Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (3): 479-487.doi: 10.12122/j.issn.1673-4254.2025.03.05
Previous Articles Next Articles
Lingwei TANG( ), Jiasong LI, Haibing XU(
), Jiasong LI, Haibing XU( )
)
Received:2024-12-18
Online:2025-03-20
Published:2025-03-28
Contact:
Haibing XU
E-mail:826283939@qq.com;haibingxu@smu.edu.cn
Supported by:Lingwei TANG, Jiasong LI, Haibing XU. Synchronized neural rhythms in rat hippocampal CA1 region and orbitofrontal cortex are involved in learning and memory consolidation in spatial goal-directed tasks[J]. Journal of Southern Medical University, 2025, 45(3): 479-487.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.03.05
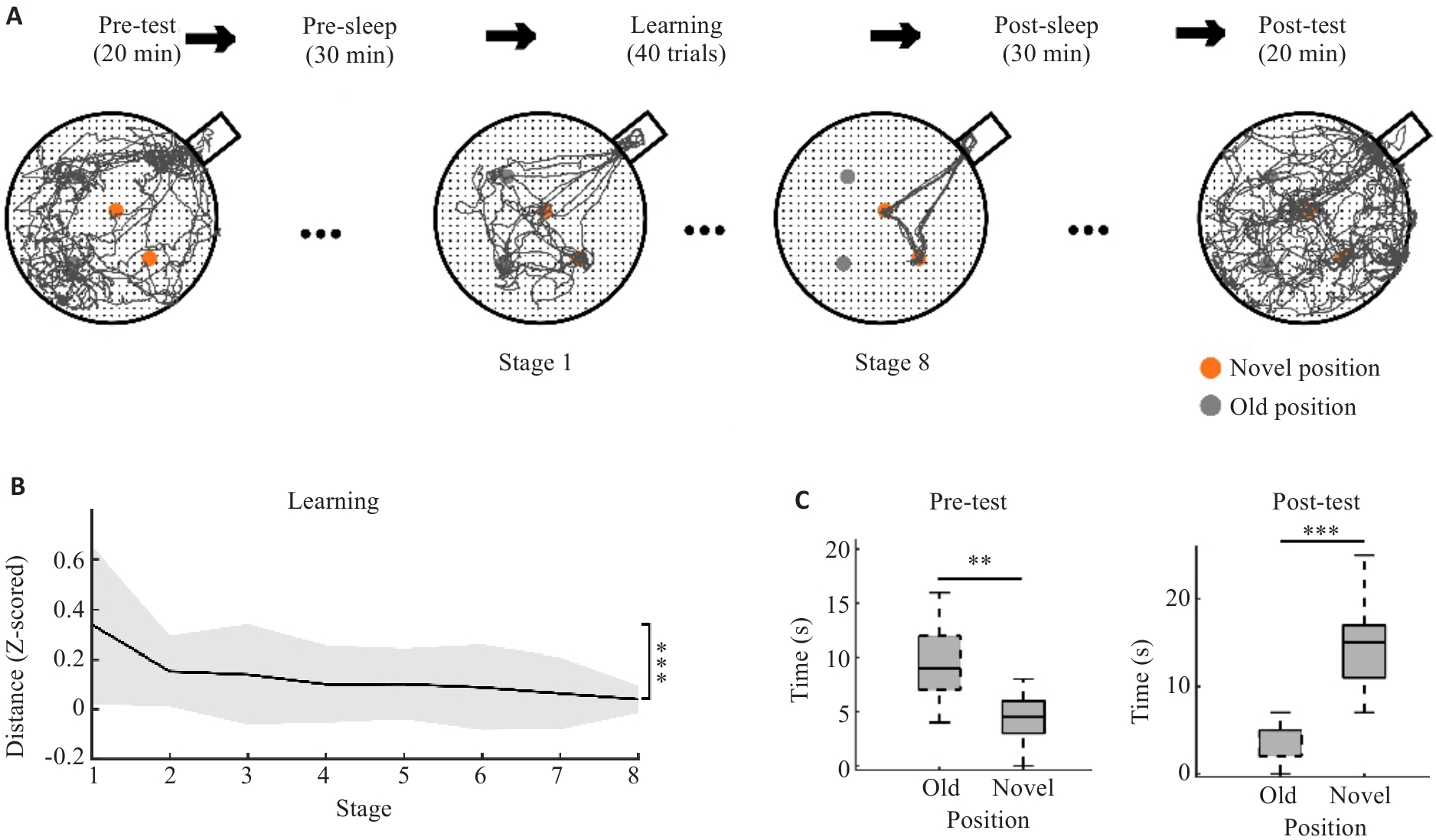
Fig.1 Performance of the rats in the spatial goal-directed task. A: Trajectory in the task. This task was divided into 5 periods, and the learning period was further divided into 8 stages. The two orange dots represent two novel reward positions; the two gray dots represent two old reward positions. B: Learning performance was assessed based on the distance of each stage (Stage 1 vs Stage 8, ***P<0.005). C: Memory performance was evaluated based on the number of times that the rats entered the old and novel reward positions during the Pre-test and Post-test, respectively (**P<0.01, ***P<0.005).
