2. 重庆医科大学附属第一医院 肾内科,重庆 400016;
3. 重庆医科大学附属第一医院 中西医结合科,重庆 400016
2. Department of Nephrology, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China;
3. Department of Integrated Traditional Chinese and Western Medicine, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
脓毒症易导致急性肾损伤,且与危重症患者的预后密切相关[1-3],其机制与细菌产物激活TLR4/NF-κB信号通路产生大量炎症因子有关[4-7],临床防治困难。细菌产生的脂多糖与TLR4的胞外部分结构结合,而MyD88与TLR4的胞内部分结合,起到信号传递的作用,激活的TLR4/MyD88募集并激活IRAKs,形成复合物结合TRAF6,TRAF6结合下游且激活TAKl,激活NF-κB信号通路,促进NF-κB p65的入核,促进多种炎症因子的产生[8]。因此,采取药物抑制促炎因子的释放是治疗脓毒症相关急性肾损伤的策略之一。而槲皮素可以减少炎症因子缓解神经炎症导致的疼痛,还可以减轻内毒素性心肌损伤[12-13],抑制细胞的凋亡[9-11]。槲皮素可通过抑制氧化应激导致的TLR4激活有关[14]。
既然槲皮素能抑制TLR4的表达改善血管平滑肌细胞钙化,那么槲皮素能否通过抑制TLR4改善脓毒症相关的急性肾损伤?其具体的机制是否与TLR4/NF- κB信号通路有关?因此,本研究拟通过腹腔注射脂多糖构建小鼠的脓毒症急性肾损伤模型,观察槲皮素预处理能否改善脂多糖导致的急性肾功能损害,并观察小鼠血中炎症因子的表达情况,进一步分析TLR4/NF-κB信号通路关键分子TLR4、MyD88、TRAF-6以及核内p65的变化,探讨槲皮素对脂多糖诱导急性肾损伤的影响及其可能的机制,为槲皮素治疗脓毒症相关急性肾损伤提供理论依据。
1 材料和方法 1.1 主要材料和仪器6~8周龄的雄性BALB/c小鼠40只,体质量22~25 g,购自重庆医科大学动物实验中心,动物按照实验动物要求进行适应性饲养1周。槲皮素试剂(美国Sigma,Q4951);TNF-α、IL-1β、IL-6检测用ELISA试剂盒(美国R & D Systems),TLR4、MyD88、TRAF-6、NF-κB p65,Histone-H3,β-actin(美国Santa Cruz)。罗氏自动生化分析仪(Roche Diagnostics, Mannheim, Germany),酶标仪(Bio-RAD, American),半干转印仪(Bio-Rad),垂直电泳仪(Bio-Rad)。
1.2 动物分组及脓毒症小鼠模型的建立脂多糖诱导急性肾损伤模型参照文献[15]进行,将上述40只BALB/c小鼠按照随机数字表法随机分为4组,每组10只。对照组:生理盐水0.2 mL腹腔注射;脂多糖组:腹腔注射等体积脂多糖(15 mg/kg);低剂量组:在脂多糖处理前3 d予以25 mg/(kg · d)槲皮素胃注,实验当天胃注槲皮素1 h后予以腹腔注射脂多糖(15 mg/kg);高剂量组:在脂多糖处理前3 d予以50 mg/(kg·d)的槲皮素胃注,实验当天胃注槲皮素1 h后予以腹腔注射脂多糖(15 mg/kg)。各组小鼠在脂多糖处理24 h后以乙醚麻醉方法处死并采集血液及肾脏组织标本进行相关指标的检测。
