Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (10): 1887-1897.doi: 10.12122/j.issn.1673-4254.2024.10.07
Previous Articles Next Articles
Ziping SONG1,2( ), Lei HAN2, Zhuochao LIN2, Guangsen SHI1,2(
), Lei HAN2, Zhuochao LIN2, Guangsen SHI1,2( )
)
Received:2024-05-18
Online:2024-10-20
Published:2024-10-31
Contact:
Guangsen SHI
E-mail:2321976315@qq.com;shiguangsen@zidd.ac.cn
Supported by:Ziping SONG, Lei HAN, Zhuochao LIN, Guangsen SHI. Behavioral changes of transgenic mice carrying Adrb1-A187V mutation with short sleep duration under different dietary conditions[J]. Journal of Southern Medical University, 2024, 44(10): 1887-1897.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.10.07
| Stage | Temperature (℃) | Time | Cycle |
|---|---|---|---|
| 1 | 95 | 3 min | 1× |
| 95 | 30 s | ||
| 2 | 60 | 30 s | 35× |
| 72 | 30 s | ||
| 3 | 72 | 5 min | 1× |
| 4 | 16 | ∞ | 1× |
Tab.1 PCR program setting
| Stage | Temperature (℃) | Time | Cycle |
|---|---|---|---|
| 1 | 95 | 3 min | 1× |
| 95 | 30 s | ||
| 2 | 60 | 30 s | 35× |
| 72 | 30 s | ||
| 3 | 72 | 5 min | 1× |
| 4 | 16 | ∞ | 1× |
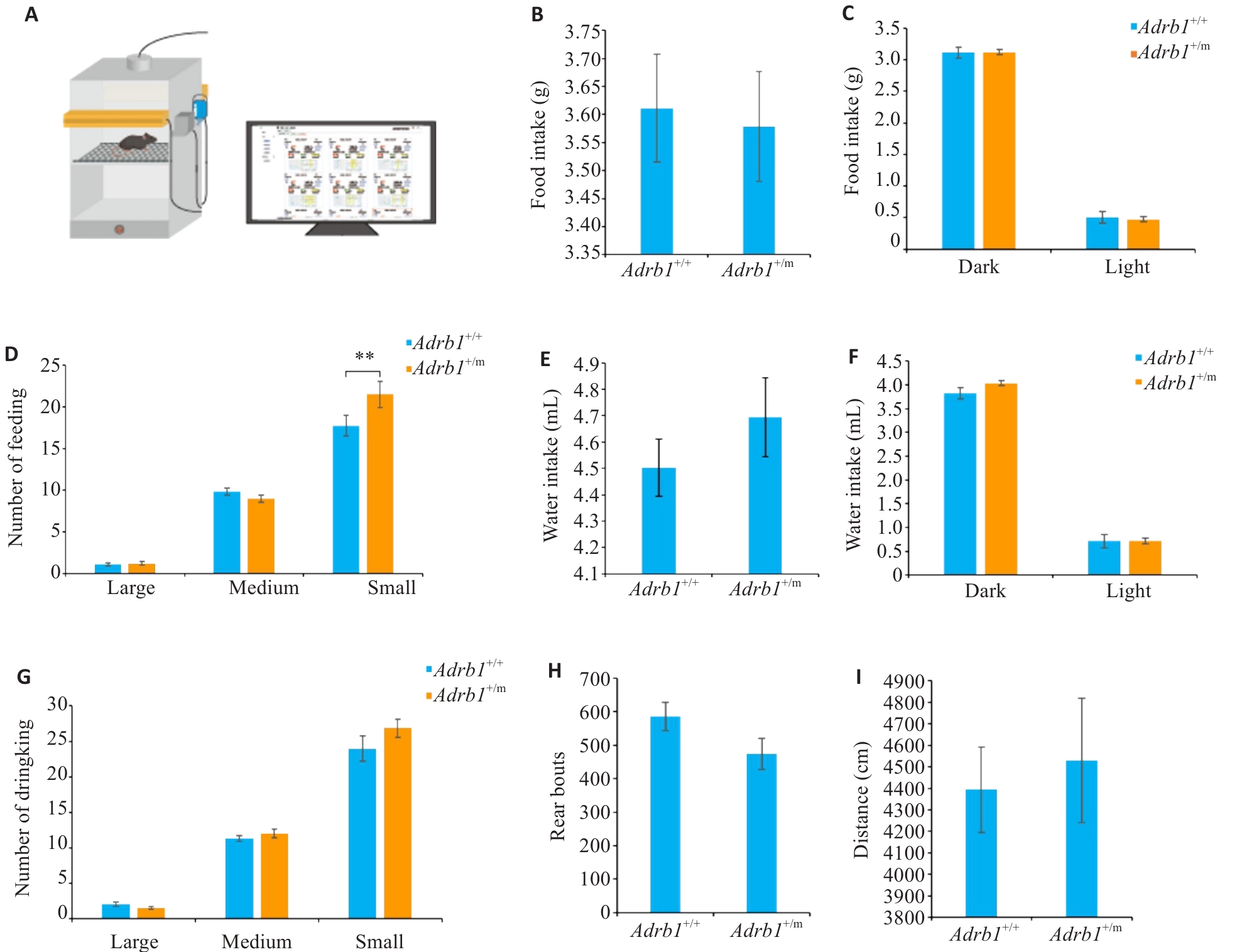
Fig.1 Activity and food/water intake of Adrb1+/+ and Adrb1+/m mice with free access to normal chow. A: Schematic of the mouse behavioral and feeding/drinking recording system. B: Total daily amount of food intake of Adrb1+/+ and Adrb1+/m mice. C: Total amount of food intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phases. D: Daily number of "big meal", " medium meal" and "small meal" in Adrb1+/+ and Adrb1+/m mice. E: Total daily amount of water intake of Adrb1+/+ and Adrb1+/m mice. F: Total amount of wake intake of Adrb1+/+ and Adrb1+/m mice in dark and light phases. G: Daily numbers of "big water", " medium water " and "small water " in Adrb1+/+ and Adrb1+/m mice. H: Total daily numbers of rearing of Adrb1+/+ and Adrb1+/m mice. I: Total daily distance traveled by Adrb1+/+ and Adrb1+/m mice. Adrb1+/+ mice (n=25), Adrb1+/m mice (n=26), **P<0.01.
