南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (9): 1637-1644.doi: 10.12122/j.issn.1673-4254.2024.09.02
收稿日期:2024-09-06
出版日期:2024-09-20
发布日期:2024-09-30
通讯作者:
孟靖昕
E-mail:zhangsen20@mails.ucas.ac.cn;mengjx628@mail.ipc.ac.cn
作者简介:张 森,博士,E-mail: zhangsen20@mails.ucas.ac.cn基金资助:
Yingbo LI, Han BAO, Sen ZHANG( ), Jingxin MENG(
), Jingxin MENG( )
)
Received:2024-09-06
Online:2024-09-20
Published:2024-09-30
Contact:
Jingxin MENG
E-mail:zhangsen20@mails.ucas.ac.cn;mengjx628@mail.ipc.ac.cn
摘要:
循环肿瘤细胞(CTCs)是从肿瘤组织上分离并进入淋巴系统或血液的细胞,虽然在体液中数量极少却与肿瘤的转移和复发密切相关。CTCs包含完整病理信息,可通过对CTCs的分离、富集和分析来提取这些能够指导癌症诊治的信息,从而显著改善癌症的监测效率和预后状况。与传统组织活检相比,使用CTCs作为生物标志物的液体活检技术可以实现肿瘤信息的特异性检测和动态持续检测并减轻患者痛苦。为了实现CTCs的检测,首先必须将CTCs从体液中分离,分离后则需要将其释放富集,本文将详细描述CTCs的响应性分离(包括光、介电泳、声波电泳和磁泳)、化学分离(特异性分子和拓扑结构)和响应性释放(包括光、电、热、pH、酶响应以及可降解基底)的最新研究进展。响应性分离利用CTCs与血细胞物理性质的差异进行分离,化学分离利用特异性识别来捕获CTCs。这些先进的分离和释放的技术具备细胞损伤低和特异性高的特点,为进一步分析提供了条件。目前CTCs检测可应用于多种癌症的检测,本文在最后讨论了CTCs对各种癌症(如肺癌、肝癌、结直肠癌和前列腺癌)的早期诊断和预后评估等方面的作用。
李英博, 包寒, 张森, 孟靖昕. 循环肿瘤细胞的响应性分离、释放和临床应用研究进展[J]. 南方医科大学学报, 2024, 44(9): 1637-1644.
Yingbo LI, Han BAO, Sen ZHANG, Jingxin MENG. Recent advances in responsive isolation, release and clinical application of circulating tumor cells[J]. Journal of Southern Medical University, 2024, 44(9): 1637-1644.
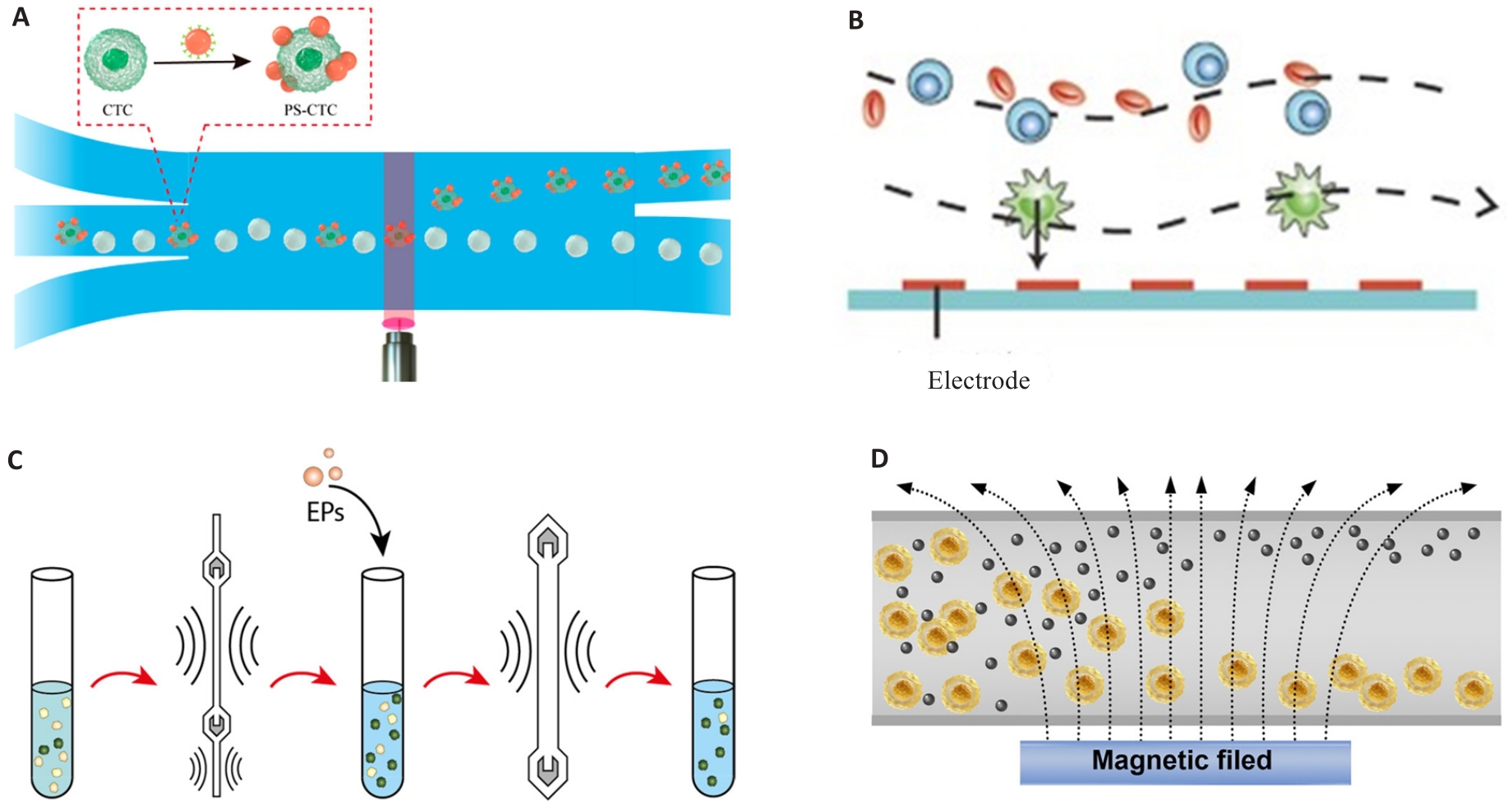
图1 基于物理特征的CTC响应性分离
Fig.1 CTC responsive isolation based on physical characteristics. A: Light manipulation. Reproduced with permission[27]. Copyright 2022, American Chemical Society. B: Dielectrophoresis. Reproduced with permission[28]. Copyright 2015, Wiley-VCH. C: Acoustophoresis. Reproduced with permission[31]. Copyright 2021, American Chemical Society. D: Magnetophoresis. Reproduced with permission[35]. Copyright 2023, Wiley-VCH.
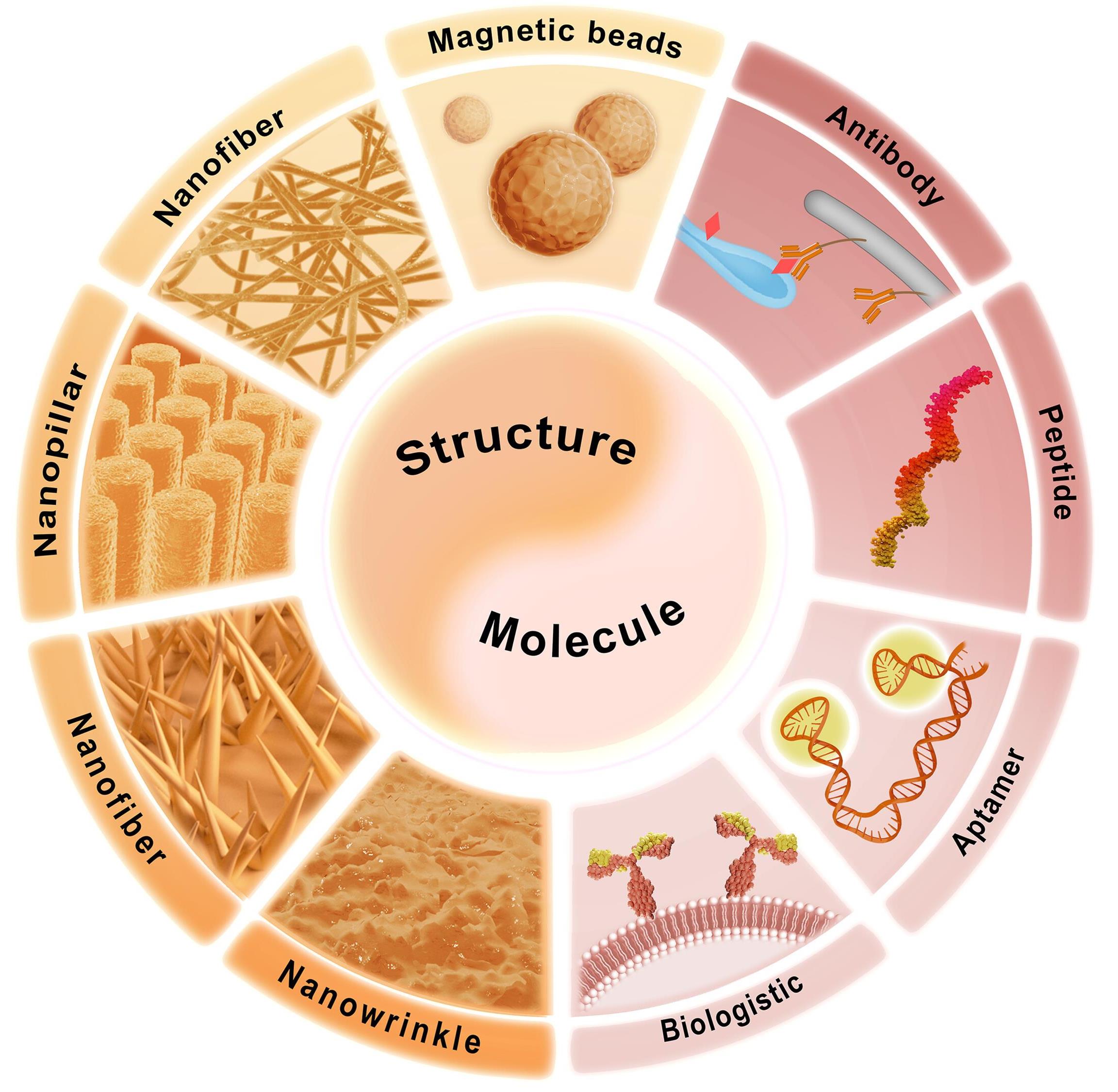
图2 基于化学特征的CTC分离
Fig.2 CTC isolation based on chemical features. The combination of specific molecule and topological structure enables the isolation of CTCs. Specific molecules include antibody, peptide, aptamer, and hybrid cell membrane. The topological structure includes magnetic beads, nanofiber, nanopillar, nanowire, and nanowrinkle. Reproduced with permission[35]. Copyright 2023, Wiley-VCH.
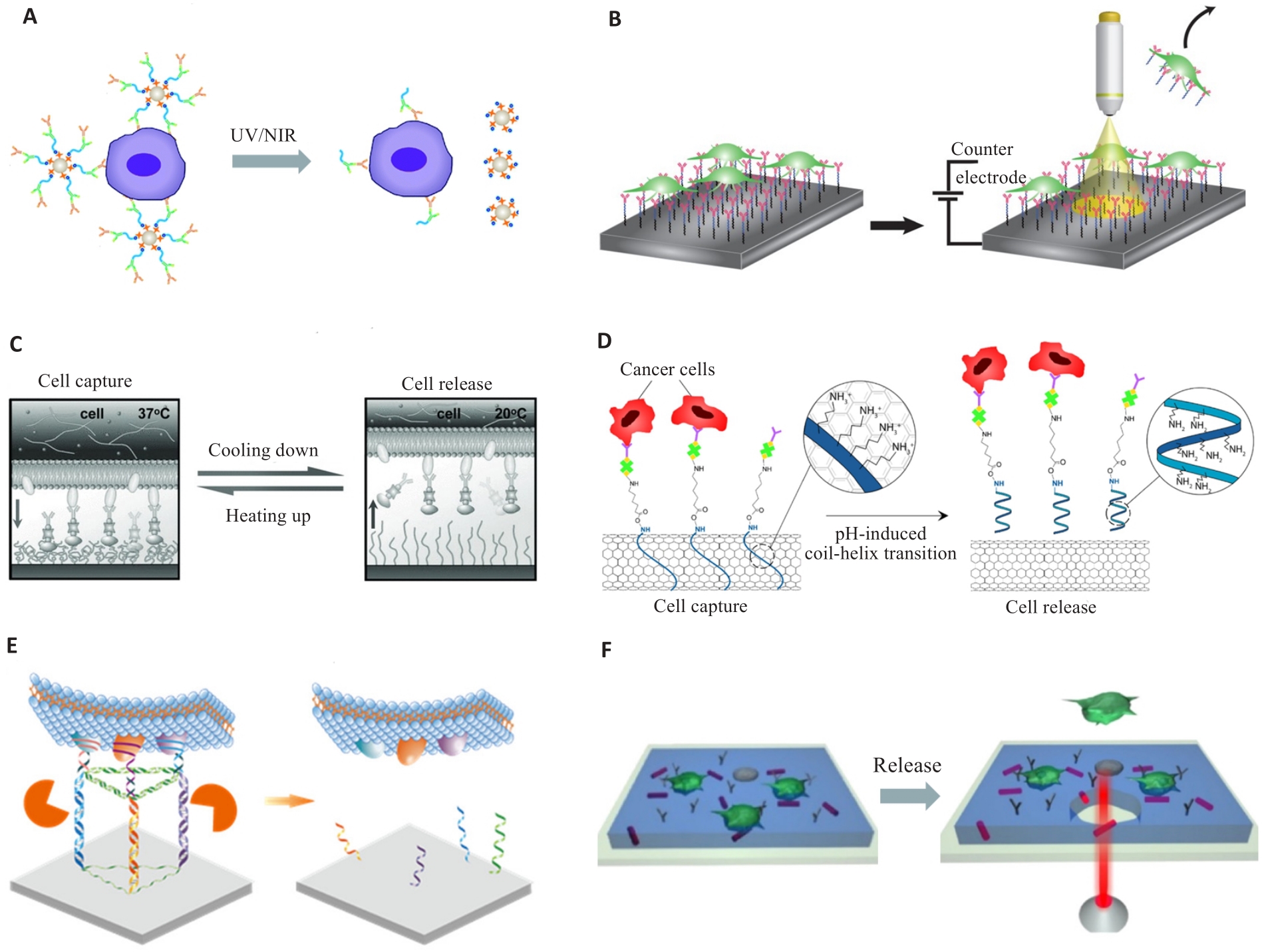
图3 CTC的响应性释放