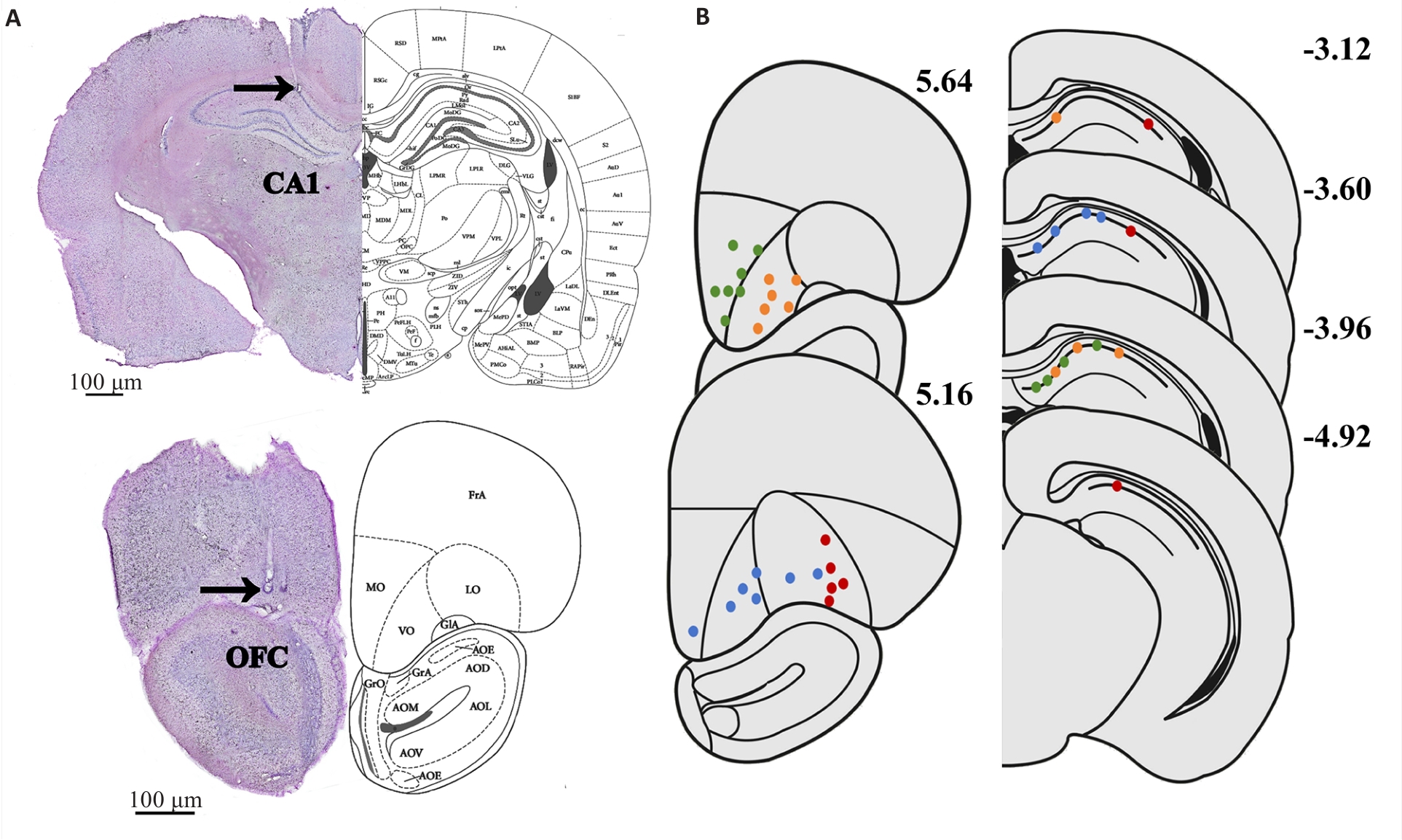
Fig.2 Hippocampal CA1 and OFC tetrodes placement. A: An example of tetrode trajectories from lw016. The arrowhead indicates the end of tetrodes. B: Tetrode positions in the 4 rats. Orange dot: lw016; green dot: lw015; blue dot: js001; red dot: lw014. Numbers represent the distance from the bregma.
