2. Cardiovascular Research Center, School of Basic Medical Sciences, Xi'an Jiaotong University Health Science Center, Xi'an 710061, China ;
3. Department of Physiology, Xi'an Medical University, Xi'an 710021, China
2. 西安交通大学医学部 心血管研究中心, 陕西 西安 710061 ;
3. 西安医学院基础医学部生理学教研室, 陕西 西安 710021
Stroke, most commonly ischemic stroke, is the second leading cause of death and the leading cause of long-term disability in adults in China[1]. Loss of regular oxygen and glucose supply even for a short period of time can initiate ischemia/reperfusion injury of the brain, which leads to cell death and permanent brain damage[2, 3]. The imbalance between the generation and clearance of reactive oxygen species (ROS) that causes oxidative damage of neurons is one of the most important factors contributing to ischemia/reperfusion injury of brain tissue[4]. In in vitro cell models of stroke, hypoxia/ reoxygenation simulates the effect of ischemia/ reperfusion to induceneuronal damage via enhanced ROS production[5].
Ample evidence suggests that nicotinamide adenine dinucleotide phosphate-reduced oxidases (Nox) are the major contributors to ROS generation during hypoxia/reoxygenation in different organs[6]. In mammalian tissues, 7 members of the Nox family have been identified and defined by the specific catalytic subunits Nox (Nox1-5) and Duox (Duox1-2). Nox2 (also known as gp91phox) is the major enzyme responsible for production of cellular ROS in brain tissues, although Nox1 and Nox4 also have been shown to play a role [6-8]. In experimental stroke models, pharmacological agents that inhibit Nox function or genetic deletion of the gp91phox subunit were shown to lessen brain ischemic damage[9, 10] and ameliorate complications of thrombo- lytic therapy by protecting the integrity of the blood- brain barrier and reducing edema formation and hemorrhage[11].
Protein tyrosine kinases (PTKs) are primarily classified into receptor PTKs and non-receptor PTKs. The Src family of tyrosine kinases includes a group of classical non-receptor PTKs ubiquitously expressed in neurons at 500-fold higher levels compared with other cell types. Five members of the Src family of PTKs (Src, Fyn, Yes, Lck, and Lyn) are highly expressed in mammalian central nervous system. Src is regulated by many stimuli to generate ROS, including hypoxia/ reoxygenation, stretch, integrins, growth factors, and vasoactive agonists (such as angiotensin II and thrombin), and has been implicated in hypoxia/ reoxygenation injury signaling pathways[12].
Equol [7-hydroxy-3-(4'-hydroxyphenyl)-chroman] is a predominant active metabolite of daidzein (a major isoflavone in soybean)[13], which is generated by the intestinal microbial flora[14]. As a nonsteroidal estrogen, isoflavone displays estrogenic activity via its affinity for estrogen receptor α (ERα) and β (ERβ)[15]. Equol also serves as an antioxidant that is more potent than genistein and daidzein[16-19]. In mice exposed to the chemical carcinogen 7, 12-dimethylbenz(a)anthracene, oral administration of equol (5 or 25 mg/kg) for two weeks prior to the exposure decreased the levels of oxidative stress biomarkers by increasing catalase and superoxide dismutase activities[20]. In addition, intake of oflavone-rich soy or other dietary isoflavones for two weeks was found to decrease the cerebral infarct size in rat models of ischemic stroke[21-23], but the molecular mechanisms underlying the neuroprotective effects of isoflavones and their metabolites are not fully understood.
ROS was reported to induce cell death in cultured neurons[24] and in rat pheochromocytoma neuronal cell line PC12[25]. PC12 cells have been exploited as a cell model for studying neuronal cell death under diverse conditions, including hypoxia[26]. In this study, we aimed to investigate the neuroprotective effect of equol and explore the roles that the signaling molecule Src and the ROS-generating enzyme Nox2 may play in hypoxia/ reoxygenation injury of PC12 cells. Our results demonstrate that equol confers effective neuroprotection against hypoxia/reoxygenation injury in PC12 cells by inhibiting ROS generation, probably by blocking Src phosphorylation-mediated down-regulation of Nox2.