Fig.3 Responsive release of the CTCs. A: Light-response. Reproduced with permission[72]. Copyright 2015, The Royal Society of Chemistry. B: Electro-response. Reproduced with permission[68]. Copyright 2018, Springer Nature. C: Thermo-response. Reproduced with permission[69]. Copyright 2013, WILEY-VCH. D: pH-response. Reproduced with permission[21]. Copyright 2022, American Chemical Society. E: Enzyme-response. Reproduced with permission[73]. Copyright 2022, American Chemical Society. F: Substrate break. Reproduced with permission[71]. Copyright 2016, American Chemical Society.
| 1 | Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer[J]. Nat Rev Clin Oncol, 2017, 14(9): 531-48. |
| 2 | Nonaka T, Wong DTW. Saliva diagnostics[J]. Annu Rev Anal Chem, 2022, 15(1): 107-21. |
| 3 | Oshi M, Murthy V, Takahashi H, et al. Urine as a source of liquid biopsy for cancer[J]. Cancers, 2021, 13(11): 2652. |
| 4 | Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease-latest advances and implications for cure[J]. Nat Rev Clin Oncol, 2019, 16: 409-24. |
| 5 | De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis[J]. Trends Pharmacol Sci, 2019, 40(3): 172-86. |
| 6 | Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma[J]. Hepatology, 2021, 73(1): 422-36. |
| 7 | Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy[J]. Cancer Discov, 2016, 6(5): 479-91. |
| 8 | Wang BS, Zhang SD, Meng JX, et al. Evaporation-induced rGO coatings for highly sensitive and non-invasive diagnosis of prostate cancer in the PSA gray zone[J]. Adv Mater, 2021, 33(40): e2103999. |
| 9 | Luo Q, Wang CM, Peng BJ, et al. Circulating tumor-cell-associated white blood cell clusters in peripheral blood indicate poor prognosis in patients with hepatocellular carcinoma[J]. Front Oncol, 2020, 10: 1758. |
| 10 | Zeinali M, Lee M, Nadhan A, et al. High-throughput label-free isolation of heterogeneous circulating tumor cells and CTC clusters from non-small-cell lung cancer patients[J]. Cancers, 2020, 12(1): 127. |
| 11 | Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer[J]. N Engl J Med, 2004, 351(8): 781-91. |
| 12 | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis[J]. Science, 2011, 331(6024): 1559-64. |
| 13 | Nel I, Morawetz EW, Tschodu D, et al. The mechanical fingerprint of circulating tumor cells (CTCs) in breast cancer patients[J]. Cancers, 2021, 13(5): 1119. |
