2. 南昌大学第一附属医院儿科, 江西 南昌 330006
2. Department of Pediatrics, First Affiliated Hospital of Nanchang University, Nanchang 330006, China
米诺环素是二代半合成四环素类抗生素,以往临床主要利用其抗菌作用而用于痤疮等疾病的治疗。近年来研究发现,除抗菌作用外,该药作为中枢神经系统小胶质细胞抑制剂,在缺血及外伤后脑损伤、脊髓损伤、以及阿尔兹海默症等脑退行性病变等疾病中发挥良好神经保护作用[1]。此外,米诺环素在多种炎性痛及神经病理性痛模型中表现出良好的镇痛作用,主要机制可能与抑制小胶质细胞活化、减少炎性介质的释放和抑制胞内信号通路有关[2-4]。
脊髓背角分布有介导Ih的超极化激活环核苷酸门控阳离子(HCN)通道[5]。Ih具有稳定细胞膜静息电位、参与细胞动作电位编码等功能,对调控神经元兴奋性具有重要作用。脊髓背角Ⅱ层又称胶状质区(SG区),是伤害性信息向中枢传递的初级门户,主要由兴奋性中间神经元组成[6],对疼痛的传入与整合具有重要调控作用。动物模型表明,腹腔或鞘内注射Ih抑制剂ZD7288可减轻部分坐骨神经结扎所致神经病理性痛及福尔马林所致炎性痛,并减少SG神经元自发性兴奋性突触后电流(sESPC)的发放频率,提示抑制突触前Ih可产生良好镇痛作用[7]。在SG区,米诺环素可抑制低电压诱发EPSCs振幅,表明米诺环素通过突触前机制降低SG神经元兴奋性[8]。但是米诺环素对SG神经元突触后机制尤其是作用于HCN通道从而调节其兴奋性尚无报道。因此,我们采用全细胞膜片钳技术,研究米诺环素对大鼠SG神经元Ih的作用。
1 材料与方法 1.1 溶液解剖液s-ACSF(mmol/L:240 sucrose,25 NaHCO3,2.5 KCl,1.25 NaH2PO4 · 2H2O,0.5 CaCl2 · 2H2O,3.5MgCl2 ·6H2O,0.4 ascorbic acid,2 pyruvate,pH=7.4,310 mOsm);细胞外液ACSF(mmol/L:117 NaCl,3.6 KCl,1.2 NaH2PO4·2H2O,2.5 CaCl2·2H2O,1.2 MgCl2·6H2O,25 NaHCO3,11 glucose,0.4 ascorbic acid,2 pyruvate,pH=7.4,310 mOsm);电极内液(mmol/L:130 K-gluconate,5 KCl,0.5 EGTA,10 HEPES,4 Mg-ATP,0.3 Li-GTP,10 phosphocreatine,pH=7.2,300 mOsm);米诺环素、ZD7288 分别用去离子水配置成20 mmol/L、10 mmol/L 的母液,分装后-20 °C 保存,实验当天用ACSF配置成终浓度;neurobiotin 488用电极内液配置,其终浓度为0.1%;ZD7288购自Tocris,K-gluconate购自 Wako,neurobiotin 488 购自VECTOR,余试剂均购自Sigma。
1.2 脊髓标本的制备选取3~5周龄雄性SD大鼠(由江西中医药大学动物中心提供,证书编号2004-0009),腹腔注射乌拉坦(1.2 g/kg)麻醉,用预先充氧(95% O2+5% CO2)的0~4 ℃ s-ACSF行心脏灌流,灌流同时迅速分离脊柱旁肌肉、离断两侧肋骨,取出脊柱并完全浸入s-ACSF中;以腹侧入路切除椎板,以腰骶膨大部为中心取出脊髓(腰1-骶3),剥离脊髓表面的硬脊膜、软脊膜及附着神经根,放置于琼脂块。以胶水将琼脂块及脊髓固定于载物台上,用振动切片机(VT1000 S,Leica)切取300 μm厚的脊髓横/纵切片(纵切片用于形态学研究)。
1.3 记录前准备将脊髓片置于32 ℃充氧的ACSF液体中孵育30 min后,将其移入记录槽(体积约为0.5 mL),以ACSF液体持续灌流,速度约为3~5 mL/min。