MATERIAL AND METHODS ReagentsMonoclonal mouse antibodies against Src and phospho-Src (Tyr416) were purchased from Upstate Biotechnology (Lake Placid, NY, USA). Mouse antigp91phox antibodies were obtained from BD Transduction LaboratoriesTM (Bedford, MA, USA). Anti-tubulin antibody used for Western blotting was acquired from CMCTAG, Inc (San Diego, CA, USA). Equol powder (50% R-equol, 50% S-equol) was sourced from Nanjing Laiyin Medicine Technology Limited Company (Nanjing, Jiangsu, China). Assay kits for measurement of lactate dehydrogenase (LDH) activity and malondialdehyde (MDA) content were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Dihydroethidine (DHE) and 2', 7'-dichlorofluorescein diacetate (DCF-DA) were ordered from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). Other chemicals used in this study were supplied by Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and treatmentRat pheochromocytoma cell line (PC12) used in this study was provided by Shanghai Institute of Cellular Biology of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin (100 U/mL) and streptomycin (100 mg/mL), and incubated at 37 ℃ in a humidified atmosphere with 5% CO2 in 100 mmdiameter dishes for 48 h. For equol treatment, the cells at 70% confluence were incubated in high-glucose DMEM containing various concentrations of equol for 1 h. Before the culture dishes were transferred into the multigas incubator for hypoxia/reoxygenation treatment, the medium was changed into low-glucose DMEM containing equol, and hypoxia was induced in a multigas incubator at 37 ℃ (1% O2 with 5% CO2 and 94% N2; Thermo Scientific, MA, USA). After a 12-h incubation in hypoxia, the medium was replaced with high-glucose medium containing equol and the dishes were returned to a standard normoxic atmosphere for a 6-h incubation [27]. For cells in the hypoxia/reoxygenation (H/R) model group, no equol was added in the medium, and the cells in the control group were incubated consistently at 37 ℃ in a humidified atmosphere with 5% CO2. The cells exposed to gradient equol doses at 10-9 to 10-5 mol/L following hypoxia/reoxygenation were examined for cell viability using 3-(4, 5-dimethylthiazol- 2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and for LDH activity and MDA content following the protocols described previously[23]. Based on the cell viability results, 10-5 mol/L and 10-6 mol/L of equol were selected as the optimal concentrations for ROS activity measurement and Western blotting experiments.
Cell viability assayAfter pretreatment with the indicated concentrations of equol for 1 h, PC12 cells in 96-well plates were exposed to hypoxia (1% O2) for 12 h followed by incubation in a normoxic atmosphere for 6 h. The cell vitality was detected by incubating the cells with MTT (5 mg/mL) for 4 h at 37 ℃. After the cells were rinsed twice with ice-cold phosphate-buffered saline (PBS), 150 μL of dimethyl sulfoxide was added to each well to dissolve the purple formazan deposit, and the absorbance at 490 nm was measured with a microplate reader.
LDH activity and MDA content measurementAfter pretreatment with various concentrations of equol for 1 h, PC12 cells were exposed to hypoxia/ reoxygenation (12 h/6 h). The culture medium was collected, and assay kits were used to spectrophotometrically determine the activity of LDH at 450 nm. The content of MDA, a biomarker of oxidative stress, was measured at 532 nm according to the manufacturer's instructions.
Measurement of intracellular ROSTo assess the effect of equol on ROS production in PC12 cells subjected to hypoxia/reoxygenation injury, intracellular ROS generation in the exposed cells was measured with the fluorescent probes of dihydroethidium (DHE) and 2', 7'-dichlorofluorescein diacetate (DCF-DA). The cells were washed with PBS, loaded with 10-5 mol/L DHE or 10-5 mol/L DCF-DA, and incubated for 30 min at 37 ℃ in darkness. Differential interference contrast images were obtained using fluorescence microscopy (Olympus IX71, Tokyo, Japan). The fluorescence intensities were quantified with image analysis software (Adobe Photoshop CS3), and the values were presented as the mean fluorescence intensity.