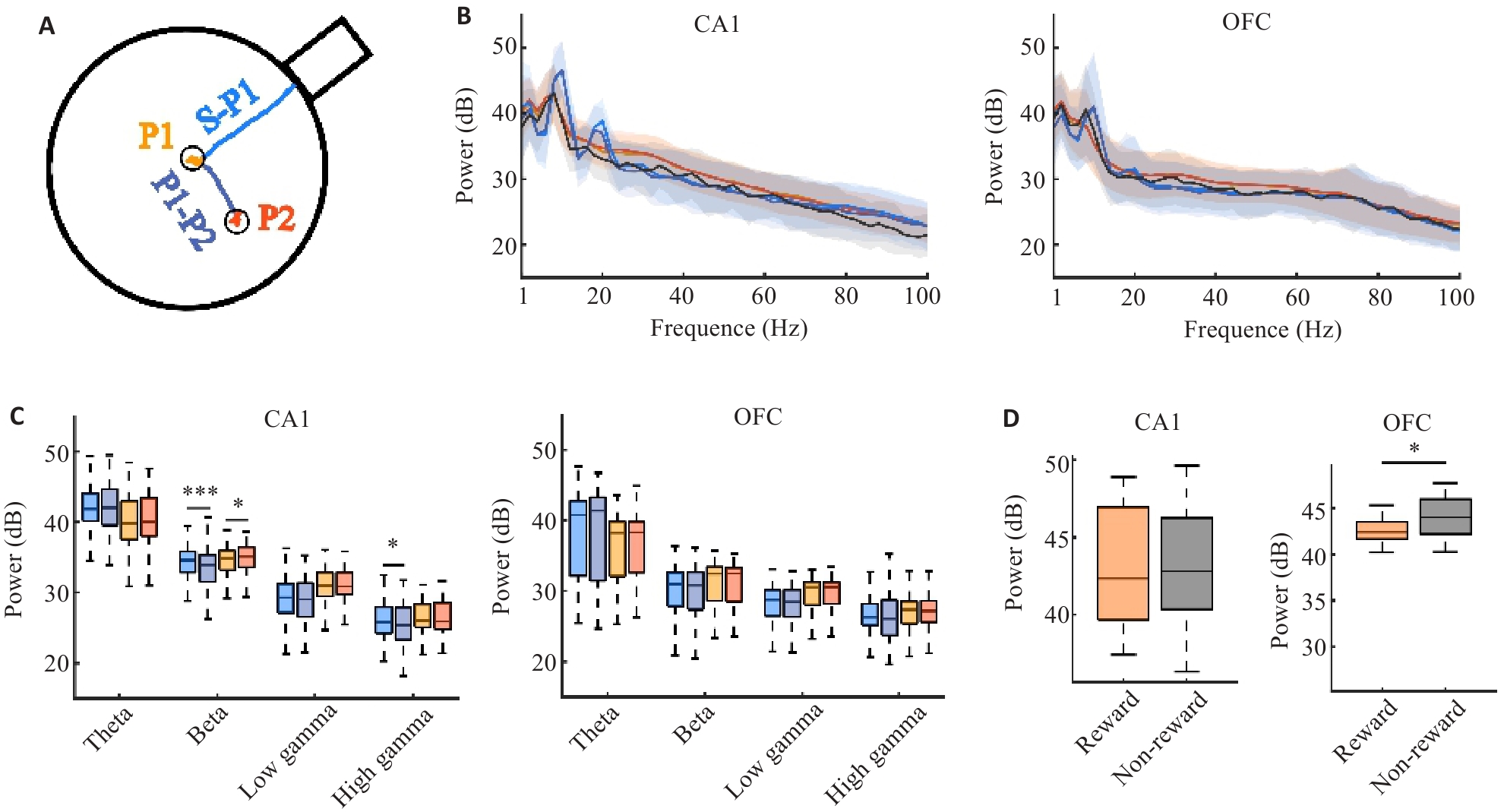
Fig.3 Differences in neural rhythm responses between the hippocampal CA1 and OFC in different task states. A: The task state was divided into goal navigation process (S-P1 and P1-P1) and reward acquisition process (P1 and P2). B: Distribution of power across 1-100 Hz. C: Differences in power strength of different rhythms across different processes. D: Comparison of power between reward (average of two novel positions) and non-reward (average of two old positions). *P<0.05, ***P<0.005.