1.4 全细胞膜片钳记录实验在室温下(22~25 ℃)进行。用电极拉制仪(P-97,Sutter)将玻璃微电极(TW150F-4,WPI)拉制成充灌电极内液后阻抗为3-6 MΩ的微电极,在电压钳模式下进行全细胞膜片钳(EPC-10 膜片钳放大器及Patchmaster记录软件)记录封接,封接成功后电极阻抗可上升至2~5 GΩ,此过程中进行快、慢电容补偿;破膜后电阻降至200~500 MΩ,对70%的串联电阻进行补偿。全细胞记录状态稳定约5 min后可记录SG神经元Ih:电压钳模式下将细胞钳制在-50 mV,给予细胞一系列刺激时程1000 ms、间隔400 ms的超极化电压刺激(从-60~-130 mV,阶跃为-10 mV)以激活Ih,比较米诺环素给药(3 min)前后Ih的变化情况;Ih幅值为电流到达稳态水平的稳态电流(Iss)与电流激活起始时的瞬间电流(Iins)之差;注入超极化电压刺激15~20 ms后测量Iins以避免漏电流的影响[9-11]。电流钳模式下给予细胞一系列刺激时程1000 ms的超极化电流刺激(从-30~-300 pA,阶跃为-30 pA)可记录到去极化“sag”。在-130 mV电压下,以Ih起始后500 ms的电流变化情况分析其激活时间常数,采用单指数方程拟合:I(h t)=A*exp(-t/τ)+C,式中I(h t)为t时的电流幅值,A为Ih幅值,τ为时间常数,C为Iss[9]。
1.5 SG神经元形态学实验部分细胞电极内液中加入0.1% neurobiotin 488进行全细胞膜片钳记录至少10 min,并通过Meta-Fluo成像系统对所记录神经元进行图像采集。电生理记录完成后,将脊髓切片移入4%多聚甲醛中4 ℃固定1晚,以0.01 mol/L的PBS(含0.02% NaN3)清洗3次后封片,于激光共聚焦显微镜(LSM 700,Zeiss)下观察神经元形态并进行分类[12-13]。
1.6 统计学分析所有数据均以均数±标准差表示,给药前后差异显著 性采用t检验进行分析,以P<0.05为差异有统计学意义。
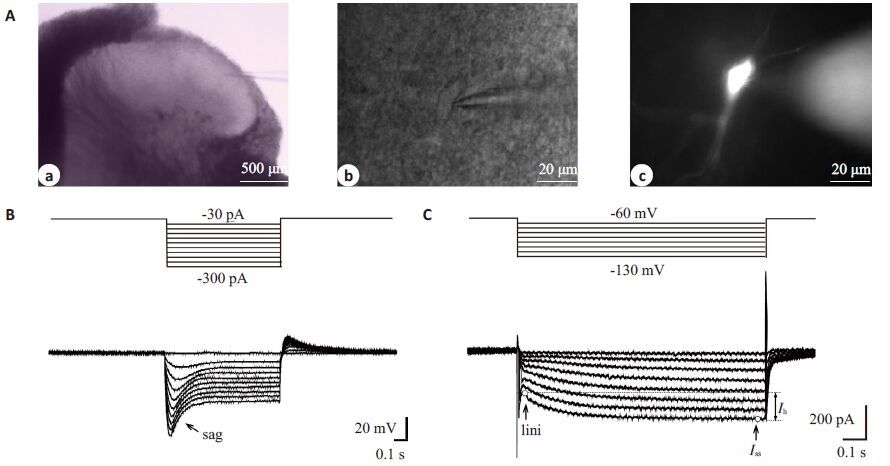
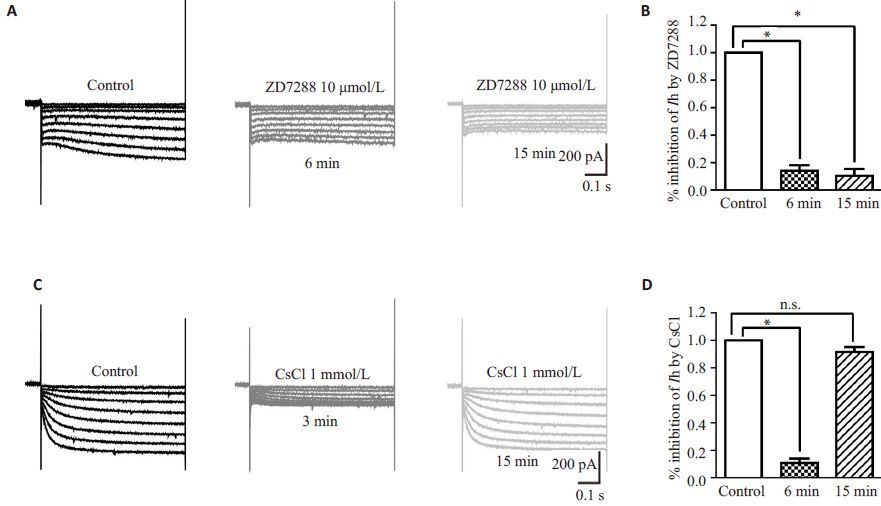
2 结果 2.1 大鼠脊髓SG神经元Ih的电生理学特点低倍显微镜(BX51WI,Olympus)下脊髓背角Ⅱ层为半透明区(图 1Aa);高倍红外微分干涉相差镜(IR-1000,Dage-MIT)下观察该区神经元形态,选择轮廓清晰光滑、立体感好、有相对完好突起的健康神经元进行全细胞膜片钳记录,电极尖端轻触细胞时可在神经元表面形成小凹(图 1Ab)。与以往研究类似[9],在电流钳下,给予一系列超极化电流刺激后,可记录到去极化sag(图 1B);当给同一细胞予电压钳时,可记录到一系列随电压脉冲变化的Ih电流(图 1C)。本实验中,约50%(113/210)的SG神经元可记录到Ih,我们选取表达Ih的细胞进行下一步实验。为确定该内向电流是否为Ih,我们在灌流液中加入HCN 通道非特异阻滞剂CsCl(1 mmol/L,3 min)或特异性阻滞剂ZD7288(10 μmol/L,6 min),发现该电流可显著被阻断,CsCl的阻断作用迅速且可逆,而ZD7288对Ih的阻断作用缓慢持久(图 2)。为排除我们记录的Ih中是否混有其它如钙或钾通道电流,我们在灌流液中分别加入钙及钾通道阻滞剂NiCl2(0.1 mmol/L)、4-AP(1 mmol/L)、TEA(20 mmol/L)及BaCl(2 1 mmol/L),结果发现以上4种药物对Ih无显著影响(表 1),表明我们记录的Ih为HCN通道介导。
|
图 1 大鼠脊髓背角SG神经元显微镜下形态及神经元Ih Figure 1 Morphologic features and representative Ih/sag in SG neurons of rat spinal cord. A: IR-DIC and Meta-Fluo images showing the SG and the position of the recording electrode on a SG neuron in a spinal transverse slice. Ac is the same cell revealed by intracellular dialysis with neurobiotin 488 10 min after whole-cell configuration. B: Voltage sag of SG neuron evoked by a series of hyperpolarizing current steps under current-clamp mode. C: Ih currents activated by 1000 ms hyperpolarizing voltage steps from -60 to -130 mV in -10 mV increment. |
