Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (11): 2444-2455.doi: 10.12122/j.issn.1673-4254.2025.11.17
Previous Articles Next Articles
Xue GONG1( ), Yongyang FAN2(
), Yongyang FAN2( ), Kaiyuan LUO2, Yi YAN2, Zhonghao LI2(
), Kaiyuan LUO2, Yi YAN2, Zhonghao LI2( )
)
Received:2025-04-03
Online:2025-11-20
Published:2025-11-28
Contact:
Zhonghao LI
E-mail:503253172@qq.com;760445401@qq.com;525838244@qq.com
Supported by:Xue GONG, Yongyang FAN, Kaiyuan LUO, Yi YAN, Zhonghao LI. Construction of cardiac organoids derived from human induced pluripotent stem cells for cardiac disease modeling and drug evaluation[J]. Journal of Southern Medical University, 2025, 45(11): 2444-2455.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.11.17
| Gene | Forward Primer (5'→3') | Reverse Primer (5'→3') |
|---|---|---|
| OCT4 | CTCTGAGGTGTGGGGGATTC | TCAGGCTGAGAGGTCCCAAG |
| Nanog | TTTGTGGGCCTGAAGAAAACT | AGGGCTGTCCTGAATAAGCAG |
| SOX2 | GCCGAGTGGAAACTTTTGTCG | GGCAGCGTGTACTTATCCTTCT |
| TNNT2 | GGAGGAGTCCAAACCAAAGCC | TCAAAGTCCACTCTCTCTCCATC |
| NKX2.5 | CCAAGTGTGCGTCTGCCTTT | CGCACAGCTCTTTCTTTTCGG |
| RYR2 | GGCAGCCCAAGGGTATCTC | ACACAGCGCCACCTTCATAAT |
| KCNJ2 | GTGCGAACCAACCGCTACA | CCAGCGAATGTCCACACAC |
| MYH7 | GGCAAGACAGTGACCGTGAAG | CGTAGCGATCCTTGAGGTTGTA |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
Tab.1 Primer sequences for RT-qPCR
| Gene | Forward Primer (5'→3') | Reverse Primer (5'→3') |
|---|---|---|
| OCT4 | CTCTGAGGTGTGGGGGATTC | TCAGGCTGAGAGGTCCCAAG |
| Nanog | TTTGTGGGCCTGAAGAAAACT | AGGGCTGTCCTGAATAAGCAG |
| SOX2 | GCCGAGTGGAAACTTTTGTCG | GGCAGCGTGTACTTATCCTTCT |
| TNNT2 | GGAGGAGTCCAAACCAAAGCC | TCAAAGTCCACTCTCTCTCCATC |
| NKX2.5 | CCAAGTGTGCGTCTGCCTTT | CGCACAGCTCTTTCTTTTCGG |
| RYR2 | GGCAGCCCAAGGGTATCTC | ACACAGCGCCACCTTCATAAT |
| KCNJ2 | GTGCGAACCAACCGCTACA | CCAGCGAATGTCCACACAC |
| MYH7 | GGCAAGACAGTGACCGTGAAG | CGTAGCGATCCTTGAGGTTGTA |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |

Fig.1 Construction of the Cardiac Organoids derived from human induced pluripotent stem cells. A: Schematic diagram depicting the protocol for constructing cardiac organoids derived from human induced pluripotent stem cells. B: Brightfield images of the developing cardiac organoids. C, D: Proportion of cardiomyocytes and endothelial cells in cardiac organoids determined by flow cytometry. E: Frozen sections of the developing cardiac organoids. F: Diameter of the developing cardiac organoids (n=10). G: The mRNA expressions in cardiac organoids determined by RT-qPCR. ***P<0.001, ****P<0.0001 vs D0.