| 14 | Katz RL, Zaidi TM, Pujara D, et al. Identification of circulating tumor cells using 4-color fluorescence in situ hybridization: validation of a noninvasive aid for ruling out lung cancer in patients with low-dose computed tomography-detected lung nodules[J]. Cancer Cytopathol, 2020, 128(8): 553-62. |
| 15 | Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions[J]. Cancer Lett, 2007, 253(2): 180-204. |
| 16 | Zhu S, Jiang FT, Han Y, et al. Microfluidics for label-free sorting of rare circulating tumor cells[J]. Analyst, 2020, 145(22): 7103-24. |
| 17 | Luo LA, He YQ. Magnetically driven microfluidics for isolation of circulating tumor cells[J]. Cancer Med, 2020, 9(12): 4207-31. |
| 18 | Min L, Wang BS, Bao H, et al. Advanced nanotechnologies for extracellular vesicle-based liquid biopsy[J]. Adv Sci, 2021, 8(20): e2102789. |
| 19 | Sun N, Yang YY, Miao H, et al. Discovery and characterization of circulating tumor cell clusters in neuroendocrine tumor patients using nanosubstrate-embedded microchips[J]. Biosens Bioelectron, 2022, 199: 113854. |
| 20 | Huang C, Yang G, Ha Q, et al. Multifunctional “smart” particles engineered from live immunocytes: toward capture and release of cancer cells[J]. Adv Mater, 2015, 27(2): 310-3. |
| 21 | Neoh KH, Cheng SKS, Wu HS, et al. pH-responsive carbon nanotube film-based microfluidic chip for efficient capture and release of cancer cells[J]. ACS Appl Nano Mater, 2022, 5(5): 6911-24. |
| 22 | Luo ZY, He Y, Li M, et al. Tumor microenvironment-inspired glutathione-responsive three-dimensional fibrous network for efficient trapping and gentle release of circulating tumor cells[J]. ACS Appl Mater Interfaces, 2023, 15(20): 24013-22. |
| 23 | Hu XJ, Zhu DM, Chen M, et al. Precise and non-invasive circulating tumor cell isolation based on optical force using homologous erythrocyte binding[J]. Lab Chip, 2019, 19(15): 2549-56. |
| 24 | Moon HS, Kwon K, Kim SI, et al. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP)[J]. Lab Chip, 2011, 11(6): 1118-25. |
| 25 | Wu ZZ, Jiang HQ, Zhang LL, et al. The acoustofluidic focusing and separation of rare tumor cells using transparent lithium niobate transducers[J]. Lab Chip, 2019, 19(23): 3922-30. |
| 26 | Liu Y, Zhao WJ, Cheng R, et al. Fundamentals of integrated ferrohydrodynamic cell separation in circulating tumor cell isolation[J]. Lab Chip, 2021, 21(9): 1706-23. |