1.3 肾脏功能检测脂多糖处理24 h后,处死小鼠,取小鼠腹主动脉血液,静置20 min后2000 r/min充分离心20 min后,用罗氏自动生化分析仪检测小鼠的血肌酐和尿素氮。
1.4 肾脏组织损伤检测取各组小鼠的肾脏,左肾放入4%的多聚甲醛固定,石蜡包埋,切片机切片,用HE染色后在光镜(×200)下观察。采用盲法原则让3位与实验无关的医师观察肾脏病理改变结果,并对肾脏病理损伤按文献[4]进行评定。肾小管损伤定义为:管状上皮增生,刷状缘消失,空泡变性,管型形成以及上皮脱落。急性肾损伤的评分标准分为0~4分,共5个等级:0分为正常肾组织;肾小管受损面积 < 25%评为1分;肾小管受损面积25%~50%评为2分;肾小管受损面积50%~75%评为3分;肾小管受损面积 > 75%评为4分。
1.5 ELISA法检测血清中TNF-α、IL-1β、IL-6浓度测定脂多糖处理24 h后处死小鼠,通过腹主动脉取各组小鼠的血液,静置20 min后2000 r/min充分离心20 min后检测。ELISA法检测血清中TNF-α、IL-1β、IL-6的表达量,实验操作步骤严格按照试剂盒的protocol进行操作。
1.6 Western blot检测各组肾脏胞浆中的TLR4、MyD88、TRAF-6及胞核内NF-κB p65的表达脂多糖处理24 h后收集不同组小鼠的肾脏组织,取等量部分肾脏组织冰上研磨后,采取组织蛋白提取试剂盒进行抽提肾脏组织的胞浆蛋白和胞核蛋白,BCA法蛋白定量后,取等量蛋白上样电泳,电转蛋白至PVDF膜,去脂奶粉封闭后,与一抗TLR4(1:500)、MyD88(1:500)、TRAF-6(1:500)、NF-κB p65(1:500)、β-actin(1:1000)以及Histone-H3(1:1000)室温孵育2 h,然后37 ℃孵育二抗1 h,然后采取ECL发光试剂盒显影成像,然后使用Image J软件分析各蛋白的相对表达。
1.7 统计学处理实验数据采用SPSS16.0进行数据分析,计量资料用均数±标准差表示,多组间均数比较采用单因素方差分析,组间两两比较采用LSD检验,P < 0.05为差异具有统计学意义。
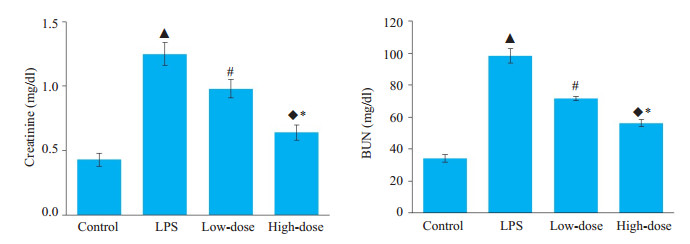
2 结果 2.1 槲皮素可以缓解LPS引起的小鼠肾功能损害结果显示,低剂量组与高剂量组槲皮素预处理的小鼠均可抑制LPS诱导的肌酐、尿素氮的升高,高剂量组的肌酐、尿素氮水平均小于低剂量组各组,各组间比较差异具有统计学意义(P < 0.05,图 1)。
|
图 1 槲皮素对脂多糖诱导的急性肾损伤小鼠血肌酐、尿素氮的影响 Fig.1 Effects of quercetin on BUN and creatinine levels in serum in mice with LPS-induced AKI. ▲P < 0.05 vs Control group; #P < 0.05 vs LPS group; ◆P < 0.05 vs LPS group; *P < 0.05 vs low-dose group |
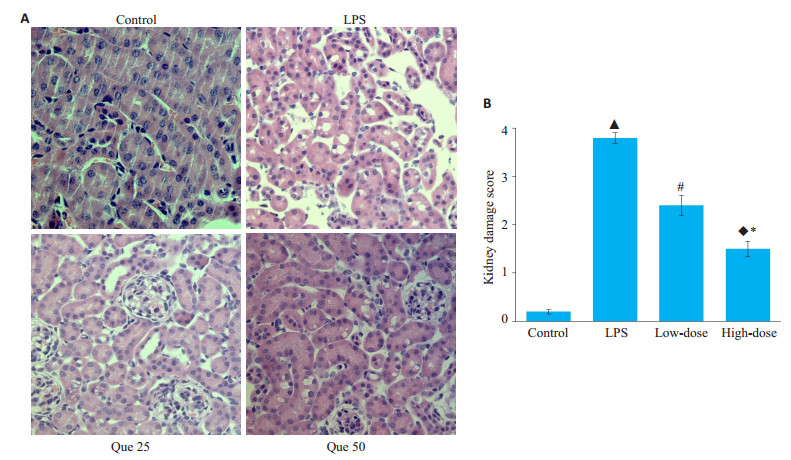
小鼠肾脏HE染色结果发现,脂多糖干预的小鼠肾小管较对照组均有不同程度的损害,低剂量和高剂量的槲皮素预处理均可以改善肾小管损害的评分,高剂量组肾脏损伤评分比低剂量组的肾脏损伤改善更明显,各组间肾小管损伤评分差异具有统计学意义(P < 0.05,图 2)。
|