Western blottingPC12 cells exposed to hypoxia/reoxygenation were lysed with ice-cold modified Radio immunoprecipitation assay (RIPA) buffer (containing 6×10-5 mol/L Tris-HCl, 0.25% sodium dodecyl sulphate, 10-3 mol/L sodium fluoride, 10-3 mol/L sodium orthovanadate, 10 μg/mL aprotinin and leupeptin). The cell lysates were centrifuged at 14 000 ×g for 20 min at 4 ℃, and the supernatant was stored at -80 ℃ until use. The protein concentrations in the supernatant were determined using a bicinchoninic acid protein assay. Immunoblotting was performed as described previously[28]. Briefly, the PVDF membranes were incubated with mouse monoclonal antigp91phox antibody (1∶1000 dilution), mouse mono-clonal anti-phospho-Src antibody (Tyr416) (1∶500), or mouse monoclonal anti-Src antibody (1∶500) at 4 ℃ overnight, washed with Tris-buffered saline 0.05% Tween-20, and incubated with horseradish peroxidase-conjugated secondary antibodies (1∶ 5000) for 1 h at room temperature. The bound antibodies were detected with an enhanced chemiluminescence detection system (Amersham, USA) and quantified by densitometry using the Chemigenius Bioimaging System (Syngene, Cambridge, UK). To ensure equal sample loading and to quantify the relative protein expression levels, the ratio of band intensity to tubulin intensity was calculated.
Statistical analysisAll the data were expressed as Mean ± SE. Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Student's t-test for comparison between the group means when appropriate. A P value <0.05 was considered to indicate a statistically significant difference.
RESULTS Equol increases viability of PC12 cells exposed to hypoxia/ reoxygenationMTT assay showed that hypoxia/reoxygenation caused a decreased PC12 cell viability by (47.15±4.1)% compared with the control cells (P<0.01, Fig. 1). Equol pre-treatment at 10-7, and 10-6, and 10-5 mol/L for 1 h protected PC12 cells against hypoxia/reoxygenationinduced injury and significantly increased the cell viability to (82.19±1.03)%, (58.5±3.7)%, and (53.25± 4.7)% , respectively, as compared with that of cells in hypoxia/reoxygenation model group (P<0.05 or 0.01).
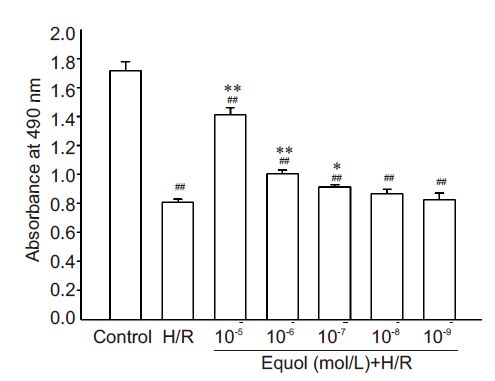
|
Figure 1 Effect of equol on cell viability of PC12 cells exposed to hypoxia/reoxygenation. PC12 cells were pretreated with the indicated concentrations of equol for 1 h and exposed to hypoxia (1% O2) for 12 h and to a normoxic atmosphere for 6 h. The cell viability was detected using MTT assay by measuring the absorbance at 490 nm (n=8). ##P<0.01 vs control; *P< 0.05, **P<0.01 vs hypoxia/reoxygenation (H/R). |
LDH activity and MDA content in the culture medium of PC12 cells were measured to evaluate the effect of equol on the membrane integrity and oxidative stress in cells exposed to hypoxia/reoxygenation injury (Fig. 2). The results showed that LDH activity and malondialdehyde content were both significantly increased in the medium of PC12 cells following the exposure to hypoxia/reoxygenation injury (P<0.01), suggesting severe membrane damage and oxidative stress in the cells. Pre-treatment with 10-7, 10-6, and 10-5 mol/L equol significantly reduced LDH activity and MDA levels in PC12 cells with hypoxia/reoxygenation injury (P<0.01), indicating lessened damage and/or oxidative stress in the neuronal cells.