| 27 | Chen B, Zheng JJ, Gao KF, et al. Noninvasive optical isolation and identification of circulating tumor cells engineered by fluorescent microspheres[J]. ACS Appl Bio Mater, 2022, 5(6): 2768-76. |
| 28 | Qian WY, Zhang Y, Chen WQ. Capturing cancer: emerging microfluidic technologies for the capture and characterization of circulating tumor cells[J]. Small, 2015, 11(32): 3850-72. |
| 29 | Kwizera EA, Sun MR, White AM, et al. Methods of generating dielectrophoretic force for microfluidic manipulation of bioparticles[J]. ACS Biomater Sci Eng, 2021, 7(6): 2043-63. |
| 30 | Montoya Mira J, Sapre AA, Walker BS, et al. Label-free enrichment of rare unconventional circulating neoplastic cells using a microfluidic dielectrophoretic sorting device[J]. Commun Biol, 2021, 4(1): 1130. |
| 31 | Undvall Anand E, Magnusson C, Lenshof A, et al. Two-step acoustophoresis separation of live tumor cells from whole blood[J]. Anal Chem, 2021, 93(51): 17076-85. |
| 32 | Jiang YQ, Chen J, Xuan WP, et al. Numerical study of particle separation through integrated multi-stage surface acoustic waves and modulated driving signals[J]. Sensors, 2023, 23(5): 2771. |
| 33 | Cui MY, Kim M, Weisensee PB, et al. Thermal considerations for microswimmer trap-and-release using standing surface acoustic waves[J]. Lab Chip, 2021, 21(13): 2534-43. |
| 34 | Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma[J]. Int J Cancer, 2013, 133(9): 2165-71. |
| 35 | Bao H, Min L, Bu FQ, et al. Recent advances of liquid biopsy: interdisciplinary strategies toward clinical decision-making[J]. Interdiscip Med, 2023, 1(4): e20230021. |
| 36 | Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology[J]. Nature, 2007, 450(7173): 1235-9. |
| 37 | Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells[J]. N Engl J Med, 2008, 359(4): 366-77. |
| 38 | Riethdorf S, Müller V, Zhang LL, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial[J]. Clin Cancer Res, 2010, 16(9): 2634-45. |
| 39 | Liu H, Wang ZL, Chen CC, et al. Dual-antibody modified PLGA nanofibers for specific capture of epithelial and mesenchymal CTCs[J]. Colloids Surf B Biointerfaces, 2019, 181: 143-8. |
| 40 | Zhang T, Boominathan R, Foulk B, et al. Development of a novel c-MET-based CTC detection platform[J]. Mol Cancer Res, 2016, 14(6): 539-47. |