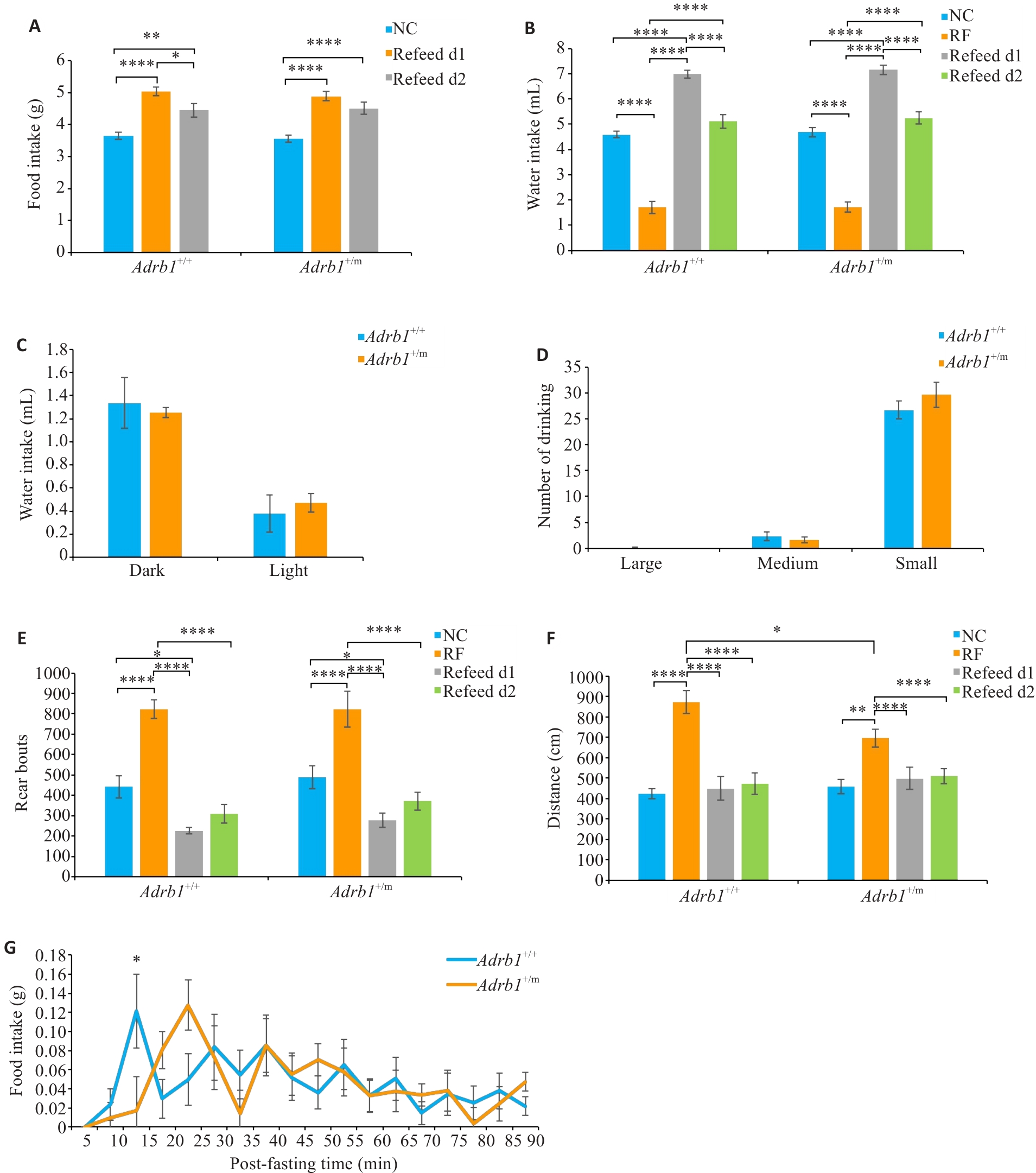
Fig.2 Changes in food intake and activity of Adrb1+/+ and Adrb1+/m mice during and after odor-retention fasting regime. A: Total daily food intake of Adrb1+/+ and Adrb1+/m mice during NC and Refeed. B: Total daily water intake of Adrb1+/+ and Adrb1+/m mice during NC, RF, and Refeed. C: Water intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under RF conditions. D: Total daily number of "big water", "medium water " and "small water" of Adrb1+/+ and Adrb1+/m mice under RF conditions. E: Total daily number of rears of Adrb1+/+ and Adrb1+/m mice during NC, RF and Refeed. F: Total daily distance traveled by Adrb1+/+ and Adrb1+/m mice under NC, RF, and Refeed. G: Time course recording of food intake after odor retention fasting (RF) plotted every 5 min for 90 minu in Adrb1+/+ and Adrb1+/m mice. Adrb1+/+ mice (n=17), Adrb1+/m mice (n=19). NC: Normal condition; RF: Odor retetion fasting. *P<0.05, **P<0.01, ****P<0.0001.