|
图 2 CsCl及ZD788显著抑制SG神经元Ih Figure 2 CsCl and ZD7288 inhibited Ih in SG neurons. A: Representative current traces of Ih measured in ACSF (left), in the presence (middle) and washout (right) of 10 μmol/L ZD7288 for the time indicated. B: Bar graphs showing the effect of ZD7288 on Ih inhibition (n=5). C: Representative current traces of Ih measured in ACSF (left), 3 min after addition of 1 mmol/ L CsCl (middle), or after washout of CsCl (right). D: Bar graphs showing the effect of CsCl on Ih inhibition (n=5). In this and subsequent figures, vertical lines accompanied by bars indicate SEM; *P<0.05; **P<0.01; ***P<0.001; n.s.: No significant difference. |
| 表 1 NiCl2、4-AP、TEA及BaCl2对Ih的作用 Table 1 Effect of NiCl2, 4-AP, TEA and BaCl2 on Ih amplitude |
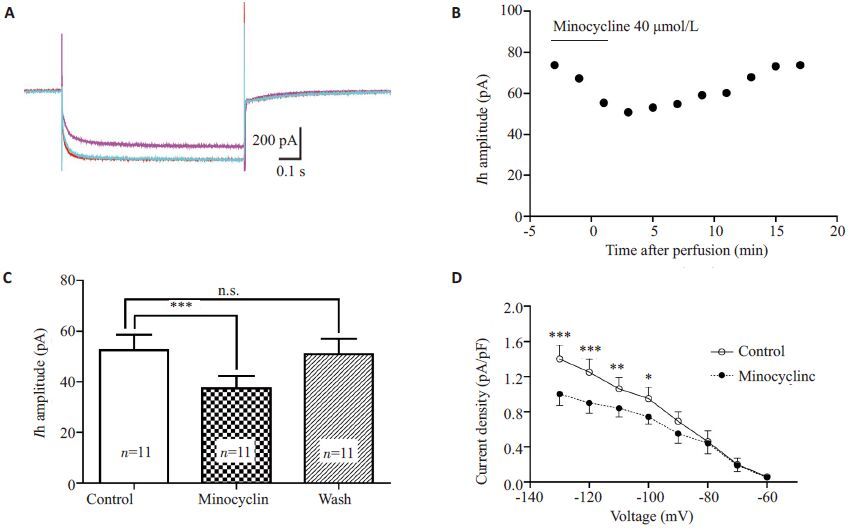
全细胞封接、破膜完成后,给予神经元上述超极化电压刺激,将ACSF灌流时所得Ih记为对照组。以40 μmol/L的米诺环素灌流3 min后Ih幅值逐渐减小,在灌流后1~3 min达到作用峰值,其幅值降至用药前(71±2.4)%(n=11,P<0.001),洗脱后完全恢复(97±1.8)%(图 3)。同时,米诺环素可显著减小钳制电位为-130~-100 mV时的Ih电流密度(图 3D)。
|
图 3 米诺环素40 μmol/L显著抑制SG神经元Ih Figure 3 Minocycline (40 μmol/L) significantly inhibited Ih in SG neurons. A: Representative superimposed Ih traces at -130 mV in the absence (red), presence (purple), and washout (green) of minocycline (40 μmol/L, 3 min) showing that minocycline-induced Ih inhibition was reversible. B: Time course of the effect of minocycline on Ih amplitude. C: Summary of Ih amplitude before, during and after exposure to minocycline (n=11, P<0.001). D: Plot of Ih current density versus membrane potential; the current density (at -130 mV, -120 mV, -110 mV and -100 mV) was significantly decreased compared with the control (n=11). |