图 2 槲皮素对脂多糖诱导的急性肾损伤肾脏组织病理改变的作用 Fig.2 Effects of quercetin on histopathological changes in the kidney tissues of mice with LPS-induced AKI (HE staining, original magnification:×200). A: HE staining of the kidney tissues; B: kidney damage score; ▲P < 0.05 vs control group; #P < 0.05 vs LPS group; ◆P < 0.05 vs LPS group; *P < 0.05 vs low dose group |
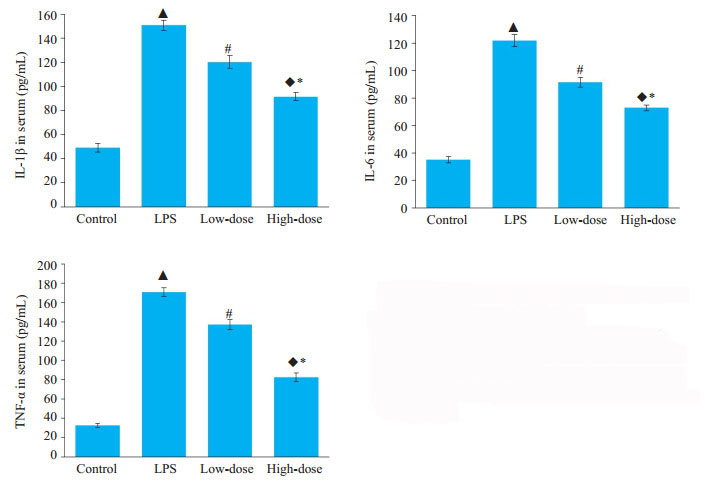
脂多糖诱导组的TNF-α、IL-1β、IL-6的表达高于对照组,低剂量和高剂量的槲皮素预处理均可不同程度的减少以上3种炎症因子在血清中的浓度,高剂量组槲皮素抑制效果更为明显,各组间差异均具有统计学意义(P < 0.05,图 3)。
|
图 3 槲皮素对脂多糖诱导小鼠急性肾损伤血清中TNF-α、IL-1β、IL-6浓度的影响 Fig.3 Inhibitory effects of quercetin on LPSinduced increases in serum TNF-α, IL-6 and IL- 1β levels. ▲ P < 0.05 vs control group; #P < 0.05 vs LPS group; ◆P < 0.05 vs LPS group; *P < 0.05 vs low dose group |
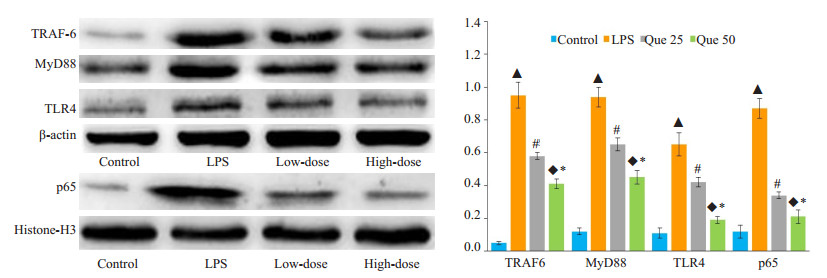
低剂量和高剂量槲皮素对脂多糖诱导的急性肾损伤胞浆中TLR4、MyD88、TRAF-6表达有明显的抑制作用,对胞核内NF-κB p65蛋白的表达也有明显的抑制作用,各组间相对表达量差异具有统计学意义(P < 0.05,图 4)。
|
图 4 Western blotting检测槲皮素对TLR4、MyD88、TRAF-6、和NF-κB p65蛋白表达的影响 Fig.4 Inhibitory effects of quercetin on LPS-induced TLR4, MyD88 and TRAF-6 expressions and NF-κB activation detected by Western blotting. ▲P < 0.05 vs control group; #P < 0.05 vs with LPS group; ◆P < 0.05 vs LPS group; *P < 0.05 vs low dose group |
脓毒症相关急性肾损伤是危重患者最常见的脏器功能损害之一,目前尚无可靠的预防与治疗的药物且预后差[16-20]。研究发现,TLR4是一种模式识别受体,它是脂多糖诱导炎症反应的传感器,其作用是激活会引发炎症的因子[21-23]。进一步激活NF-κB通路,从而导致p65蛋白的入核,增加促炎因子TNF-α、IL-1β、IL-6的表达[24-28]。TLR4/NF-κB信号通路是导致脓毒症相关急性肾损伤的最主要机制之一[15]。槲皮素具有抗炎作用,可以减轻多种疾病的炎症反应,具有抑制炎症相关信号通路的作用[29-30]。研究表明,槲皮素可以抑制氧化应激导致的TLR4的表达改善Ox-LDL诱导的血管平滑肌细胞钙化[14]。基于以上机制,本研究探讨槲皮素对脓毒症相关急性肾损伤是否也能起到保护作用,它对脓毒症相关急性肾损伤的保护作用是否通过抑制TLR4/NF-κB信号通路起作用?