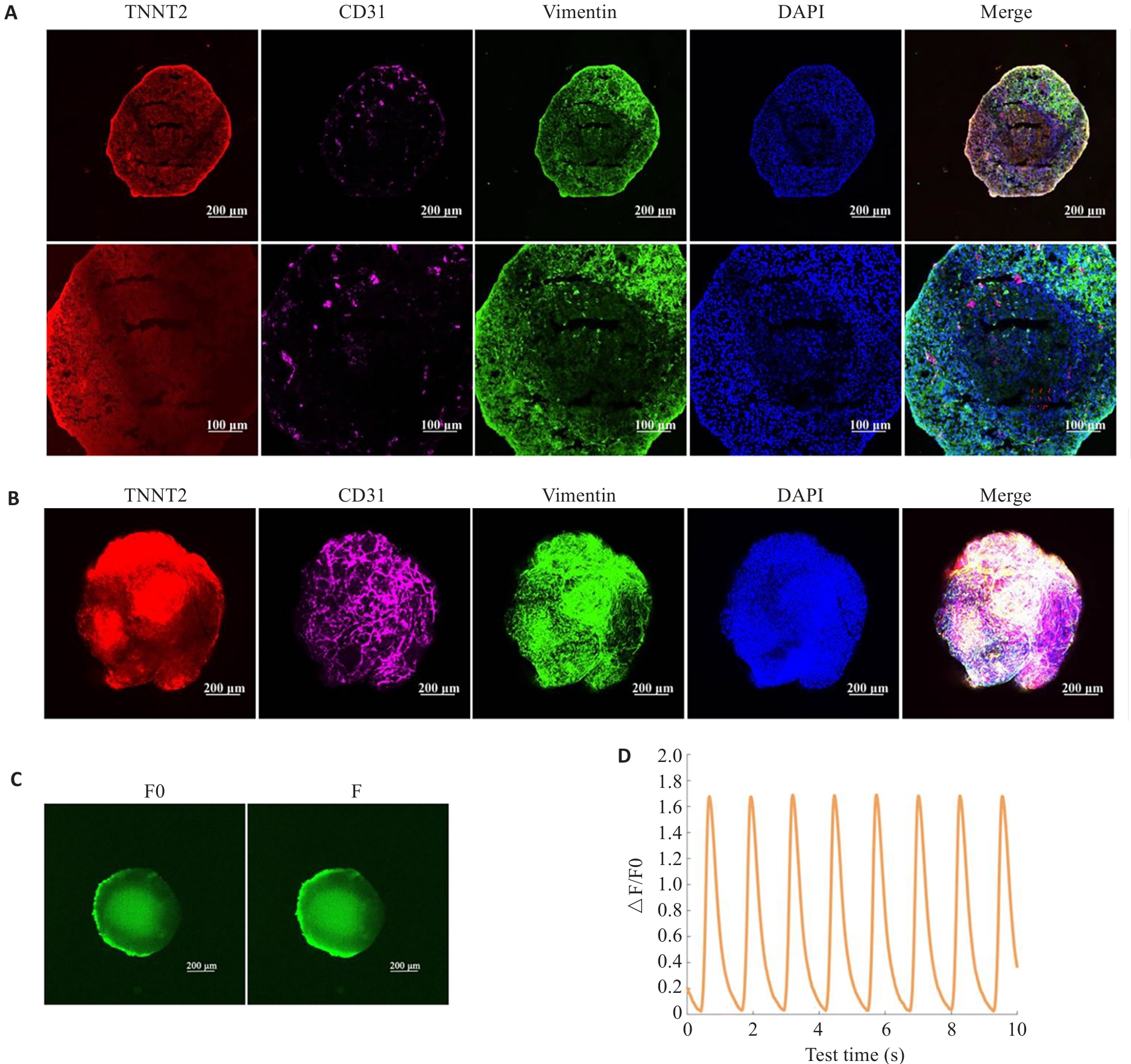
Fig.2 Characterization of the constructed cardiac organoids. A: Immunofluorescent staining of frozen sections of the cardiac organoids. B: Whole-mount staining of the cardiac organoids. C, D: Calcium transient assay of the cardiac organoids (n=3).