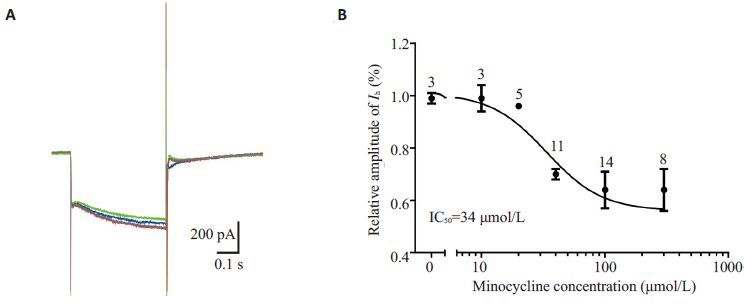
同一神经元在不同米诺环素浓度(1~300 μmol/L)灌流后给予重复超极化刺激,记录所得电流。米诺环素40、100 μmol/L可分别使钳制电压为-130 mV时的Ih幅值降至用药前(71±0.02)%(n=11)、(64±0.24)%(n=14);用Hill 方程进行拟合,得到米诺环素对Ih抑制作用的IC50约为34 μmol/L,Hill相关系数为1.921(图 4)。
|
图 4 米诺环素对SG神经元Ih的抑制呈剂量依赖关系 Figure 4 Minocycline inhibited Ih of SG neurons in a concentration-dependent manner. A: Superimposed Ih traces at -130 mV before (red) and after application of minocycline at different concentrations (blue: 40 μmol/L; green: 100 μmol/L), and washout (gray). B: Dose-response curve for the effects of minocycline on Ih. The curve was fitted by Hill equation (IC50=34 μmol/L): y=Imax/(1 + IC50/x), where x is minocycline concentration (in μmol/L). Each point represents the average of 3 to 14 cells. |
介导Ih电流的HCN通道有4种亚型(HCN1-4),为进一步明确SG表现出Ih的神经元类型及HCN通道亚型,我们对神经元形态及Ih的激活时间常数进行了分析。本实验中,参照Yasaka等的分类方法[12-13],SG神经元根据胞体大小、树突分布及轴突走形等特征分为islet、vertical、central、radial 和unclassified 五大类(图 5A),各类神经元均不同比例地表达Ih(图 5B)。另外,不同形态SG神经元Ih激活时间常数主要集中于40~400(217.2±33.2)ms(图 5C)。
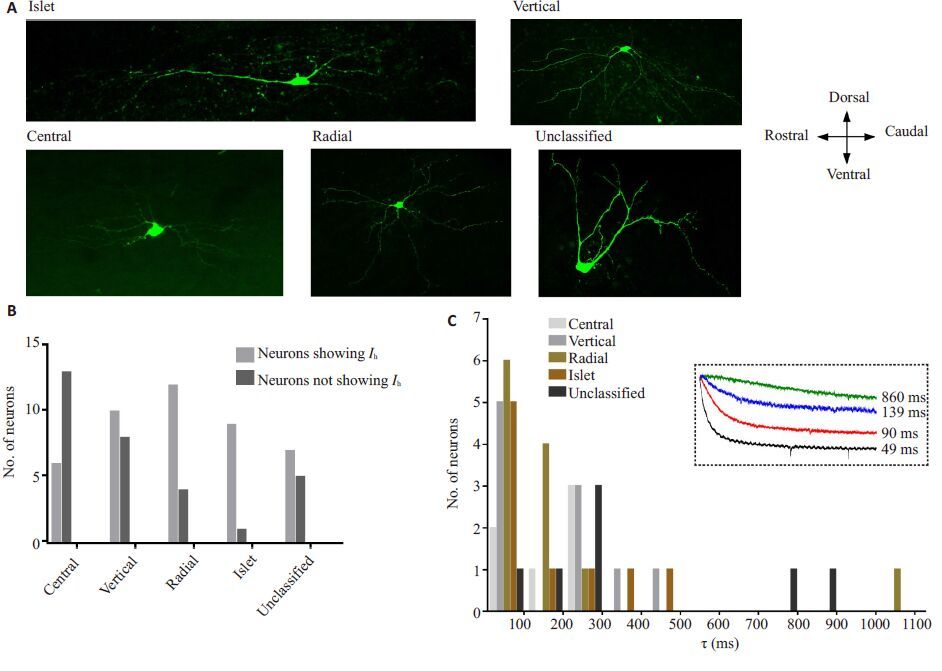
|
图 5 大鼠SG神经元形态分类及各类神经元Ih特性 Figure 5 Morphology of rat SG neurons and properties of Ih in 5 types of SG neurons. A: Representative confocal images showing the morphology of SG neurons which were classified as: islet, vertical, central, radial, and unclassified. Bar=50 μm. B: Histogram showing numbers of neurons showing Ih or not with respect to cell morphological classification. C: Histogram showing τ values (obtained at -130 mV) in the 5 types of rat SG neurons. Dashed inset in C showed representative Ih currents with different τ values. |
本实验以离体大鼠脊髓背角SG神经元为标本,研究了米诺环素对Ih的作用。主要结果有:(1)大鼠脊髓背角SG神经元Ih基本特征与背根神经节(DRG)等其他神经元一致,即能被CsCl、ZD7288所阻断而不为NiCl2、4-AP、TEA、BaCl2等影响;(2)米诺环素对大鼠SG神经元Ih的抑制作用呈可逆性及剂量依赖性;(3)大鼠SG不同形态的神经元均不同比例地表达Ih。