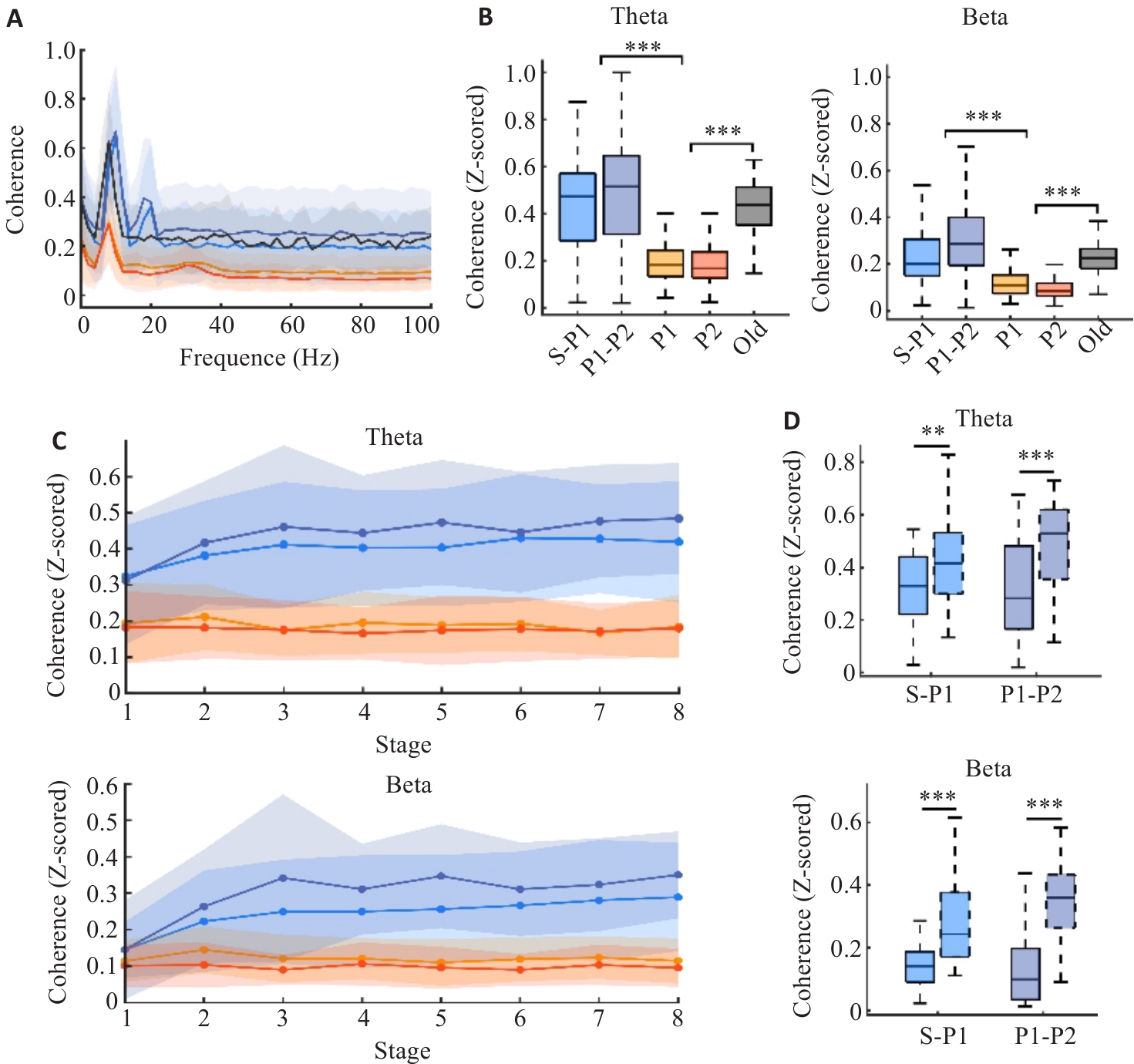
Fig.4 Coherence of hippocampal CA1 region and the OFC. A: Distribution of coherence across 1-100 Hz. B: Coherence in two goal navigation processes, two reward acquisition processes, and non-reward processes. C: The distribution of coherence across learning phases. D: Comparison of coherence differences in Theta and Beta rhythms during Stage 1 and Stage 8 in two goal navigation processes (**P<0.01, ***P<0.005).