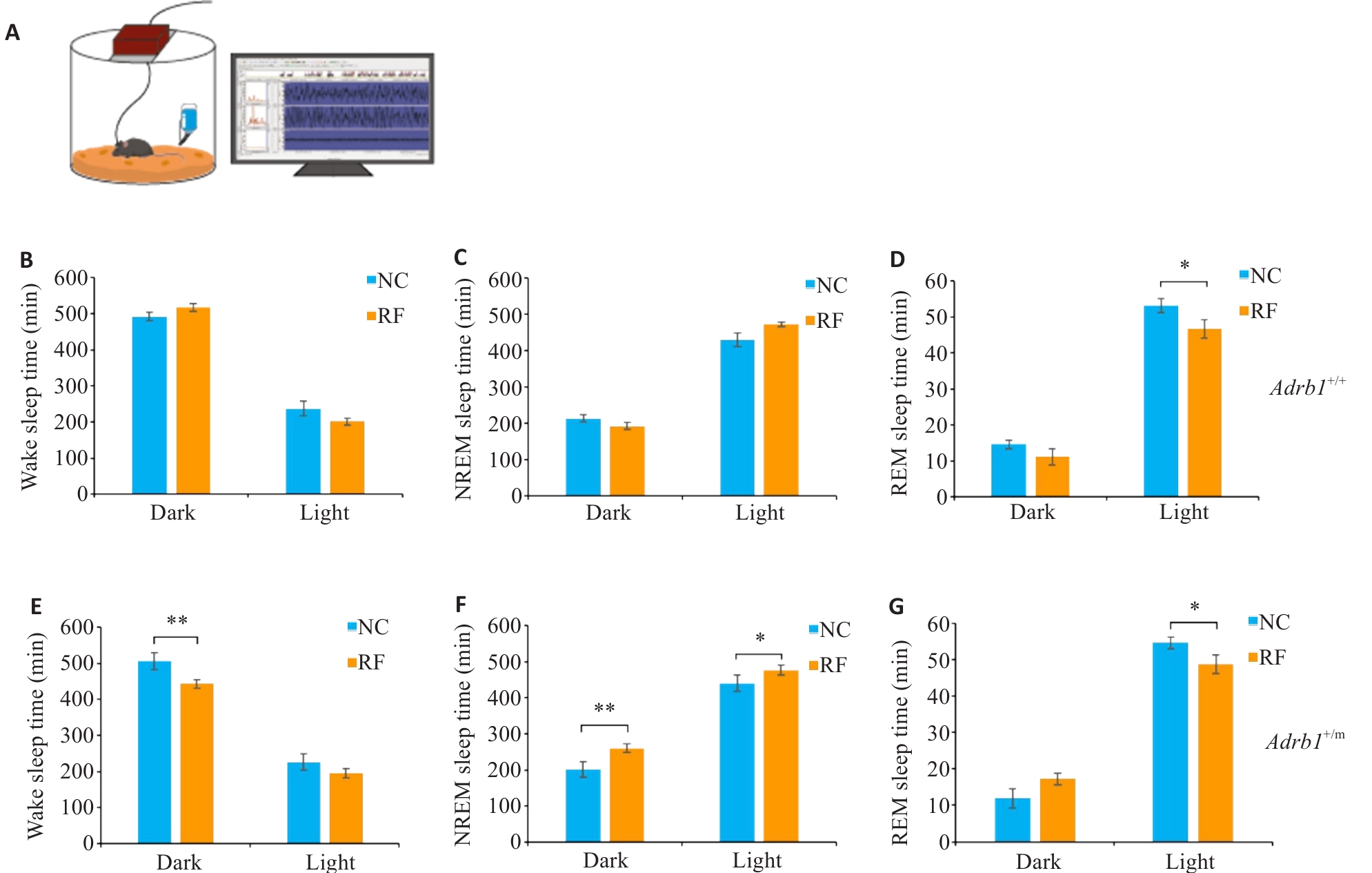
Fig.3 Sleep changes in Adrb1+/+ and Adrb1+/m mice under odor retention fasting condition. A: The multichannel EEG recording system. B-D: Total Wake (B), NREM (C), and REM (D) sleep time of Adrb1+/+ mice in the dark and light phase under NC and RF conditions. E-G: Total Wake (E), NREM (F), and REM (G) sleep time of Adrb1+/m mice in the dark and light phase under NC and RF conditions. Adrb1+/+ mice (n=6), Adrb1+/m mice (n=6), *P<0.05, **P<0.01.
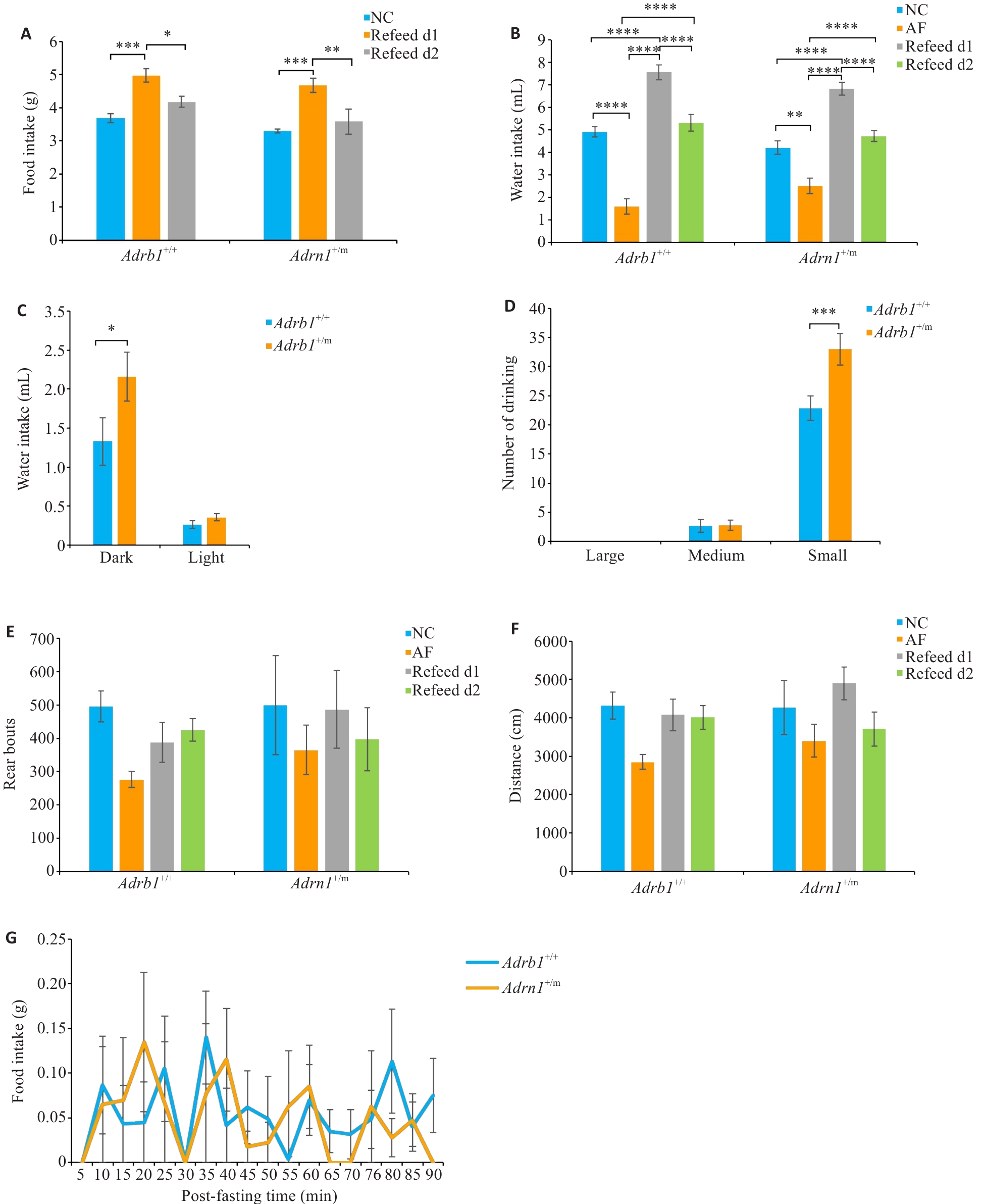
Fig.4 Changes in food intake and activity of Adrb1+/+ and Adrb1+/m mice during and after absolute fasting condition (AF). A: Total daily food intake of Adrb1+/+ and Adrb1+/m mice during NC and Refeed. B: Total daily water intake of Adrb1+/+ and Adrb1+/m mice during NC, AF, and Refeed. C: Water intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under AF conditions. D: Total daily number of "big water", " medium