首先,本研究采用脂多糖腹腔注射成功构建了脓毒症相关急性肾损伤的小鼠模型。研究发现不同剂量的槲皮素均可以改善由脂多糖诱导的急性肾损伤,可降低脓毒症小鼠的肌酐和尿素氮的水平,随着槲皮素剂量的增加,肌酐、尿素氮水平下降更为明显。同时,槲皮素可改善脓毒症小鼠的肾小管损害的评分,经不同剂量的槲皮素预处理的小鼠光镜下肾脏的损伤评分均有显著改善,且随着槲皮素剂量的增加,肾损伤的评分改善更加明显。研究结果表明,槲皮素可以减轻脂多糖诱导的小鼠的急性肾损伤,且呈剂量依赖性。
本研究发现不同剂量的槲皮素均可以降低脓毒症小鼠血清中TNF-α、IL-1β、IL-6等炎症因子的产生,而且随着剂量的增加,各炎症因子的水平下降更加显著,具有药物剂量依赖性。有研究发现脓毒症相关急性肾损伤与促炎因子(TNF-α、IL-1β、IL-6)的过度释放有关[31-33]。而本研究发现槲皮素可以减轻脂多糖诱导TNF-α、IL- 1β、IL-6的过度表达,呈现出剂量依赖性,因此,槲皮素可以通过减少TNF-α、IL-1β、IL-6等炎症因子的释放从而减轻脂多糖诱导的小鼠脓毒症相关的急性肾损伤。
在脂多糖诱导的急性肾损伤模型中,TLR4的表达明显增加,采用不同剂量的槲皮素均可以抑制脂多糖诱导的急性肾损伤模型肾脏组织中TLR4的表达,随着剂量的增加抑制效果更为明显。有研究显示MyD88、TRAF-6是LPS激活的TLR4/NF-κB信号通路中关键的蛋白,导致p65入核[5,7]。为探讨槲皮素改善脓毒症相关急性肾损伤的机制,本研究检测经过槲皮素预处理的小鼠肾脏组织中MyD88、TRAF-6蛋白表达情况。实验得出不同剂量的槲皮素均可以明显减少小鼠肾脏组织中MyD88、TRAF-6蛋白表达,减少p65蛋白的入核,从而减少TNF-α、IL-1β、IL-6等促炎因子的生成,进而缓解脂多糖诱导的小鼠急性肾损伤,这与血清中TNF-α、IL- 1β、IL-6浓度减少的结果相一致。通过以上研究初步得出槲皮素抑制促炎因子TNF-α、IL-1β、IL-6的产生减轻急性肾损伤的机制可能与TLR4/NF-κB信号通路受到抑制有关。
综上所述,本研究发现槲皮素可以改善脂多糖诱导的小鼠的肌酐、尿素氮水平,减轻肾小管损伤的评分,改善脂多糖诱导的急性肾损伤。另一方面,槲皮素可以通过下调脂多糖诱导的TLR4表达,然后抑制MyD88、TRAF-6蛋白的表达,从而减少p65的入核,减少血清中促炎因子(TNF-α、IL-1β、IL-6)的生成,从而改善脓毒症相关的急性肾损伤。该研究为今后槲皮素治疗脓毒症相关急性肾损伤提供理论依据,但是槲皮素是不是只通过TLR4/NF-κB通路起作用,以及如何下调TLR4的表达,有待进一步研究。
| [1] |
Skube SJ, Katz SA, Chipman JG, et al. Acute kidney injury and sepsis[J]. Surg Infect, 2018, 19(2): 216-24. DOI:10.1089/sur.2017.261 |
| [2] |
Alobaidi R, Basu RK, Goldstein SL, et al. Sepsis-associated acute kidney injury[J]. Semin Nephrol, 2015, 35(1): 2-11. |
| [3] |
Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies[J]. Curr Opin Crit Care, 2014, 20(6): 588-95. DOI:10.1097/MCC.0000000000000153 |
| [4] |
Shi M, Zeng X, Guo F, et al. Anti-Inflammatory pyranochalcone derivative attenuates LPS-induced acute kidney injury via inhibiting TLR4/NF-κB pathway[J]. Molecules, 2017, 22(10): 1683-95. DOI:10.3390/molecules22101683 |
| [5] |
Zhang L, Sun D, Bao Y, et al. Nerolidol protects against LPS-induced acute kidney injury via inhibiting TLR4/NF-κB signaling[J]. Phytoth Res, 2017, 31(3): 459-65. DOI:10.1002/ptr.v31.3 |
| [6] |
Ye HY, Jin J, Jin LW, et al. Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/ NF-κB signal pathway[J]. Inflammation, 2017, 40(2): 523-9. DOI:10.1007/s10753-016-0498-9 |
| [7] |
Zhou SJ, Wang G, Zhang WB. Effect of TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory distress syndrome in rats, via regulation of macrophage activation and inflammatory response[J]. Exp Ther Med, 2018, 15(4): 3376-84. |
| [8] |