| 41 | Jia F, Wang YH, Fang ZG, et al. Novel peptide-based magnetic nanoparticle for mesenchymal circulating tumor cells detection[J]. Anal Chem, 2021, 93(14): 5670-5. |
| 42 | Wu ZE, Pan Y, Wang ZL, et al. A PLGA nanofiber microfluidic device for highly efficient isolation and release of different phenotypic circulating tumor cells based on dual aptamers[J]. J Mater Chem B, 2021, 9(9): 2212-20. |
| 43 | Chang ZM, Zhang R, Yang C, et al. Cancer-leukocyte hybrid membrane-cloaked magnetic beads for the ultrasensitive isolation, purification, and non-destructive release of circulating tumor cells[J]. Nanoscale, 2020, 12(37): 19121-8. |
| 44 | Li YY, Lu QH, Liu HL, et al. Antibody-modified reduced graphene oxide films with extreme sensitivity to circulating tumor cells[J]. Adv Mater, 2015, 27(43): 6848-54. |
| 45 | Wang WS, Yang G, Cui HJ, et al. Bioinspired pollen-like hierarchical surface for efficient recognition of target cancer cells[J]. Adv Healthc Mater, 2017, 6(15): 1700003. |
| 46 | Wang ST, Liu K, Liu J, et al. Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers[J]. Angew Chem Int Ed, 2011, 50(13): 3084-8. |
| 47 | Genna A, Vanwynsberghe AM, Villard AV, et al. EMT-associated heterogeneity in circulating tumor cells: sticky friends on the road to metastasis[J]. Cancers, 2020, 12(6): 1632. |
| 48 | Liang NX, Liu L, Li P, et al. Efficient isolation and quantification of circulating tumor cells in non-small cell lung cancer patients using peptide-functionalized magnetic nanoparticles[J]. J Thorac Dis, 2020, 12(8): 4262-73. |
| 49 | Carmona-Ule N, Gal N, Abuín Redondo C, et al. Peptide-functionalized nanoemulsions as a promising tool for isolation and ex vivo culture of circulating tumor cells[J]. Bioengineering, 2022, 9(8): 380. |
| 50 | Wu LL, Wang YD, Xu X, et al. Aptamer-based detection of circulating targets for precision medicine[J]. Chem Rev, 2021, 121(19): 12035-105. |
| 51 | Ling JJ, Liu D, Zhang JL, et al. Thermodynamic and kinetic modulation of microfluidic interfaces by DNA nanoassembly mediated merit-complementary heteromultivalency[J]. ACS Nano, 2022, 16(12): 20915-21. |
| 52 | Li JX, Yuan YG, Gan HY, et al. Double-tetrahedral DNA probe functionalized Ag nanorod biointerface for effective capture, highly sensitive detection, and nondestructive release of circulating tumor cells[J]. ACS Appl Mater Interfaces, 2022, 14(29): 32869-79. |