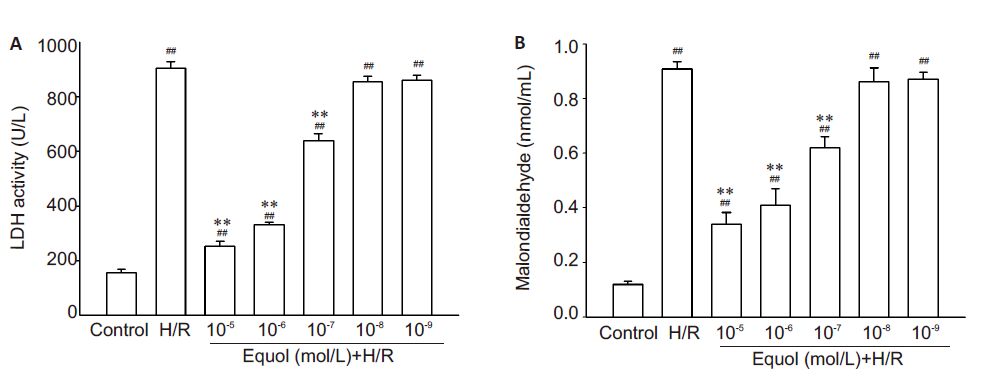
|
Figure 2 Effects of equol on LDH activity and MDA content in PC12 culture medium. PC12 cells were pretreated with the indicated concentrations of equol for 1 h, exposed to hypoxia (1% O2) for 12 h and to a normoxic atmosphere for 6 h. LDH activity (A) and MDA content (B) in the culture medium were determined by spectrophotometry (n=5). ##P<0.01 vs control; **P<0.01 vs H/R. |
Compared with the control cells, PC12 cells exposed to hypoxia/reoxygenation injury exhibited markedly increased ROS activity as indicated by increased fluorescence intensity of the fluorescent probes DHE and DCF-DA (P<0.05 or 0.01 vs control; Fig. 3). Pre-treatment with 10-5 and 10-6 mol/L of equol significantly lowered the fluorescence intensity in the cells exposed to hypoxia/reoxygenation (P<0.01, Fig. 3). These results demonstrate that an increased ROS production is involved in hypoxia/reoxygenationinduced damage in PC12 cells and that the protective effect of equol against this cell damage is mediated very likely by lowering the intracellular ROS level.
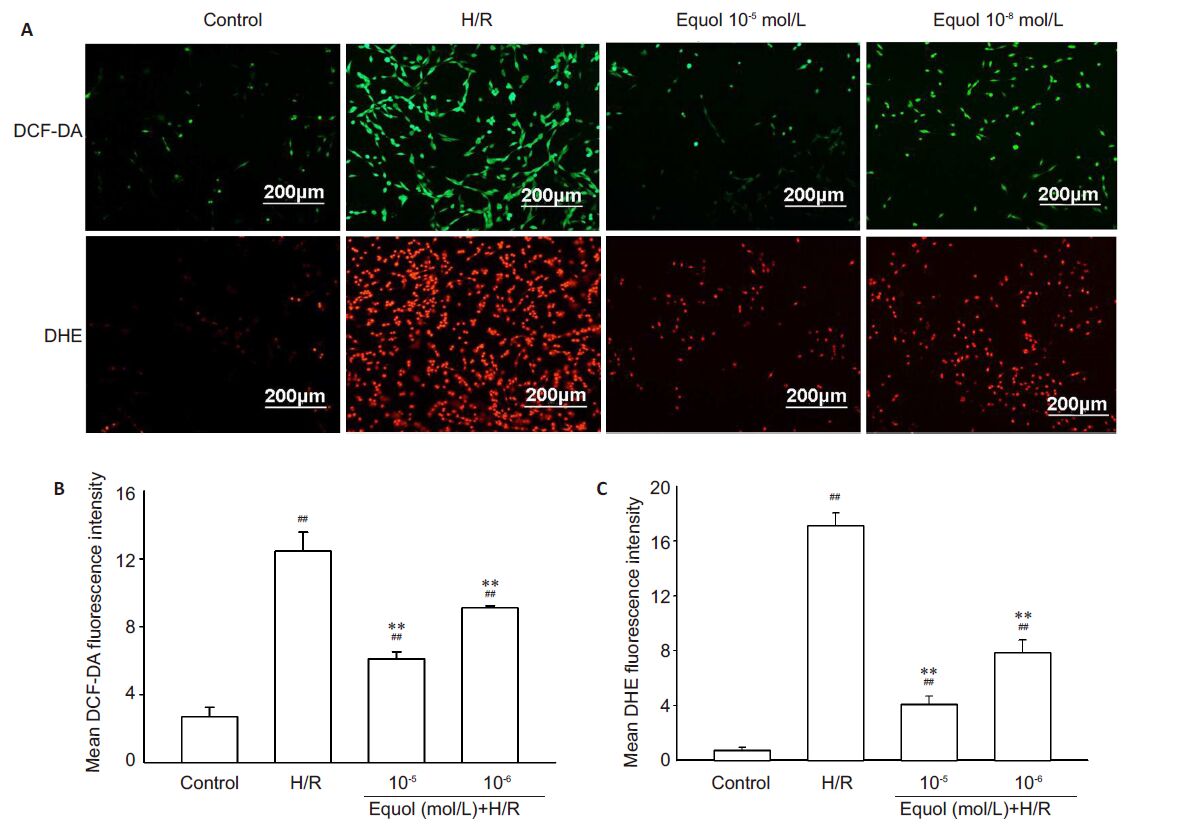
|
Figure 3 Effect of equol on ROS levels in cultured PC12 cells exposed to hypoxia/reoxygenation. PC12 cells were pretreated with the indicated concentrations of equol for 1 h, exposed to hypoxia (1% O2) for 12 h and to a normoxic atmosphere for 6 h. PC12 cells were incubated with 10-5 mol/L DCF-DA or 10-5 mol/L DHE for 30 min in darkness. The fluorescence images show ROS levels (A) detected by DCF-DA (green) and DHE (red) in cultured PC12 cells (scale bar=200 μm). Quantitative analysis of DCF-DA (B) and DHE (C) staining in PC12 cells showed significantly lowered mean fluorescence intensity in equol-treated cells (n=7). ##P<0.01 vs control; **P<0.01 vs H/R. |