Molteni M, Gemma S, Rossetti C. The role of Toll-Like receptor 4 in infectious and noninfectious inflammation[J]. Mediators Inflamm, 2016, 20(11): 936-49. |
| [9] |
Qu X, Qi D, Dong F, et al. Quercetin improves hypoxia- ischemia induced cognitive deficits via promoting remyelination in neonatal rat[J]. Brain Research, 2014, 1553(12): 31-40. |
| [10] |
Wei X, Meng X, Yuan Y, et al. Quercetin exerts cardiovascular protective effects in LPS-induced dysfunction in vivo by regulating inflammatory cytokine expression, NF-κB phosphorylation and caspase activity[J]. Molecul Cellul Biochem, 2018, 32(4): 1-10. |
| [11] |
Lu J, Zheng Y, Luo L, et al. Quercetin reverses d-galactose induced neurotoxicity in mouse brain[J]. Behav Brain Res, 2006, 171(2): 251-60. DOI:10.1016/j.bbr.2006.03.043 |
| [12] |
李坚, 张剑, 董欣敏, 等. 槲皮素对内毒素性心肌损伤的保护作用及机制[J]. 南方医科大学学报, 2015, 35(7): 1068-72. DOI:10.3969/j.issn.1673-4254.2015.07.27 |
| [13] |
司海超, 司小萌, 刘展. 槲皮素减轻坐骨神经慢性缩窄性损伤大鼠的神经病理性疼痛及其相关机制[J]. 第三军医大学学报, 2017, 39(1): 54-9. |
| [14] |
梁青春, 陈燕亭, 李传翔, 等. 槲皮素调节ROS/TLR4信号通路抑制Ox-LDL诱导的血管平滑肌细胞钙化[J]. 南方医科大学学报, 2018, 38(8): 980-5. |
| [15] |
Fu H, Hu Z, Di X, et al. Tenuigenin exhibits protective effects against LPS-induced acute kidney injury via inhibiting TLR4/NF-κB signaling pathway[J]. Europ J Pharmacol, 2016, 791(6): 229-34. |
| [16] |
Anderberg SB, Luther T, Frithiof R. Physiological aspects of Tolllike receptor 4 activation in sepsis-induced acute kidney injury[J]. Acta Physiolog, 2017, 219(3): 573-88. |
| [17] |
Bagshaw SM, George C, Bellomo R, et al. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units[J]. Crit Care, 2007, 11(3): R68-80. DOI:10.1186/cc5949 |
| [18] |
Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States[J]. Critical Care Med, 2013, 41(5): 1167-74. DOI:10.1097/CCM.0b013e31827c09f8 |
| [19] |
Zarjou A, Agarwal A. Sepsis and acute kidney injury[J]. J Am Soc Nephrol, 2011, 22(6): 999-1006. DOI:10.1681/ASN.2010050484 |
| [20] |
赵娜, 田焕焕, 李志, 等. 脓毒症并发急性肾损伤的危险因素分析与早期诊断[J]. 中华危重病急救医学, 2013, 25(9): 542-5. DOI:10.3760/cma.j.issn.2095-4352.2013.09.009 |
| [21] |
Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury[J]. Mediators Inflamm, 2009, 16(8): 137072-85. |
| [22] |
Huang RS, Zhou JJ, Feng YY, et al. Pharmacological inhibition of macrophage toll-like receptor 4/nuclear factor-kappa B alleviates rhabdomyolysis-induced acute kidney injury[J]. Chin Med J, 2017, 130(18): 2163-9. DOI:10.4103/0366-6999.213406 |
| [23] |