脊髓背角SG区接收、整合并向中枢高级部位投射由外周不同传入神经(主要为C纤维及Aδ纤维)介导的伤害性信息,同时汇聚着来自脑干和大脑皮质的下行抑制性投射神经纤维;其作为“闸门控制理论”的核心组成部分在慢性疼痛的起病与维持过程中发挥至关重要的作用[14]。近年随着方法学的改进、神经科学领域的发展,关于SG神经元的形态、神经递质、功能及细胞间相互联系等的研究不断深入,经典的“闸门控制理论”在受到质疑的同时也被不断补充完善[13, 15-17];但直至今日,SG区在疼痛传递及调制中的重要意义依旧毫无争议。Santos等研究表明,SG神经元中85%为兴奋性中间神经元[6],因此,降低SG神经元的兴奋性,对疼痛治疗具有重大的意义。
Ih是由HCN通道介导的超极化电压激活的内向电流,通过负反馈调节机制发挥稳定细胞膜静息电位、整合突触信息等功能,并参与中脑导水管周围灰质、DRG等痛觉传导区域神经元兴奋性的调控;Ih振幅、频率及激活动力学特性的改变可能是病理痛外周及中枢敏化的机制之一[18-19]。Yasaka及Tadros等[13, 20]的电生理研究对SG区Ih的比例进行了报道,尽管他们的研究结果存在差异,但都提示该区域Ih的表达与部分神经元功能活动调节密切相关。本研究中,SG区表现Ih的神经元比例约占50%左右,且五种不同形态类型的SG神经元均存在Ih。以往研究指出,SG区islet、radial神经元分别为抑制性和兴奋性中间神经元,vertical神经元则以兴奋性为多(约80%),而central 及unclassified 中两种类型神经元比例相近[13]。因此,我们认为,虽Ih在各类神经元中均有表达,但参照以往的文献,本研究中表达Ih的SG神经元仍以兴奋性神经元为主。疼痛状态下Ih参与调节SG神经元兴奋性的改变,鞘内或静脉注射ZD7288可有效缓解神经病理性痛或炎性痛小鼠的机械及热痛敏,体外灌流ZD7288可减少SG神经元兴奋性突触后电流频率[7]。由此推测,抑制SG神经元Ih进而改变动作电位发放可能会发挥良好的中枢镇痛作用。
近年研究发现,小胶质细胞的激活是神经病理性痛发生发展的必要条件,其抑制剂米诺环素在多种疼痛动物模型中的良好镇痛效应已得到验证[2]。同时,米诺环素的临床镇痛疗效在最新一项对坐骨神经痛患者进行的临床试验中亦已证实[21]。大量研究通过应用免疫组化、Western、PCR等技术方法指出,米诺环素可通过抑制脊髓背角小胶质细胞活化[2]、减少TNF-α、IL-1β及脑源性神经营养因子等多种炎性介质的表达及释放[3, 22]、抑制p38 MAPK、JNK等多条胞内信号通路[4, 23]而产生镇痛作用。除作用于胶质细胞外,近年有学者发现米诺环素可直接作用于神经元并调节其兴奋性。鞘内注射米诺环素可通过降低炎性痛大鼠SG神经元EPSCs频率而抑制动物热痛敏反应[24]。我们的前期研究显示,米诺环素可阻断炎性介质IL-1β对兴奋性神经递质AMPA诱发电流的增强作用、降低NMDA诱导电流峰值振幅[25]。
本实验中,米诺环素可减低Ih幅值、减小Ih电流密度,且该作用呈剂量依赖性,IC50约为34 μmol/L;我们推测对SG区HCN通道的抑制可能是其镇痛的作用机制之一。由于米诺环素抑制Ih作用迅速且呈可逆性,我们认为与以往报道的米诺环素对胶质细胞的抑制作用无关。近年免疫组化实验证实,HCN通道的四种亚型在脊髓背角均有分布,其中SG区又以HCN2 为主[5, 26]。Santoro 等应用PCR 分析脊髓背角HCN 各亚型的mRNA表达情况后发现,该区HCN2表达量最多,其次为HCN1[27]。而不同通道介导的Ih激活时间常数存在明显差异,HCN1激活时间常数最快、HCN4最慢[28]。本研究中,约40% Ih的激活时间常数较为迅速,为HCN1介导。50%相对缓慢,可能由HCN2介导。因此,我们初步推测米诺环素可作用于SG神经元HCN1、HCN2 通道,从而对疼痛进行调控,但其对HCN通道作用的确切亚型及具体胞内、胞外位点有待进一步研究。
本研究首次阐明了米诺环素对脊髓背角SG神经元Ih的抑制作用,不仅有助于进一步了解米诺环素镇痛的脊髓机制,而且为其临床应用提供新的理论依据。