To investigate whether equol decreases MDA content and intracellular ROS by directly regulating the expression of the ROS-generating enzyme Nox2 in PC12 cells, Western blotting for gp91phox was performed (Fig. 4). The expression of gp91phox protein was significantly enhanced in PC12 cells exposed to hypoxia/ reoxygenation injury (P<0.01), but this increment in gp91phox protein was significantly antagonized by pretreatment with 10-5 and 10-6 mol/L of equol (P<0.01). These results suggest that the neuroprotective effect of equol (particularly in reducing ROS generation) is likely mediated by antagonizing hypoxia/reoxygenation-ind uced gp91phox up-regulation in PC12 cells.
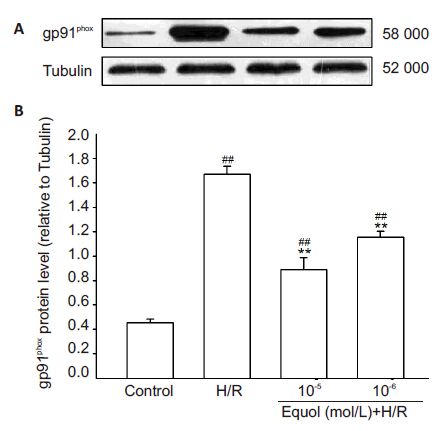
|
Figure 4 Inhibition of hypoxia/reoxygenation-induced up-regulation of gp91phox expression by equol in PC12 cells shown by Western blotting (A) and relative protein level of gp91phox to tubulin (B) (n=4). ##P<0.01 vs control; **P<0.01 vs H/R. |
To understand the role of Src tyrosine kinase in the neuroprotective effect of equol, we measured the levels of phosphorylated Src (p-Src) in PC12 cells exposed to hypoxia/reoxygenation injury using Western blotting (Fig. 5). In PC12 cells exposed to hypoxia/reoxygenation, the level of p-Src (Tyr416) protein was markedly increased (P<0.01 vs control) but was significantly reduced in cells pretreated with 10-5 and 10-6 mol/L of equol (P<0.01). No significant difference was found in the levels of total Src protein expression between the control cells, PC12 cells subjected to hypoxia/ reoxygenation, and equol-pretreated PC12 cells with hypoxia/reoxygenation (Fig. 5A). These results suggest that equol protects against hypoxia/reoxygenation injury by inhibiting Src kinase activity in PC12 cells.