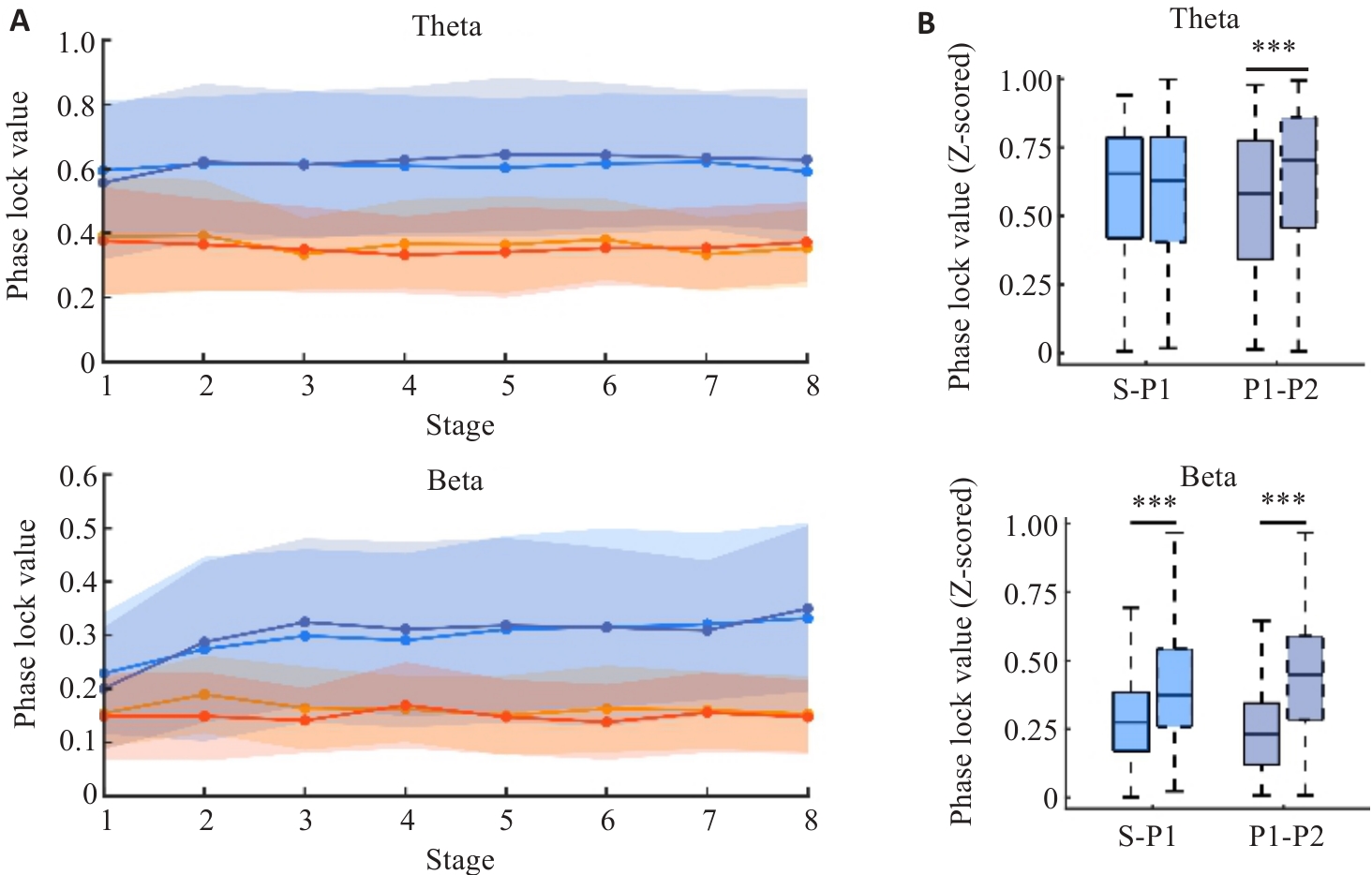
Fig.5 Phase locking of hippocampal CA1 and the OFC. A: Distribution of phase locking across the learning phases. B: Comparison of phase locking differences in Theta and Beta rhythms during Stage 1 and Stage 8 in the two goal navigation processes (***P<0.005).