| [1] | Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic[J]. Br J Pharmacol,2013, 169 (2) : 337-52. DOI: 10.1111/bph.12139. |
| [2] | Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat[J]. Mol Pain,2012, 8 : 43. DOI: 10.1186/1744-8069-8-43. |
| [3] | Amin B, Hajhashemi V, Hosseinzadeh H, et al. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat[J]. Neuroscience,2012, 224 : 15-25. DOI: 10.1016/j.neuroscience.2012.07.058. |
| [4] | Chang YW, Waxman SG. Minocycline attenuates mechanical allodynia and central sensitization following peripheral seconddegree burn injury[J]. J Pain,2010, 11 (11) : 1146-54. DOI: 10.1016/j.jpain.2010.02.010. |
| [5] | Papp I, Szucs P, Holló K, et al. Hyperpolarization-activated and cyclic nucleotide-gated cation Channel subunit 2 ion channels modulate synaptic transmission from nociceptive primary afferents containing substance P to secondary sensory neurons in laminae I-IIo of the rodent spinal dorsal Horn[J]. Eur J Neurosci,2006, 24 (5) : 1341-52. DOI: 10.1111/ejn.2006.24.issue-5. |
| [6] | Santos SF, Rebelo S, Derkach VA, et al. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat[J]. J Physiol,2007, 581 . |
| [7] | Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain[J]. Pain,2010, 151 (1) : 87-96. DOI: 10.1016/j.pain.2010.06.020. |
| [8] | Cho IH, Chung YM, Park CK, et al. Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat[J]. Brain Res,2006, 1072 (1) : 208-14. DOI: 10.1016/j.brainres.2005.12.039. |
| [9] | Gao LL, Mcmullan S, Djouhri L, et al. Expression and properties of hyperpolarization-activated current in rat dorsal root ganglion neurons with known sensory function[J]. J Physiol,2012, 590 (19) : 4691-705. DOI: 10.1113/jphysiol.2012.238485. |