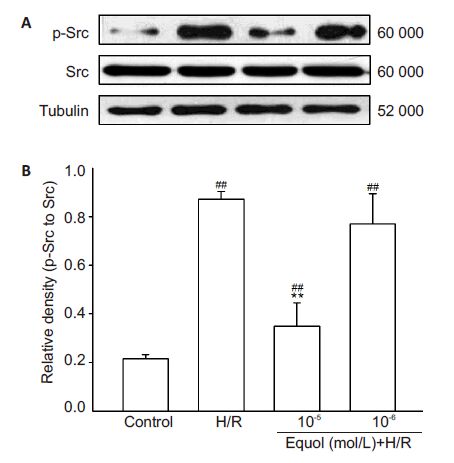
|
Figure 5 Inhibition of hypoxia/reoxygenation-induced upregulation of Src phosphorylation by equol in PC12 cells shown by Western blotting of the total (Src) and phosphorylated Src (p-Src) and tubulin levels (A) and quantification of their relative levels (B) (n=4). ##P< 0.01 vs control; **P<0.01 vs H/R. |
In the present study, we demonstrated that the isoflavone metabolite equol offered strong neuroprotection of PC12 cells against hypoxia/ reoxygenation injury possibly by inhibiting ROS generation, down-regulating the ROS-generating enzyme gp91phox, and inhibiting Src phosphorylation. Previous studies have also demonstrated a more potent antioxidative activity of equol than the main soy isoflavones genistein and daidzein[16-19], suggesting the potential of equol as a useful therapeutic agent. However, only 33% to 50% of healthy adults are capable of producing equol from gut microflora after dietary soy intake[29].
Hypoxic injury has been implicated in many diseases, including stroke and neurodegenerative diseases[30]. Hypoxic cell damage is often exacerbated by reoxygenation [31]. Our study showed that equol significantly inhibited PC12 cell death after hypoxia/ reoxygenation injury. Hypoxia/reoxygenation injury caused substantial oxidative damage in PC12 cells, as shown by significantly increased LDH activity and MDA content in the culture medium. Pretreatment with equol effectively lessened such cell damage in a dosedependent manner, suggesting the protective effects of equol against hypoxia/reoxygenation injury in the cultured neuronal cells.
Richardson et al[32] recently reported that the Rand S-forms of equol were equally effective in attenuating ROS generation and preventing cell death against L-buthionine (S, R)-sulfoximine-induced oxidative insult in cultured fibroblasts with Friedreich's ataxia. The antioxidant effect of equol was mediated by inhibition of superoxide anion production[33, 34]. Hypoxia followed by reoxygenation causes the overproduction of ROS, e.g., the superoxide anion and hydrogen peroxide[35], and our findings show that pre-treatment with equol can effectively suppress hypoxia/ reoxygenation-induced ROS over-production in PC12 cells to attenuate the cellular oxidative stress.
ROS is generated from many sources, including the mitochondrial electron transport system, the Nox, and other cellular oxidant-generating systems in cells exposed to hypoxia or hyperoxia [36-38]. Substantial evidence suggests that Nox2 is the most important Nox to mediate cerebral injury by facilitating the transport of electrons from NADPH to O2, resulting in the generation of superoxide anion[6, 10]. ROS also plays an important role as second messengers to activate such cellular signaling pathways as PTK, MAP kinases, and PLCgamma [38]. The Src family of non-receptor PTK is abundantly expressed in the brain and plays a prominent role in mediating ischemic alteration in neurons. The Src kinase comprises 6 distinct functional domains, and the regulation of its catalytic domain by Tyr416 phosphorylation is critical for its kinase activity[39].
In this context, we examined the effect of equol on Nox2 protein levels in PC12 cells exposed to hypoxia/ reoxygenation injury. The results showed that equol significantly attenuated the increment of gp91phox protein expression in PC12 cells exposed to hypoxia/ reoxygenation, demonstrating that the neuroprotective effect of equol relied on inhibition of Nox2 expression as the main ROS-generating enzymes in the neurons. In addition, the expression level of p-Src was significantly increased in PC12 cells following hypoxia/reoxygenation injury, and was attenuated by pretreatment with equol. We therefore infer that equol alleviates hypoxia/ reoxygenation-induced neuronal injury and cell death partially via suppressing Src activation. In summary, our data demonstrate that equol confers neuroprotection against hypoxia/reoxygenation injury by inhibiting the generation of ROS. The effect is very likely mediated by the inhibition of Src-mediated cell signaling and the down-regulation of the main neuronal ROS generating enzyme gp91phox.