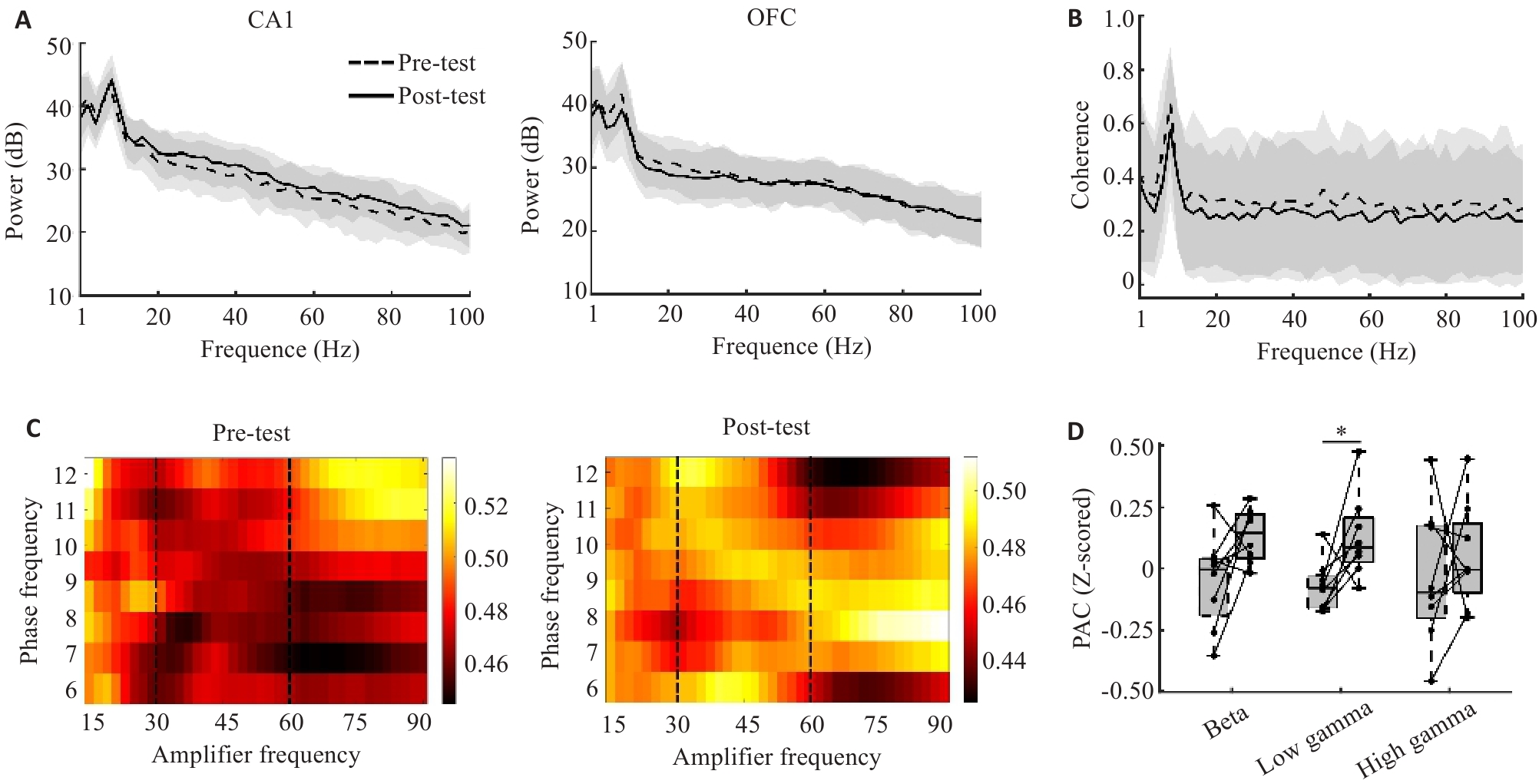
Fig.6 Neural activity in the hippocampal CA1 region and the OFC during long-term memory and short-term memory. A: Distribution of power across 1-100 Hz. Dashed line represents Pre-test; solid line represents Post-test. B: Distribution of power across 1-100 Hz. C: Phase-amplitude coupling in Pre-test and Post-test. Y-axis represents the data of OFC in the 15-90 Hz range; X-axis represents the data of CA1 in the Theta rhythm. The color map indicates the phase-amplitude coupling value. The area between the two dashed lines represents the Low gamma rhythm. D: Hippocampal CA1 Theta phase-OFC Low gamma amplitude cross-coupling in the Post-test was greater than in the Pre-test (*P<0.05).
| 1 | de Wit S, Dickinson A. Associative theories of goal-directed behaviour: a case for animal-human translational models[J]. Psychol Res, 2009, 73(4): 463-76. |
| 2 | Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates[J]. Neuropharmacology, 1998, 37(4/5): 407-19. |
| 3 | Balleine BW, Peak J, Matamales M, et al. The dorsomedial striatum: an optimal cellular environment for encoding and updating goal-directed learning[J]. Curr Opin Behav Sci, 2021, 41: 38-44. |
| 4 | van Wingerden M, van der Meij R, Kalenscher T, et al. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning[J]. J Neurosci, 2014, 34(2): 493-505. |
| 5 | Womelsdorf T, Vinck M, Stan Leung L, et al. Selective Theta-synchronization of choice-relevant information subserves goal-directed behavior[J]. Front Hum Neurosci, 2010, 4: 210. |
| 6 | Buzsáki G. Two-stage model of memory trace formation: a role for "noisy" brain states[J]. Neuroscience, 1989, 31(3): 551-70. |
| 7 | O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat[J]. Brain Res, 1971, 34(1): 171-5. |
| 8 | Speers LJ, Bilkey DK. Disorganization of oscillatory activity in animal models of schizophrenia[J]. Front Neural Circuits, 2021, 15: 741767. |
| 9 | Shimbo A, Izawa EI, Fujisawa S. Scalable representation of time in the hippocampus[J]. Sci Adv, 2021, 7(6): eabd7013. |
| 10 | Drieu C, Zugaro M. Hippocampal sequences during exploration: mechanisms and functions[J]. Front Cell Neurosci, 2019, 13: 232. |