| [10] | Liu Z, Bunney EB, Appel SB, et al. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C[J]. J Neurophysiol,2003, 90 (5) : 3201-12. DOI: 10.1152/jn.00281.2003. |
| [11] | Kwak J. Capsaicin blocks the Hyperpolarization-Activated inward currents via TRPV1 in the rat dorsal root ganglion neurons[J]. Exp Neurobiol,2012, 21 (2) : 75-82. DOI: 10.5607/en.2012.21.2.75. |
| [12] | Yasaka T, Kato G, Furue H, et al. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal Horn in vitro[J]. J Physiol,2007, 581 (2) : 603-18. DOI: 10.1113/jphysiol.2006.123919. |
| [13] | Yasaka T, Tiong SY, Hughes DI, et al. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal Horn revealed by a combined electrophysiological and anatomical approach[J]. Pain,2010, 151 (2) : 475-88. DOI: 10.1016/j.pain.2010.08.008. |
| [14] | 王群, 吕岩. 疼痛特异性学说与闸门控制学说: 争论还在持续[J]. 中国疼痛医学杂志,2014, 9 (9) : 609-13. |
| [15] | Todd AJ. Neuronal circuitry for pain processing in the dorsal Horn[J]. Nat Rev Neurosci,2010, 11 (12) : 823-36. DOI: 10.1038/nrn2947. |
| [16] | Lu Y, Dong H, Gao Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia[J]. J Clin Invest,2013, 123 (9) : 4050-62. DOI: 10.1172/JCI70026. |
| [17] | Braz J, Solorzano C, Wang X, et al. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control[J]. Neuron,2014, 82 (3) : 522-36. DOI: 10.1016/j.neuron.2014.01.018. |
| [18] | Du L, Wang SJ, Cui J, et al. The role of HCN channels within the periaqueductal gray in neuropathic pain[J]. Brain Res,2013, 1500 : 36-44. DOI: 10.1016/j.brainres.2013.01.035. |
| [19] | Emery EC, Young GT, Berrocoso EM, et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain[J]. Science,2011, 333 (648) : 1462-6. |
| [20] | Tadros MA, Harris BM, Anderson WB, et al. Are all spinal segments equal: intrinsic membrane properties of superficial dorsal Horn neurons in the developing and mature mouse spinal cord[J]. J Physiol,2012, 590 . |