| 53 | Rao L, Meng QF, Huang QQ, et al. Platelet-leukocyte hybrid membrane-coated immunomagnetic beads for highly efficient and highly specific isolation of circulating tumor cells[J]. Adv Funct Mater, 2018, 28(34): 1803531. |
| 54 | Ronvaux L, Riva M, Coosemans A, et al. Liquid biopsy in glioblastoma[J]. Cancers, 2022, 14(14): 3394. |
| 55 | Jelski W, Mroczko B. Molecular and circulating biomarkers of gastric cancer[J]. Int J Mol Sci, 2022, 23(14): 7588. |
| 56 | Zigeuner RE, Riesenberg R, Pohla H, et al. Isolation of circulating cancer cells from whole blood by immunomagnetic cell enrichment and unenriched immunocytochemistry in vitro [J]. J Urol, 2003, 169(2): 701-5. |
| 57 | Song YY, Dong XF, Shang DY, et al. Unusual nanofractal microparticles for rapid protein capture and release[J]. Small, 2021, 17(36): e2102802. |
| 58 | Xiao YC, Lin LZ, Shen MW, et al. Design of DNA aptamer-functionalized magnetic short nanofibers for efficient capture and release of circulating tumor cells[J]. Bioconjug Chem, 2020, 31(1): 130-8. |
| 59 | Winograd P, Hou S, Court CM, et al. Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors[J]. Hepatol Commun, 2020, 4(10): 1527-40. |
| 60 | Sun N, Zhang C, Wang J, et al. Hierarchical integration of DNA nanostructures and NanoGold onto a microchip facilitates covalent chemistry-mediated purification of circulating tumor cells in head and neck squamous cell carcinoma[J]. Nano Today, 2023, 49: 101786. |
| 61 | Meng JX, Liu HL, Liu XL, et al. Hierarchical biointerfaces assembled by leukocyte-inspired particles for specifically recognizing cancer cells[J]. Small, 2014, 10(18): 3735-41. |
| 62 | Gu CC, Hou T, Zhang SX, et al. Light-driven ultrasensitive self-powered cytosensing of circulating tumor cells via integration of biofuel cells and a photoelectrochemical strategy[J]. J Mater Chem B, 2019, 7(14): 2277-83. |
| 63 | Huang X, Hu XJ, Song S, et al. Triple-enhanced surface plasmon resonance spectroscopy based on cell membrane and folic acid functionalized gold nanoparticles for dual-selective circulating tumor cell sensing[J]. Sensor Actuat B-Chem, 2020, 305: 127543. |
| 64 | Ho LC, Wu WC, Chang CY, et al. Aptamer-conjugated polymeric nanoparticles for the detection of cancer cells through "turn-on" retro-self-quenched fluorescence[J]. Anal Chem, 2015, 87(9): 4925-32. |