| 11 | Iwasaki S, Sasaki T, Ikegaya Y. Hippocampal beta oscillations predict mouse object-location associative memory performance[J]. Hippocampus, 2021, 31(5): 503-11. |
| 12 | Leung LS, Gill RS, Shen B, et al. Cholinergic and behavior-dependent beta and gamma waves are coupled between olfactory bulb and hippocampus[J]. Hippocampus, 2024, 34(9): 464-90. |
| 13 | Naggar I, Stewart M, Orman R. High frequency oscillations in rat hippocampal slices: origin, frequency characteristics, and spread[J]. Front Neurol, 2020, 11: 326. |
| 14 | Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience[J]. Nat Rev Neurosci, 2005, 6(9): 691-702. |
| 15 | Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat[J]. J Comp Neurol, 2011, 519(18): 3766-801. |
| 16 | Mar AC, Walker AL, Theobald DE, et al. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat[J]. J Neurosci, 2011, 31(17): 6398-404. |
| 17 | Rolls ET, Critchley HD, Mason R, et al. Orbitofrontal cortex neurons: role in olfactory and visual association learning[J]. J Neurophysiol, 1996, 75(5): 1970-81. |
| 18 | Stalnaker TA, Liu TL, Takahashi YK, et al. Orbitofrontal neurons signal reward predictions, not reward prediction errors[J]. Neurobiol Learn Mem, 2018, 153(pt b): 137-43. |
| 19 | Gardner MPH, Sanchez D, Conroy JC, et al. Processing in lateral orbitofrontal cortex is required to estimate subjective preference during initial, but not established, economic choice[J]. Neuron, 2020, 108(3): 526-37. e4. |
| 20 | Amodeo LR, McMurray MS, Roitman JD. Orbitofrontal cortex reflects changes in response-outcome contingencies during probabilistic reversal learning[J]. Neuroscience, 2017, 345: 27-37. |
| 21 | Rich EL, Wallis JD. Spatiotemporal dynamics of information encoding revealed in orbitofrontal high-gamma[J]. Nat Commun, 2017, 8(1): 1139. |
| 22 | Roullet F, Datiche F, Liénard F, et al. Learning-stage dependent Fos expression in the rat brain during acquisition of an olfactory discrimination task[J]. Behav Brain Res, 2005, 157(1): 127-37. |
| 23 | Thonnard D, Callaerts-Vegh Z, D'Hooge R. Effects of orbitofrontal cortex and ventral hippocampus disconnection on spatial reversal learning[J]. Neurosci Lett, 2021, 750: 135711. |