water " and "small water " of Adrb1+/+ and Adrb1+/m mice under AF conditions. E: Total daily number of rears of Adrb1+/+ and Adrb1+/m mice during NC, AF and Refeed. F: Total daily distance traveled by Adrb1+/+ and Adrb1+/m mice under NC, AF, and Refeed. G: Time course recording of food intake after absolute fasting (AF) plotted every 5 min for 90 min in Adrb1+/+ and Adrb1+/m mice. Adrb1+/+ mice (n=6), Adrb1+/m mice (n=4-5), *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
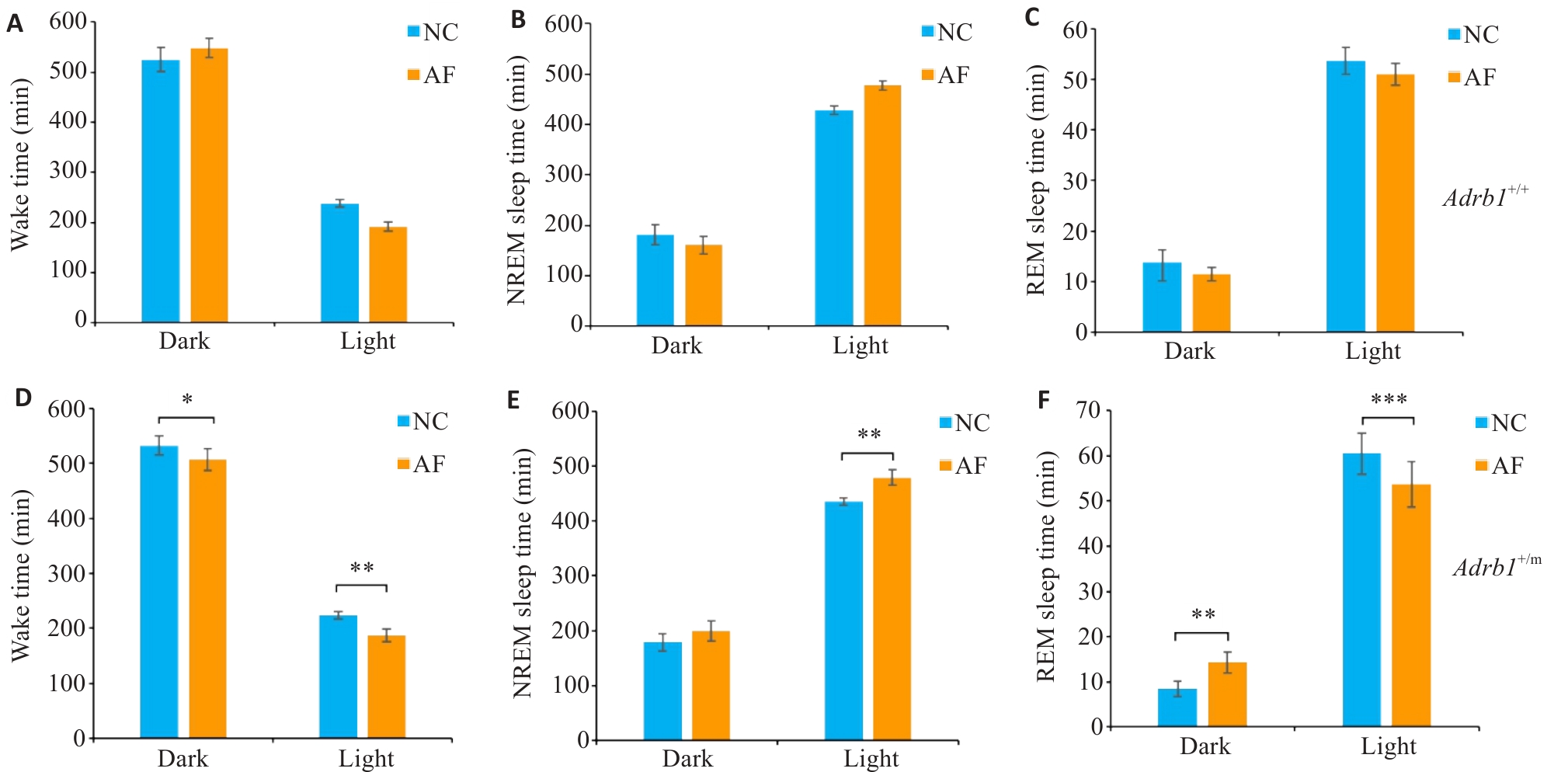
Fig.5 Sleep changes in Adrb1+/+ and Adrb1+/m mice under absolute fasting (AF) condition. A-C: Total Wake (A), NREM (B), and REM (C) sleep time of Adrb1+/+ mice in the dark and light phase under NC and AF conditions. D-F: Total Wake (D), NREM (E), and REM (F) sleep time of Adrb1+/m mice in the dark and light phase under NC and AF conditions. Adrb1+/+ mice (n=6), Adrb1+/m mice n=6), *P<0.05, **P<0.01, ***P<0.001.