Zhao L, Xu L, Tao X, et al. Protective effect of the total flavonoids from rosa laevigata michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation[J]. Molecules, 2016, 21(7): 185-96. |
| [24] |
Mir SM, Ravuri HG, Pradhan RK, et al. Ferulic acid protects lipopolysaccharide-induced acute kidney injury by suppressing inflammatory events and upregulating antioxidant defenses in Balb/ c mice[J]. Biomed Pharmacoth, 2018, 100(3): 304-15. |
| [25] |
Feng D, Wang Y, Liu Y, et al. DC-SIGN reacts with TLR-4 and regulates inflammatory cytokine expression via NF-κB activation in renal tubular epithelial cells during acute renal injury: DC-SIGN/ TLR-4 regulates NF-κB activation[J]. Clin Experim Immunol, 2018, 191(1): 107-15. DOI:10.1111/cei.2018.191.issue-1 |
| [26] |
Zhang D, Li Y, Liu Y, et al. Paclitaxel ameliorates lipopolysaccharideinduced kidney injury by binding myeloid differentiation protein-2 to block toll-like receptor 4-mediated nuclear factor-B activation and cytokine production[J]. J Pharmacol Experim Therapeut, 2013, 345(1): 69-75. DOI:10.1124/jpet.112.202481 |
| [27] |
Song J, Fan H, Li H, et al. Zingerone ameliorates lipopolysaccharideinduced acute kidney injury by inhibiting Toll-like receptor 4 signaling pathway[J]. Europ J Pharmacol, 2016, 772(2): 108-14. |
| [28] |
Fan HY, Qi D, Yu C, et al. Paeonol protects endotoxin-induced acute kidney injury: potential mechanism of inhibiting TLR4-NF-κB signal pathway[J]. Oncotarget, 2016, 7(26): 39497-510. |
| [29] |
Beckmann DV, Carvalho FB, Mazzanti CM, et al. Neuroprotective role of quercetin in locomotor activities and cholinergic neurotransmission in rats experimentally demyelinated with ethidium bromide[J]. Life Sci, 2014, 103(2): 79-87. DOI:10.1016/j.lfs.2014.03.033 |
| [30] |
檀昕, 程安玮, 孙金月, 等. 茶多酚和槲皮素对炎性脂肪细胞信号通路和炎性因子的影响[J]. 中国农学通报, 2018, 34(5): 25-30. |
| [31] |
Ho AW, Wong CK, Lam CW. Tumor necrosis factor-alpha upregulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways[J]. Immunobiology, 2008, 213(7): 533-44. DOI:10.1016/j.imbio.2008.01.003 |
| [32] |
Leemans JC, Butter LM, Teske GD, et al. The toll interleukin-1 receptor (IL-1R)8/single Ig domain IL-1R-related molecule modulates the renal response to bacterial infection[J]. Infec Immun, 2012, 80(11): 3812-20. DOI:10.1128/IAI.00422-12 |
| [33] |
Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis[J]. Nat Rev Nephrol, 2014, 10(9): 493-503. DOI:10.1038/nrneph.2014.114 |
 2019, Vol. 39
2019, Vol. 39