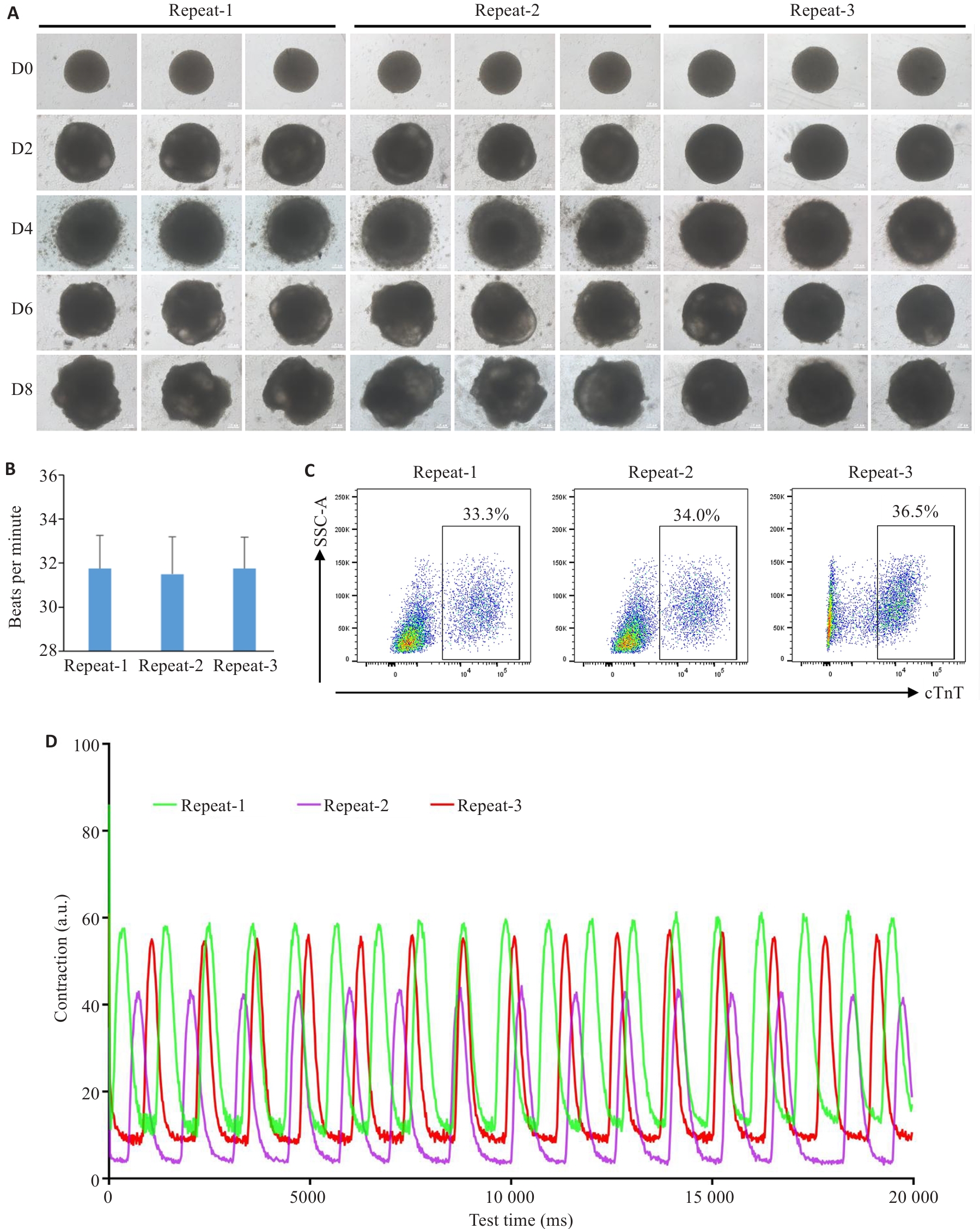
Fig.3 Consistency tests of the cardiac organoids from different batches. A: Brightfield images of the developing cardiac organoids from different batches. B: Beating frequency of the cardiac organoids from different batches (n=8). C: Proportion of cardiomyocytes in the cardiac organoids from different batches determined by flow cytometry. D: Measurement of the contractile ability of cardiac organoids using Image J.

Fig.4 Long-term culture of the cardiac organoids. A: Brightfield images of the developing cardiac organoids. B: Measurement of contractile ability of the cardiac organoids using Image J. C: Immunofluorescent staining of frozen sections of the cardiac organoids.

Fig.5 Disease modeling of cardiac organoids. A: Brightfield images of the cardiac organoids with different treatments. B: Masson's staining of the cardiac organoids. C: Calcium transient assay of the cardiac organoids. D: Amplitude of Ca2+ transient and the time to peak of Ca2+ transient (n=5). E: mRNA expressions of MYH7 and TNNT2 with or without cryoinjury and captopril treatment. F: Evaluation of cTnT levels in the culture medium by ELISA (n=6). G: Masson's staining of the cardiac organoids. H: TUNEL staining of the organoid sections after H/R treatment. I: Statistical analysis of apoptosis. *P<0.05, **P<0.01, ***P<0.001 vs control group; #P<0.05 vs cryoinjury group.