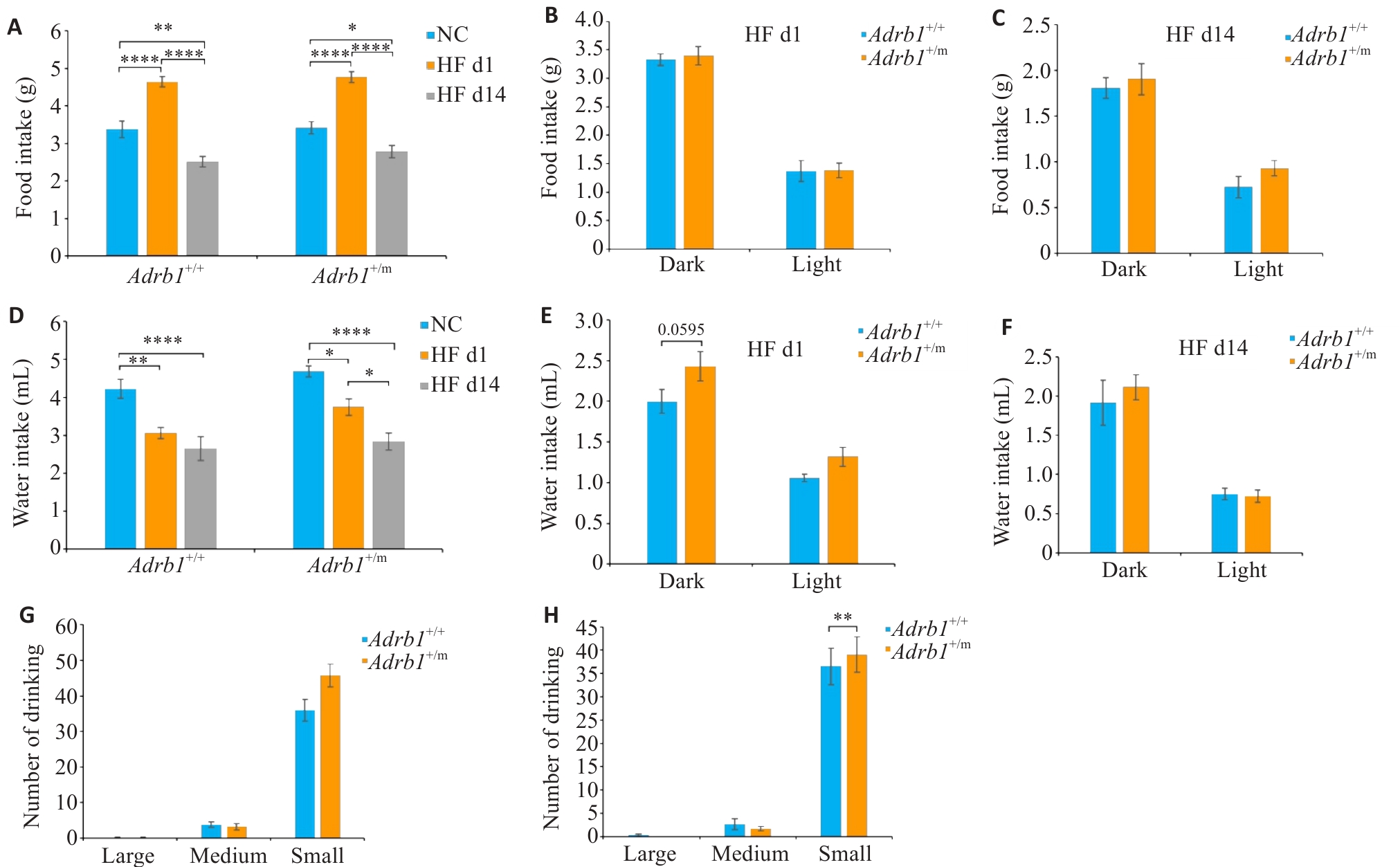
Fig.6 Effects of high-fat diet (HF) on feeding and drinking behaviors in Adrb1+/+ and Adrb1+/m mice. A: Total daily food intake of Adrb1+/+ and Adrb1+/m mice during NC, HF d1 and HF d14. B: Food intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d1. C: Total daily water intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d14. D: Total daily water intake of Adrb1+/+ and Adrb1+/m mice during NC、HF d1 and HF d14. E: Water intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d1. F: Water intake of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d14. G: Total daily number of "big water", "medium water " and "small water " of Adrb1+/+ and Adrb1+/m mice under HF d1 conditions. H: Total daily number of "big water", " medium water " and "small water " in Adrb1+/+ and Adrb1+/m mice under HF d14 conditions. Adrb1+/+ mice (n=6), Adrb1+/m mice (n=7, except n=4 for drinking at HF d14, E G I), *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
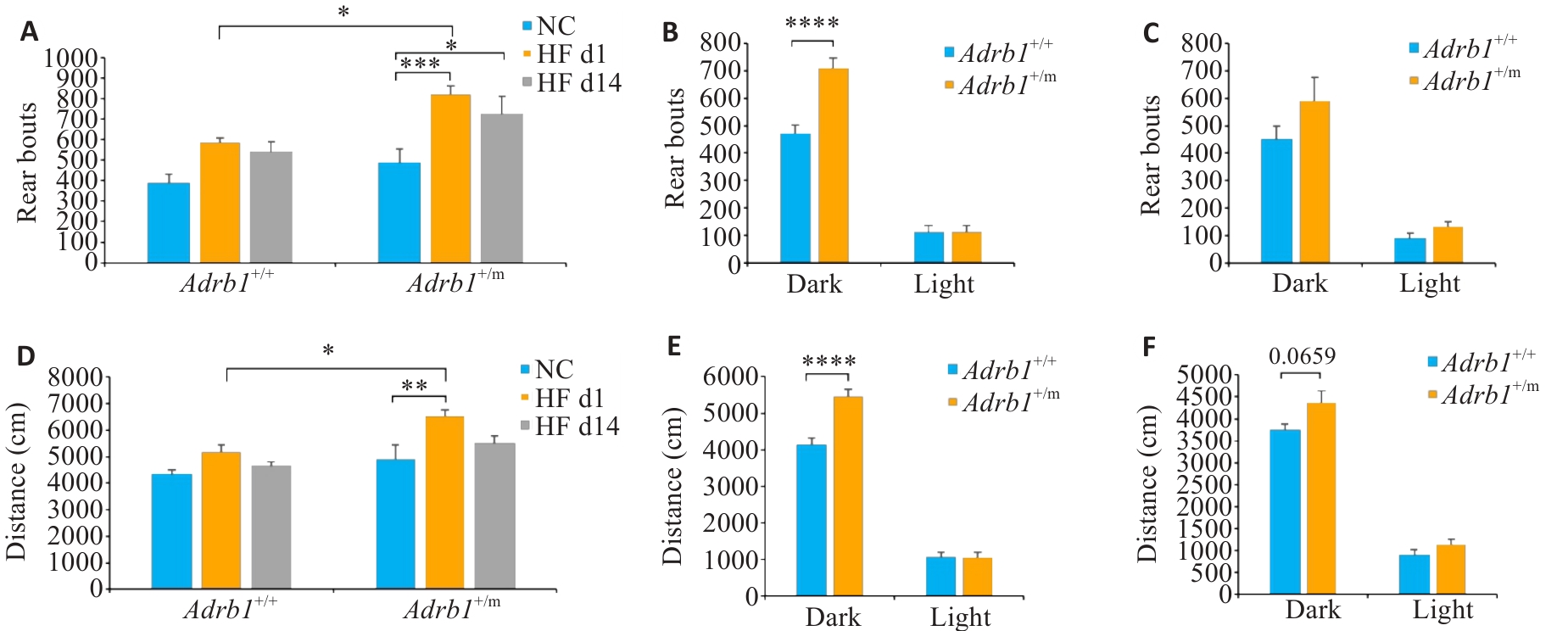