| [21] | Sumracki NM, Hutchinson MR, Gentgall M, et al. The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica[J]. PLoS One,2012, 7 (6) . |
| [22] | Zhang X, Xu YM, Wang J, et al. The effect of intrathecal administration of glial activation inhibitors on dorsal Horn BDNF overexpression and hind paw mechanical allodynia in spinal nerve ligated rats[J]. J Neural Transm,2012, 119 (3) : 329-36. DOI: 10.1007/s00702-011-0713-7. |
| [23] | Zhuang ZY, Wen YR, Zhang DR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance[J]. J Neurosci,2006, 26 (13) : 3551-60. DOI: 10.1523/JNEUROSCI.5290-05.2006. |
| [24] | Huang CY, Chen YL, Li AH, et al. Minocycline, a microglial inhibitor, blocks spinal CCL2-induced heat hyperalgesia and augmentation of glutamatergic transmission in substantia gelatinosa neurons[J]. J Neuroinflammation,2014, 11 : 7. DOI: 10.1186/1742-2094-11-7. |
| [25] | Liu T, Jiang CY, Fujita T, et al. Enhancement by interleukin-1 β of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal Horn neurons[J]. Mol Pain,2013, 9 : 16. DOI: 10.1186/1744-8069-9-16. |
| [26] | Antal M, Papp I, Bahaerguli N, et al. Expression of hyperpolarization-activated and cyclic nucleotide-gated cation Channel subunit 2 in axon terminals of peptidergic nociceptive primary sensory neurons in the superficial spinal dorsal Horn of rats[J]. Eur J Neurosci,2004, 19 (5) : 1336-42. DOI: 10.1111/ejn.2004.19.issue-5. |
| [27] | Santoro B, Chen S, Luthi A, et al. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS[J]. J Neurosci,2000, 20 (14) : 5264-75. |
| [28] | Jiang YQ, Sun Q, Tu HY, et al. Characteristics of HCN channels and their participation in neuropathic pain[J]. Neurochem Res,2008, 33 (10) : 1979-89. DOI: 10.1007/s11064-008-9717-6. |
 2015, Vol. 35
2015, Vol. 35