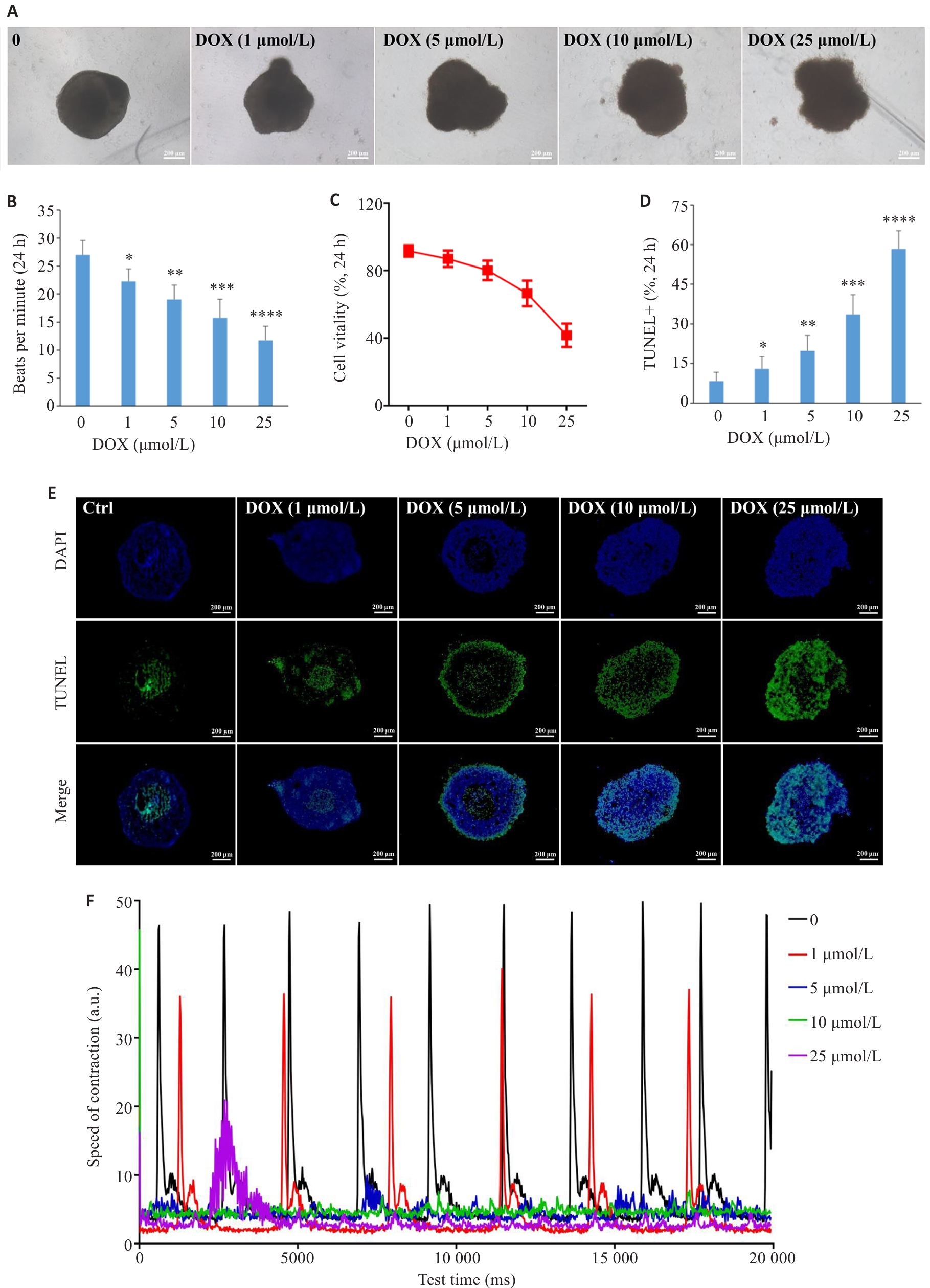
Fig.6 Effect of doxorubicin on the cardiac organoids. A: Brightfield images of the cardiac organoids treated with doxorubicin (scale bar=200 μm). B: Dose-dependent effects of doxorubicin on beating frequency of the cardiac organoids (n=8). C: Dose-dependent effects of doxorubicin on cell vitality in the cardiac organoids by CCK8 assay. D: Statistical analysis of cell apoptosis. E: TUNEL staining of the organoid sections after exposure to doxorubicin for 24 h at the indicated concentrations. F: Measurement of contractile ability of the cardiac organoids using Image J. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs control group (0).