Fig.7 Effects of high-fat diet (HF) on locomotor activity of Adrb1+/+ and Adrb1+/m mice. A: Total daily number of rears of Adrb1+/+ and Adrb1+/m mice during NC, HF d1 and HF d14. B: Rear bouts of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d1. C: Rear bouts of Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d14. D: Total daily distance traveled by Adrb1+/+ and Adrb1+/m mice under NC, HF D1 and HF d14. E: Distance traveled by Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d1. F: Distance traveled by Adrb1+/+ and Adrb1+/m mice in the dark and light phase under HF d14. Adrb1+/+ mice (n=6), Adrb1+/m mice (n=7), *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
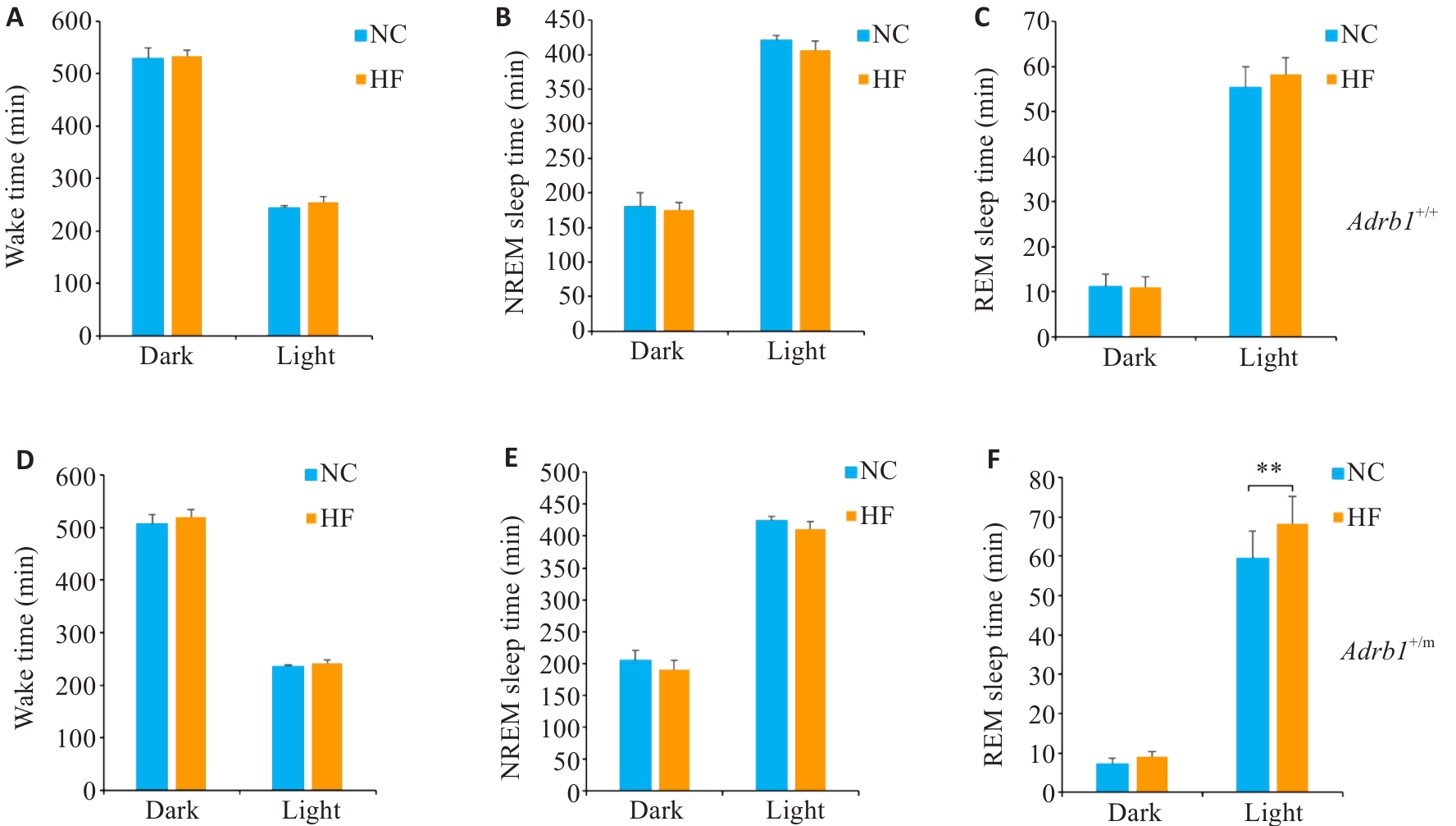
Fig.8 Sleep changes in Adrb1+/+ and Adrb1+/m mice under high-fat diet (HF) condition. A-C: Total Wake (A), NREM (B), and REM (C) sleep time in Adrb1+/+ mice in the dark and light phase under NC and HF conditions. D-F: Total Wake (D), NREM (E), and REM (F) sleep time in Adrb1+/m mice in the dark and light phase under NC and HF conditions. Data are presented as Mean±SE (n=6). **P<0.01.
