]. Science, 2008, 321(5895): 1493-5. doi:10.1126/science.1158554 |
| [35] | Haileselassie B, Joshi AU, Minhas PS, et al. Mitochondrial dysfunction mediated through dynamin-related protein 1 (Drp1) propagates impairment in blood brain barrier in septic encephalopathy[J]. J Neuroinflammation, 2020, 17(1): 36. doi:10.1186/s12974-019-1689-8 |
| [36] | Gherardi G, Corbioli G, Ruzza F, et al. CoQ10 and resveratrol effects to ameliorate aged-related mitochondrial dysfunctions[J]. Nutrients, 2022, 14(20): 4326. doi:10.3390/nu14204326 |
| [37] | Chang HC, Tai YT, Cherng YG, et al. Resveratrol attenuates high-fat diet-induced disruption of the blood-brain barrier and protects brain neurons from apoptotic insults[J]. J Agric Food Chem, 2014, 62(15): 3466-75. doi:10.1021/jf403286w |
| [1] | 王静娴, 任自敬, 周佩洋. S1PR5激动与过表达通过调控氧化应激增强脑微血管内皮细胞屏障功能抵抗氧糖剥夺/复氧复糖损伤[J]. 南方医科大学学报, 2025, 45(7): 1451-1459. |
| [2] | 张安邦, 孙秀颀, 庞博, 吴远华, 时靖宇, 张宁, 叶涛. 电针预处理通过调节肠道-大脑轴及Nrf2/HO-1信号通路抑制铁死亡减轻大鼠脑缺血再灌注损伤[J]. 南方医科大学学报, 2025, 45(5): 911-920. |
| [3] | 浦延鹏, 王震, 储浩然. 眼针疗法通过上调METTL3介导的m6A甲基化修饰促进脑皮层血管新生进而改善脑缺血再灌注损伤大鼠的神经功能[J]. 南方医科大学学报, 2025, 45(5): 921-928. |
| [4] | 李泽涵, 梁萌, 韩根成, 张学武. 菊淀粉型巴戟天寡糖降低肺炎链球菌脑膜炎小鼠的症状评分和死亡率[J]. 南方医科大学学报, 2025, 45(3): 577-586. |
| [5] | 陈洁, 刘晨旭, 王春, 李丽, 陶伟婷, 徐婧茹, 唐红辉, 黄丽. 外源性瘦素通过上调星形胶质细胞GLT-1和GLAST的表达减轻小鼠脑缺血再灌注引起的谷氨酸兴奋毒性损伤[J]. 南方医科大学学报, 2024, 44(6): 1079-1087. |
| [6] | 赵倩, 张振, 周星琦, 荣翔宇, 刘雪柔, 赵新丽, 王豪杰, 庞金龙, 李姗姗, 李娴. 川芎增强替莫唑胺对小鼠脑转移黑色素瘤脑的抑制作用[J]. 南方医科大学学报, 2024, 44(6): 1088-1097. |
| [7] | 刘昊铭, 林子诗, 叶 靖. CaMKIIγ和CaMKIIδ通过 PI3K/Akt/Erk 信号通路减轻小鼠神经元缺血再灌注损伤[J]. 南方医科大学学报, 2024, 44(3): 563-570. |
| [8] | 许光明, 高安迪, 丛 斌. 束缚应激通过激活Rho/ROCK通路诱导大鼠杏仁核血脑屏障的损伤[J]. 南方医科大学学报, 2024, 44(3): 411-419. |
| [9] | 桂建军, 孙晓东, 温 舒, 刘 欣, 覃冰清, 桑 明. 白藜芦醇对帕金森病模型小鼠多巴胺能神经元的保护作用:基于抑制TLR4/MyD88/NF-κB通路[J]. 南方医科大学学报, 2024, 44(2): 270-279. |
| [10] | 李新翼, 刘玉杰, 邓克崇, 胡义奎. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-410. |
| [11] | 叶红伟, 张钰明, 云 琦, 杜若丽, 李 璐, 李玉萍, 高 琴. 白藜芦醇可减轻高糖诱导的心肌细胞肥大:基于促进SIRT1表达维持线粒体稳态[J]. 南方医科大学学报, 2024, 44(1): 45-51. |
| [12] | 孙 伟, 陈 平, 唐小杭, 谷颖敏, 田雪松. 改良的四血管间断阻塞法制作大鼠全脑缺血再灌注损伤模型[J]. 南方医科大学学报, 2023, 43(7): 1194-1203. |
| [13] | 牛雯雯, 荣翔宇, 赵 倩, 刘雪柔, 徐廉松, 李姗姗, 李 娴. 酒川芎增强阿美替尼对EGFR突变型非小细胞肺癌脑移植瘤的抑制作用[J]. 南方医科大学学报, 2023, 43(3): 375-382. |
| [14] | 李 晒, 李 丽, 闵思敏, 刘赛赛, 秦志文, 熊志尚, 徐建国, 王博文, 丁渡山, 赵士弟. 大豆异黄酮可减轻大鼠脑缺血/再灌注损伤:基于抑制铁死亡及炎症级联反应[J]. 南方医科大学学报, 2023, 43(2): 323-330. |
| [15] | 曹天然, 刘青芳, 潘美民, 张雪红. LncRNA SNHG8通过抑制miR-494-3p表达减轻脑缺血再灌注损伤[J]. 南方医科大学学报, 2023, 43(12): 2015-2022. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||