Fig.7 Trastuzumab causes dysfunction of the cardiac organoids. A: Calcium transient assay of the cardiac organoids with Trastuzumab treatment. B: Amplitude of Ca2+ transient and the time to peak of Ca2+ transient (*P<0.05, **P<0.01 vs control group, n=5). C: Measurement of contractile ability of the cardiac organoids with Trastuzumab treatment using Image J.
| [1] | Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study[J]. Lancet, 2020, 395(10226): 795-808. doi:10.1016/s0140-6736(19)32008-2 |
| [2] | Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association[J]. Circulation, 2017, 135(10): e146-603. doi:10.1161/cir.0000000000000491 |
| [3] | Tang XY, Wu SS, Wang D, et al. Human organoids in basic research and clinical applications[J]. Signal Transduct Target Ther, 2022, 7(1): 168. doi:10.1038/s41392-022-01024-9 |
| [4] | Kapałczyńska M, Kolenda T, Przybyła W, et al. 2D and 3D cell cultures-a comparison of different types of cancer cell cultures[J]. Arch Med Sci, 2018, 14(4): 910-9. |
| [5] | Wnorowski A, Yang HX, Wu JC. Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models[J]. Adv Drug Deliv Rev, 2019, 140: 3-11. doi:10.1016/j.addr.2018.06.001 |
| [6] | Soldatow VY, Lecluyse EL, Griffith LG, et al. In vitro models for liver toxicity testing[J]. Toxicol Res (Camb), 2013, 2(1): 23-39. doi:10.1039/c2tx20051a |
| [7] | Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts[J]. Science, 1998, 282(5391): 1145-7. doi:10.1126/science.282.5391.1145 |
| [8] | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors[J]. Cell, 2006, 126(4): 663-76. doi:10.1016/j.cell.2006.07.024 |
| [9] | Thomas D, Cunningham NJ, Shenoy S, et al. Human-induced pluripotent stem cells in cardiovascular research: current approaches in cardiac differentiation, maturation strategies, and scalable production[J]. Cardiovasc Res, 2022, 118(1): 20-36. doi:10.1093/cvr/cvab115 |
| [10] | Yang DH, Gomez-Garcia J, Funakoshi S, et al. Modeling human multi-lineage heart field development with pluripotent stem cells[J]. Cell Stem Cell, 2022, 29(9): 1382-401. e8. doi:10.1016/j.stem.2022.08.007 |
| [11] | Kim H, Kamm RD, Vunjak-Novakovic G, et al. Progress in multicellular human cardiac organoids for clinical applications[J]. Cell Stem Cell, 2022, 29(4): 503-14. doi:10.1016/j.stem.2022.03.012 |
| [12] | Lee SG, Kim YJ, Son MY, et al. Generation of human iPSCs derived heart organoids structurally and functionally similar to heart[J]. Biomaterials, 2022, 290: 121860. doi:10.1016/j.biomaterials.2022.121860 |
| [13] | Arzt M, Pohlman S, Mozneb M, et al. Chemically defined production of tri-lineage human iPSC-derived cardiac spheroids[J]. Curr Protoc, 2023, 3(5): e767. doi:10.1002/cpz1.767 |
| [14] | Lewis-Israeli YR, Wasserman AH, Gabalski MA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease[J]. Nat Commun, 2021, 12(1): 5142. doi:10.1038/s41467-021-25329-5 |
| [15] | Zhang FZ, Qiu H, Dong XH, et al. Single-cell atlas of multilineage cardiac organoids derived from human induced pluripotent stem cells[J]. Life Med, 2022, 1(2): 179-95. doi:10.1093/lifemedi/lnac002 |
| [16] | Ho BX, Pang JKS, Chen Y, et al. Robust generation of human-chambered cardiac organoids from pluripotent stem cells for improved modelling of cardiovascular diseases[J]. Stem Cell Res Ther, 2022, 13(1): 529. doi:10.1186/s13287-022-03215-1 |
| [17] | Santoro R, Piacentini L, Vavassori C, et al. An in vitro model for cardiac organoid production: The combined role of geometrical confinement and substrate stiffness[J]. Mater Today Bio, 2025, 31: 101566. doi:10.1016/j.mtbio.2025.101566 |
| [18] | Song HB, Weinstein HNW, Allegakoen P, et al. Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states[J]. Nat Commun, 2022, 13(1): 141. doi:10.1038/s41467-021-27322-4 |