| 1 | Zuraikat FM, Wood RA, Barragán R, et al. Sleep and diet: mounting evidence of a cyclical relationship[J]. Annu Rev Nutr, 2021, 41: 309-32. |
| 2 | Shukla C, Basheer R. Metabolic signals in sleep regulation: recent insights[J]. Nat Sci Sleep, 2016, 8: 9-20. |
| 3 | Panagiotou M, Meijer JH, Deboer T. Chronic high-caloric diet modifies sleep homeostasis in mice[J]. Eur J Neurosci, 2018, 47(11): 1339-52. |
| 4 | Luppi M, Al-Jahmany AA, del Vecchio F, et al. Wake-sleep and cardiovascular regulatory changes in rats made obese by a high-fat diet[J]. Behav Brain Res, 2017, 320: 347-55. |
| 5 | Kang J, Park M, Oh CM, et al. High-fat diet-induced dopaminergic dysregulation induces REM sleep fragmentation and ADHD-like behaviors[J]. Psychiatry Res, 2023, 327: 115412. |
| 6 | dos Santos SO, Arias J, Ribeiro AA, et al. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis[J]. Med Vet Entomol, 1998, 12(3): 315-7. |
| 7 | Theorell-Haglöw J, Lemming EW, Michaëlsson K, et al. Sleep duration is associated with healthy diet scores and meal patterns: results from the population-based EpiHealth study[J]. J Clin Sleep Med, 2020, 16(1): 9-18. |
| 8 | Chaput JP, McHill AW, Cox RC, et al. The role of insufficient sleep and circadian misalignment in obesity[J]. Nat Rev Endocrinol, 2023, 19(2): 82-97. |
| 9 | Cheng FW, Li YP, Winkelman JW, et al. Probable insomnia is associated with future total energy intake and diet quality in men[J]. Am J Clin Nutr, 2016, 104(2): 462-9. |
| 10 | Mossavar-Rahmani Y, Weng J, Wang R, et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueño ancillary study[J]. J Sleep Res, 2017, 26(6): 739-46. |
| 11 | Fenton S, Burrows TL, Skinner JA, et al. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies[J]. J Hum Nutr Diet, 2021, 34(2): 273-85. |
| 12 | Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain[J]. Proc Natl Acad Sci U S A, 2013, 110(14): 5695-700. |
| 13 | Morselli L, Leproult R, Balbo M, et al. Role of sleep duration in the regulation of glucose metabolism and appetite[J]. Best Pract Res Clin Endocrinol Metab, 2010, 24(5): 687-702. |
| 14 | Penev PD. Sleep deprivation and energy metabolism: to sleep, perchance to eat[J]? Curr Opin Endocrinol Diabetes Obes, 2007, 14(5): 374-81. |
| 15 | Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society[J]. Sleep, 2015, 38(6): 843-4. |
| 16 | Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary[J]. Sleep Health, 2015, 1(1): 40-3. |
| 17 | Shi GS, Xing LJ, Wu D, et al. A rare mutation of β1-adrenergic receptor affects sleep/wake behaviors[J]. Neuron, 2019, 103(6): 1044-55. e7. |
| 18 | Kjaerby C, Andersen M, Hauglund N, et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine[J]. Nat Neurosci, 2022, 25(8): 1059-70. |
| 19 | Poe GR, Foote S, Eschenko O, et al. Locus coeruleus: a new look at the blue spot[J]. Nat Rev Neurosci, 2020, 21(11): 644-59. |
| 20 | Berridge CW, Schmeichel BE, España RA. Noradrenergic modulation of wakefulness/arousal[J]. Sleep Med Rev, 2012, 16(2): 187-97. |
| 21 | Szabadi E. Functional neuroanatomy of the central noradrenergic system[J]. J Psychopharmacol, 2013, 27(8): 659-93. |
| 22 | Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review[J]. Neurogastroenterol Motil, 2006, 18(4): 263-83. |
| 23 | Parkman HP, Yates KP, Hasler WL, et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis[J]. Gastroenterology, 2011, 141(2): 486-98, 498. e1-7. |
| 24 | Hassanzadeh S, Saneei P, Keshteli AH, et al. Meal frequency in relation to prevalence of functional dyspepsia among Iranian adults[J]. Nutrition, 2016, 32(2): 242-8. |
| 25 | Holmbäck I, Ericson U, Gullberg B, et al. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men[J]. Br J Nutr, 2010, 104(7): 1065-73. |
| 26 | Titan SM, Bingham S, Welch A, et al. Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): cross sectional study[J]. BMJ, 2001, 323(7324): 1286-8. |
| 27 | Ma YS, Bertone ER, Stanek EJ 3rd, et al. Association between eating patterns and obesity in a free-living US adult population[J]. Am J Epidemiol, 2003, 158(1): 85-92. |
| 28 | Ruidavets JB, Bongard V, Bataille V, et al. Eating frequency and body fatness in middle-aged men[J]. Int J Obes Relat Metab Disord, 2002, 26(11): 1476-83. |
| 29 | Carlson O, Martin B, Stote KS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women[J]. Metabolism, 2007, 56(12): 1729-34. |