ACKNOWLEDGMENTS: The authors are grateful for substantial support from Professor Chen Huang in the Department of Genetics and Cellular Biology,School of Basic Medical Sciences,Xi'an Jiaotong University Health Science Center.| [1] | Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies[J]. Lancet Neurol,2007, 6 (5) : 456-64. DOI: 10.1016/S1474-4422(07)70004-2. |
| [2] | Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain[J]. J Cereb Blood Flow Metab,2001, 21 (1) : 2-14. |
| [3] | Sun AY, Wang Q, Simonyi A, et al. Resveratrol as a therapeutic agent for neurodegenerative diseases[J]. Mol Neurobiol,2010, 41 (2-3) : 375-83. DOI: 10.1007/s12035-010-8111-y. |
| [4] | Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke[J]. Int J Stroke,2009, 4 (6) : 461-70. DOI: 10.1111/ijs.2009.4.issue-6. |
| [5] | Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+[J]. FEBS J,2005, 272 (14) : 3593-601. DOI: 10.1111/ejb.2005.272.issue-14. |
| [6] | Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox2[J]. Antioxid Redox Signal,2013, 18 (12) : 1400-17. DOI: 10.1089/ars.2012.4721. |
| [7] | Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function[J]. Antioxid Redox Signal,2006, 8 (9-10) : 1583-96. DOI: 10.1089/ars.2006.8.1583. |
| [8] | Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease[J]. Antioxid Redox Signal,2009, 11 (10) : 2481-504. DOI: 10.1089/ars.2009.2578. |
| [9] | Tang LL, Ye K, Yang XF, et al. Apocynin attenuates cerebral infarction after transient focal ischemia in rats[J]. J Int Med Res,2007, 35 (4) : 517-22. DOI: 10.1177/147323000703500411. |
| [10] | Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion[J]. J Cereb Blood Flow Metab,2009, 29 (7) : 1262-72. DOI: 10.1038/jcbfm.2009.47. |
| [11] | Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke[J]. Stroke,2007, 38 (11) : 3000-6. DOI: 10.1161/STROKEAHA.107.489765. |
| [12] | Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function[J]. Antioxid Redox Signal,2011, 15 (6) : 1531-47. DOI: 10.1089/ars.2010.3614. |
| [13] | Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids[J]. Pharmacol Ther,2001, 90 (2-3) : 157-77. DOI: 10.1016/S0163-7258(01)00137-1. |
| [14] | Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavonedaidzein: exploring the relevance to human health[J]. Exp Biol Med (Maywood),2005, 230 (3) : 155-70. |
| [15] | Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta[J]. Biol Pharm Bull,2001, 24 (4) : 351-6. DOI: 10.1248/bpb.24.351. |
| [16] | Mitchell JH, Gardner PT, McPhail DB, et al. Antioxidant efficacy of phytoestrogens in chemical and biological model systems[J]. Arch Biochem Biophys,1998, 360 (1) : 142-8. DOI: 10.1006/abbi.1998.0951. |
| [17] | Rimbach G, De Pascual-Teresa S, Ewins BA, et al. Antioxidant and free radical scavenging activity of isoflavone metabolites[J]. Xenobiotica,2003, 33 (9) : 913-25. DOI: 10.1080/0049825031000150444. |
| [18] | Rüfer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays[J]. J Agric Food Chem,2006, 54 (8) : 2926-31. DOI: 10.1021/jf053112o. |
| [19] | Turner R, Baron T, Wolffram S, et al. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein[J]. Free Radical Res,2004, 38 (2) : 209-16. DOI: 10.1080/10715760310001641854. |
| [20] | Choi EJ, Kim GH. Anticancer mechanism of equol in 7, 12- dimethylbenz(a)anthracene-treated animals[J]. Int J Oncol,2011, 39 (3) : 747-54. |
| [21] | Schreihofer DA, Do DK, Schreihofer AM. High-soy diet decreases infarct size after permanent middle cerebral artery occlusion in female rats[J]. Am J Physiol Regul Integr Comp Physiol,2005, 289 (1) : R103-8. DOI: 10.1152/ajpregu.00642.2004. |