| 24 | Dupret D, O’Neill J, Pleydell-Bouverie B, et al. The reorganization and reactivation of hippocampal maps predict spatial memory performance[J]. Nat Neurosci, 2010, 13(8): 995-1002. |
| 25 | Voigts J, Newman JP, Wilson MA, et al. An easy-to-assemble, robust, and lightweight drive implant for chronic tetrode recordings in freely moving animals[J]. J Neural Eng, 2020, 17(2): 026044. |
| 26 | Buzsáki G. Large-scale recording of neuronal ensembles[J]. Nat Neurosci, 2004, 7(5): 446-51. |
| 27 | Young JJ, Shapiro ML. Dynamic coding of goal-directed paths by orbital prefrontal cortex[J]. J Neurosci, 2011, 31(16): 5989-6000. |
| 28 | Riceberg JS, Srinivasan A, Guise KG, et al. Hippocampal signals modify orbitofrontal representations to learn new paths[J]. Curr Biol, 2022, 32(15): 3407-13.e6. |
| 29 | Wikenheiser AM, Gardner MPH, Mueller LE, et al. Spatial representations in rat orbitofrontal cortex[J]. J Neurosci, 2021, 41(32): 6933-45. |
| 30 | Sharma D, Lupkin SM, McGinty VB. Orbitofrontal high-gamma reflects spike-dissociable value and decision mechanisms[J]. bioRxiv, 2024: 2024.04.02.587758. |
| 31 | Namboodiri VMK, Otis JM, van Heeswijk K, et al. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation[J]. Nat Neurosci, 2019, 22(7): 1110-21. |
| 32 | Jarzebowski P, Hay YA, Grewe BF, et al. Different encoding of reward location in dorsal and intermediate hippocampus[J]. Curr Biol, 2022, 32(4): 834-41.e5. |
| 33 | Tamura M, Spellman TJ, Rosen AM, et al. Hippocampal-prefrontal Theta-gamma coupling during performance of a spatial working memory task[J]. Nat Commun, 2017, 8(1): 2182. |
| 34 | Wang C, Furlong TM, Stratton PG, et al. Hippocampus-prefrontal coupling regulates recognition memory for novelty discrimination[J]. J Neurosci, 2021, 41(46): 9617-32. |
| 35 | Ramirez-Gordillo D, Bayer KU, Restrepo D. Hippocampal-prefrontal θ coupling develops as mice become proficient in associative odorant discrimination learning[J]. eneuro, 2022, 9(5): ENEURO.0259-22.2022. |
| [1] | . Alterations in orbitofrontal cortex functional connectivity and decision making deficits in heroin-dependent individuals [J]. Journal of Southern Medical University, 2013, 33(08): 1117-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||