| 30 | Ngo FY, Li HH, Zhang HQ, et al. Acute fasting modulates food-seeking behavior and neural signaling in the piriform cortex[J]. Nutrients, 2022, 14(19): 4156. |
| 31 | Siegel JM. Sleep function: an evolutionary perspective[J]. Lancet Neurol, 2022, 21(10): 937-46. |
| [1] | Xinshun WU, Jingcao LI, Ying LIU, Renhong QIU, Henglin WANG, Rui XYE, Yang ZHANG, Shuo LI, Qiongyin FAN, Huajin DONG, Youzhi ZHANG, Jiangbei CAO. Cannabidiol regulates circadian rhythm to improve sleep disorders following general anesthesia in rats [J]. Journal of Southern Medical University, 2025, 45(4): 744-750. |
| [2] | Ying WANG, Wengyang DENG, Chaomei WU, Shihuan TIAN, Hua LI. Effects of larval feeding amount on development and deltamethrin resistance in Aedes albopictus [J]. Journal of Southern Medical University, 2025, 45(3): 488-493. |
| [3] | Yuejiao PEI, Huimin LIU, Yu XIN, Bo LIU. High expression of miR-124 improves cognitive function of sleep-deprived rats by modulating the PI3K/AKT signaling pathway [J]. Journal of Southern Medical University, 2025, 45(2): 340-346. |
| [4] | Menglu DONG, Tian ZHU, Junwen MA, Xiaohong DU, Yuan FENG. Reoxygenation improves reduced hypothalamic leptin responsiveness induced by intermittent hypoxia in obese rats [J]. Journal of Southern Medical University, 2024, 44(9): 1696-1703. |
| [5] | Weitao ZHONG, Weisong LI, Zelin LI, Qiang WANG, Wangming ZHANG. Causal relationship between sleep phenotype and idiopathic normal pressure hydrocephalus: a two-sample bidirectional Mendelian randomization study [J]. Journal of Southern Medical University, 2024, 44(8): 1612-1619. |
| [6] | Ping YUAN, Xiuli HU, Guojia QI, Xiu DAI, Xiangyuan CHU, Weihang CHEN, Xiuquan SHI. Poor sleep quality contributes to occurrence of posttraumatic stress disorder in trauma patients [J]. Journal of Southern Medical University, 2024, 44(6): 1166-1172. |
| [7] | ZHU Jiwei, LU Manlu, JIAO Qianqian, SUN Yunliang, LIU Lu, DING Honghong, YU Yan, PAN Lei. Analysis of gut target microbiota and species difference in patients with obstructive sleep apnea based on 16S rRNA sequencing [J]. Journal of Southern Medical University, 2024, 44(1): 146-155. |
| [8] | SU Xiaofeng, HAN Jiming, GAO Yinghui, FAN Li, HE Zijun, ZHAO Zhe, LIN Junlin, GUO Jingjing, CHEN Kaibing, GAOYan, LIU lin. A long-term ischemic stroke risk score model in patients aged 60 years and older with obstructive sleep apnea: a multicenter prospective cohort study [J]. Journal of Southern Medical University, 2022, 42(3): 338-346. |
| [9] | GU Cuifang, ZHAI Mingjian, LÜ Aijun, LIU Lu, HU Huan, LIU Xi, LI Xuan, CHENG Xiangyang. Ultrasound-guided stellate ganglion block improves sleep quality in elderly patients early after thoracoscopic surgery for lung cancer: a randomized controlled study [J]. Journal of Southern Medical University, 2022, 42(12): 1807-1814. |
| [10] | HAN Binbin, WANG Shanshan, LI Guohua, WANG Xuehui, CHEN Zhigang, ZHAO Guoan, CHEN Yingen, LI Meng, LI Yan, ZHANG Min, AI Sizhi. Objective sleep characteristics and risk factors for sleep apnea in heart failure patients with different left ventricular ejection fraction [J]. Journal of Southern Medical University, 2021, 41(9): 1415-1419. |
| [11] | . Incidence of enteral feeding intolerance and its risk factors in patients with oral and maxillofacial malignancies [J]. Journal of Southern Medical University, 2021, 41(7): 1114-1118. |
| [12] | SU Xiaofeng, HAN Jiming, GAO Yinghui, HE Zijun, ZHAO Zhe, LIN Junling, GUO Jingjing, CHEN Kaibing, GAOYan, LIU Lin. Correlation of obstructive sleep apnea with components of metabolic syndrome and implications for long‐term adverse cardiovascular risk in elderly patients [J]. Journal of Southern Medical University, 2021, 41(11): 1592-1599. |
| [13] | . Effect of general anesthesia on postoperative melatonin secretion in 4-to 6-year-old children with snoring [J]. Journal of Southern Medical University, 2021, 41(1): 128-134. |
| [14] | . Cardiac functional alterations and its risk factors in elderly patients with obstructive sleep apnea syndrome free of cardiovascular disease [J]. Journal of Southern Medical University, 2020, 40(11): 1587-1592. |
| [15] | . Efficacy of weight management combined with uvulopalatopharyngoplasty for obesityrelated obstructive sleep apnea-hypopnea syndrome [J]. Journal of Southern Medical University, 2020, 40(11): 1668-1672. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||