| [22] | Ma Y, Sullivan JC, Schreihofer DA. Dietary genistein and equol (4', 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia[J]. Am J Physiol RegulIntegr Comp Physiol,2010, 299 (3) : R871-7. DOI: 10.1152/ajpregu.00031.2010. |
| [23] | Yu W, Wang Y, Zhou DX, et al. Equol is neuroprotective during focal cerebral ischemia and reperfusion that involves p-Src and gp91phox[J]. Curr Neurovas Res,2014, 11 (4) : 367-77. DOI: 10.2174/1567202611666140908094517. |
| [24] | Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons[J]. J Neurochem,1994, 62 (1) : 376-9. |
| [25] | Vimard F, Nouvelot A, Duval D. Cytotoxic effects of an oxidative stress on neuronal-like pheochromocytoma cells (PC12)[J]. Biochem Pharmacol,1996, 51 (10) : 1389-95. DOI: 10.1016/0006-2952(96)00065-2. |
| [26] | Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor[J]. Proc Natl Acad Sci USA,1976, 73 (7) : 2424-8. DOI: 10.1073/pnas.73.7.2424. |
| [27] | Koo BS, Lee WC, Chung KH, et al. A water extract of Curcuma longa L. (Zingiberaceae) rescues PC12 cell death caused by pyrogallol or hypoxia/reoxygenation and attenuates hydrogen peroxide induced injury in PC12 cells[J]. Life Sci,2004, 75 (9) : 2363-75. |
| [28] | Zhao LM, Zhang W, Wang LP, et al. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels[J]. Pflugers Arch,2012, 464 (6) : 613-21. DOI: 10.1007/s00424-012-1165-0. |
| [29] | Lampe JW, Skor HE, Li S, et al. Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavan equol in premenopausal women[J]. J Nutr,2001, 131 (3) : 740-4. |
| [30] | Risuleo G, Cristofanilli M, Scarsella G. Acute ischemia/hypoxia in rat hippocampal neurons activates nuclear ubiquitin and alters both chromatin and DNA[J]. Mol Cell Biochem,2003, 250 (1-2) : 73-80. |
| [31] | Xiao XQ, Lee NT, Carlier PR, et al. Bis(7)-tacrine, a promising anti- Alzheimer's agent, reduces hydrogen peroxide-induced injury in rat pheochromocytoma cells: comparison with tacrine[J]. Neurosci Lett,2000, 290 (3) : 197-200. DOI: 10.1016/S0304-3940(00)01357-4. |
| [32] | Cheng C, Wang X, Weakley SM, et al. The soybean isoflavonoidequol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells[J]. J Nutr,2010, 140 (1) : 12-7. DOI: 10.3945/jn.109.110981. |
| [33] | Hwang J, Wang J, Morazzoni P, et al. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification[J]. Free Radic Biol Med,2003, 34 (10) : 1271-82. DOI: 10.1016/S0891-5849(03)00104-7. |
| [34] | Fordel E, Thijs L, Martinet W, et al. Anoxia or oxygen and glucose deprivation in SH-SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions[J]. Gene,2007, 398 (1-2) : 114-22. DOI: 10.1016/j.gene.2007.03.022. |
| [35] | Chowdhury AK, Watkins T, Parinandi NL, et al. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells[J]. J Biol Chem,2005, 280 (21) : 20700-11. DOI: 10.1074/jbc.M411722200. |
| [36] | Lambeth JD. NOX enzymes and the biology of reactive oxygen[J]. Nat Rev Immunol,2004, 4 (3) : 181-9. DOI: 10.1038/nri1312. |
| [37] | Ward JP. Point: hypoxic pulmonary vasoconstriction is mediated by increased production of reactive oxygen species[J]. J Appl Physiol (1985),2006, 101 (3) : 993-5. DOI: 10.1152/japplphysiol.00480.2006. |
| [38] | Kamata H, Hirata H. Redox regulation of cellular signaling[J]. Cell Signal,1999, 11 (1) : 1-14. DOI: 10.1016/S0898-6568(98)00037-0. |
| [39] | Tang XN, Cairns B, Kim JY, et al. NADPH oxidase in stroke and cerebrovascular disease[J]. Neurol Res,2012, 34 (4) : 338-45. DOI: 10.1179/1743132812Y.0000000021. |
 2016, Vol. 36
2016, Vol. 36