| [19] | Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research[J]. Nat Rev Genet, 2018, 19(11): 671-87. doi:10.1038/s41576-018-0051-9 |
| [20] | Rossi G, Broguiere N, Miyamoto M, et al. Capturing cardiogenesis in gastruloids[J]. Cell Stem Cell, 2021, 28(2): 230-40. e6. doi:10.1016/j.stem.2020.10.013 |
| [21] | Drakhlis L, Biswanath S, Farr CM, et al. Human heart-forming organoids recapitulate early heart and foregut development[J]. Nat Biotechnol, 2021, 39(6): 737-46. doi:10.1038/s41587-021-00815-9 |
| [22] | Song M, Choi DB, Im JS, et al. Modeling acute myocardial infarction and cardiac fibrosis using human induced pluripotent stem cell-derived multi-cellular heart organoids[J]. Cell Death Dis, 2024, 15(5): 308. doi:10.1038/s41419-024-06703-9 |
| [23] | Arhontoulis DC, Kerr CM, Richards D, et al. Human cardiac organoids to model COVID-19 cytokine storm induced cardiac injuries[J]. J Tissue Eng Regen Med, 2022, 16(9): 799-811. doi:10.1002/term.3327 |
| [24] | Richards DJ, Li Y, Kerr CM, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity[J]. Nat Biomed Eng, 2020, 4(4): 446-62. doi:10.1038/s41551-020-0539-4 |
| [25] | Gopal S, Rodrigues AL, Dordick JS. Exploiting CRISPR Cas9 in three-dimensional stem cell cultures to model disease[J]. Front Bioeng Biotechnol, 2020, 8: 692. doi:10.3389/fbioe.2020.00692 |
| [26] | Hofbauer P, Jahnel SM, Papai N, et al. Cardioids reveal self-organizing principles of human cardiogenesis[J]. Cell, 2021, 184(12): 3299-317.e22. doi:10.1016/j.cell.2021.04.034 |
| [27] | Schmidt C, Deyett A, Ilmer T, et al. Multi-chamber cardioids unravel human heart development and cardiac defects[J]. Cell, 2023, 186(25): 5587-605.e27. doi:10.1016/j.cell.2023.10.030 |
| [28] | Hoang P, Kowalczewski A, Sun SY, et al. Engineering spatial-organized cardiac organoids for developmental toxicity testing[J]. Stem Cell Reports, 2021, 16(5): 1228-44. doi:10.1016/j.stemcr.2021.03.013 |
| [29] | Yang JS, Lei W, Xiao Y, et al. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation[J]. Cell Prolif, 2024, 57(8): e13631. doi:10.1111/cpr.13631 |
| [30] | Paik DT, Chandy M, Wu JC. Patient and disease-specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics[J]. Pharmacol Rev, 2020, 72(1): 320-42. doi:10.1124/pr.116.013003 |
| [31] | Marini V, Marino F, Aliberti F, et al. Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression[J]. Front Cell Dev Biol, 2022, 10: 878311. doi:10.3389/fcell.2022.878311 |
| [32] | Filippo Buono M, von Boehmer L, Strang J, et al. Human cardiac organoids for modeling genetic cardiomyopathy[J]. Cells, 2020, 9(7): 1733. doi:10.3390/cells9071733 |
| [33] | Garreta E, Kamm RD, Chuva de Sousa Lopes SM, et al. Rethinking organoid technology through bioengineering[J]. Nat Mater, 2021, 20(2): 145-55. doi:10.1038/s41563-020-00804-4 |
| [34] | Zhang S, Wan ZP, Kamm RD. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature[J]. Lab Chip, 2021, 21(3): 473-88. doi:10.1039/d0lc01186j |
| [35] | Kim H, Wang MQ, Paik DT. Endothelial-myocardial angiocrine signaling in heart development[J]. Front Cell Dev Biol, 2021, 9: 697130. doi:10.3389/fcell.2021.697130 |
| [1] | LI Yangyang, XU Jiajia, JIANG Chengcheng, CHEN Zilong, CHEN Ying, YING Mengjiao, WANG Ao, MA Caiyun, WANG Chunjing, GUO Yu, LIU Changqing. Rho kinase inhibitor Y27632 promotes survival of human induced pluripotent stem cells during differentiation into functional midbrain dopaminergic progenitor cells in vitro [J]. Journal of Southern Medical University, 2024, 44(2): 236-243. |
| [2] | XU Jiajia, LI Yangyang, ZHONG Guangshang, FANG Zhuling, LIU Chunbo, MA Caiyun, WANG Chunjing, GUO Yu, LIU Changqing. Directed differentiation of human induced pluripotent stem cells into midbrain dopaminergic neuron progenitors in vitro [J]. Journal of Southern Medical University, 2023, 43(2): 175-182. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||