| 65 | Chen B, Wang GG, Huang CY, et al. A light-induced hydrogel responsive platform to capture and selectively isolate single circulating tumor cells[J]. Nanoscale, 2022, 14(9): 3504-12. |
| 66 | Li MR, Liu J, Wang XT, et al. Facile preparation of three-dimensional wafer with interconnected porous structure for high-performance capture and nondestructive release of circulating tumor cells[J]. Anal Chem, 2022, 94(43): 15076-84. |
| 67 | Pahattuge TN, Jackson JM, Digamber R, et al. Visible photorelease of liquid biopsy markers following microfluidic affinity-enrichment[J]. Chem Commun, 2020, 56(29): 4098-101. |
| 68 | Parker SG, Yang Y, Ciampi S, et al. A photoelectrochemical platform for the capture and release of rare single cells[J]. Nat Commun, 2018, 9(1): 2288. |
| 69 | Liu HL, Liu XL, Meng JX, et al. Hydrophobic interaction-mediated capture and release of cancer cells on thermoresponsive nanostructured surfaces[J]. Adv Mater, 2013, 25(6): 922-7. |
| 70 | Meng JX, Zhang PC, Zhang FL, et al. A self-cleaning TiO2 nanosisal-like coating toward disposing nanobiochips of cancer detection[J]. ACS Nano, 2015, 9(9): 9284-91. |
| 71 | Lv SW, Liu Y, Xie M, et al. Near-infrared light-responsive hydrogel for specific recognition and photothermal site-release of circulating tumor cells[J]. ACS Nano, 2016, 10(6): 6201-10. |
| 72 | Lv SW, Wang J, Xie M, et al. Photoresponsive immunomagnetic nanocarrier for capture and release of rare circulating tumor cells[J]. Chem Sci, 2015, 6(11): 6432-8. |
| 73 | Liu Y, Lin Z, Zheng ZW, et al. Accurate isolation of circulating tumor cells via a heterovalent DNA framework recognition element-functionalized microfluidic chip[J]. ACS Sens, 2022, 7(2): 666-73. |
| 74 | Mei ZF, Yan J, Qian L, et al. Enrichment of circulating tumor cells of lung cancer and correlation with serum leukomonocyte and tumor biomarkers: a retrospective study[J]. Technol Cancer Res Treat, 2023, 22: 15330338231167827. |
| 75 | Zhou JM, Zhang ZW, Zhou HH, et al. Preoperative circulating tumor cells to predict microvascular invasion and dynamical detection indicate the prognosis of hepatocellular carcinoma[J]. BMC Cancer, 2020, 20(1): 1047. |
| 76 | Pei XM, Wong HT, Ng SSM, et al. The diagnostic significance of CDH17-positive circulating tumor cells in patients with colorectal cancer[J]. Expert Rev Mol Diagn, 2023, 23(2): 171-9. |
| 77 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. |
| 78 | Lindsay CR, Blackhall FH, Carmel A, et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer[J]. Eur J Cancer, 2019, 117: 60-8. |
| 79 | Wang CM, Luo Q, Huang WB, et al. Correlation between circulating tumor cell DNA genomic alterations and mesenchymal CTCs or CTC-associated white blood cell clusters in hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 686365. |
| 80 | Magri V, Marino L, Nicolazzo C, et al. Prognostic role of circulating tumor cell trajectories in metastatic colorectal cancer[J]. Cells, 2023, 12(8): 1172. |
| [1] | 谭茹雪, 包晓樟, 韩亮, 李朝晖, 田男. 基于HOXA9 DNA甲基化的两位点联合预测模型可用于脑膜瘤进展风险的早期筛查[J]. 南方医科大学学报, 2024, 44(11): 2110-2120. |
| [2] | 齐淇, 周征, 马金召, 姚兵, 陈莉. 胚胎植入前遗传学检测结合三代测序在阻断遗传性痉挛性截瘫中的成功应用[J]. 南方医科大学学报, 2024, 44(11): 2184-2191. |
| [3] | 李 莹, 王 倩, 陈小鸟, 席 悦, 杨 建, 刘晓敏, 王远大, 张 利, 蔡广研, 陈香美, 董哲毅. 基于糖尿病视网膜病变的诊断模型对糖尿病肾病有较好诊断效能[J]. 南方医科大学学报, 2023, 43(9): 1585-1590. |
| [4] | 李 静, 殷丽霞, 张 敏, 夏勇生, 左芦根, 刘牧林, 胡建国. 粪便蛋白Luminex液相芯片检测系统构建及其对结直肠肿瘤早期诊断的价值[J]. 南方医科大学学报, 2023, 43(11): 1874-1880. |
| [5] | 邹青清, 王梦虹, 陆紫箫, 赵英华, 冯前进. 基于多序列MRI的3D关系注意力网络预测HLA-B27阴性中轴性脊柱关节病[J]. 南方医科大学学报, 2023, 43(11): 1955-1964. |
| [6] | 程扬眉, 夏 群, 王 俊, 解红娟, 余 奕, 刘海华, 姚志正, 胡金花. 超声S-Detect技术在乳腺肿块诊断中的价值[J]. 南方医科大学学报, 2022, 42(7): 1044-1049. |
| [7] | 高 洁, 张伦军, 彭 珂, 孙 红. 血清肿瘤标志物CEA、CYFRA21-1、SCCAg、NSE、ProGRP在不同病理分型肺癌诊断中的应用价值[J]. 南方医科大学学报, 2022, 42(6): 886-891. |
| [8] | 殷益飞, 李 红, 杨春生, 张敏敏, 黄选东, 李梦夏, 杨蓉西, 张正东. HYAL2基因 DNA 甲基化水平可用于甲状腺良恶性肿瘤的鉴别诊断[J]. 南方医科大学学报, 2022, 42(1): 123-129. |
| [9] | 梁绮红, 陈昭宇, 张 峥, 黄 爽, 安胜利. 一致性评价系数应用于无序多分类资料的效果评价[J]. 南方医科大学学报, 2021, 41(9): 1374-1380. |
| [10] | 孙巧玉, 刘 佳, 樊筱玓, 周咏春, 王效静, 崔 珍. 血浆SEPTIN9甲基化水平检测有助于食管癌诊断和放疗敏感性预测[J]. 南方医科大学学报, 2021, 41(8): 1214-1219. |
| [11] | 王 啸, 黄 鉴, 吉 祥, 珠 珠. 人工智能在结肠息肉检测与分类中的应用[J]. 南方医科大学学报, 2021, 41(2): 310-312. |
| [12] | 范宇斌, 何荣伶, 邹丽君, 孟 婕. 生物标志物在特发性肺纤维化中的临床价值[J]. 南方医科大学学报, 2020, 40(07): 1062-1064. |
| [13] | 李青峰,邢潇丹,冯前进. 基于耦合的卷积-图卷积神经网络的阿尔茨海默病的磁共振诊断方法[J]. 南方医科大学学报, 2020, 40(04): 531-537. |
| [14] | 潘洁琳,姜云萍,占颖莺,左盼莉,方义杰,李绍林,洪国斌. 基于MRI平扫的影像组学模型鉴别软骨肉瘤与内生软骨瘤[J]. 南方医科大学学报, 2020, 40(04): 483-490. |
| [15] | 周惠敏,邱小忠,沈折玉. T1-加权磁共振成像造影剂及其诊疗一体化纳米探针研究进展[J]. 南方医科大学学报, 2020, 40(03): 427-444. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||