Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (4): 751-759.doi: 10.12122/j.issn.1673-4254.2025.04.10
Shuting GUO1( ), Fuyang CAO1,2(
), Fuyang CAO1,2( ), Yongxin GUO1, Yanxiang LI1,3, Xinyu HAO1, Zhuoning ZHANG1, Zhikang ZHOU1, Li TONG1(
), Yongxin GUO1, Yanxiang LI1,3, Xinyu HAO1, Zhuoning ZHANG1, Zhikang ZHOU1, Li TONG1( ), Jiangbei CAO1(
), Jiangbei CAO1( )
)
Received:2024-12-27
Online:2025-04-20
Published:2025-04-28
Contact:
Li TONG, Jiangbei CAO
E-mail:gstanes@163.com;caofuyang840723@163.com;tongli301@aliyun.com;caojiangbei@301hospital.com.cn
Supported by:Shuting GUO, Fuyang CAO, Yongxin GUO, Yanxiang LI, Xinyu HAO, Zhuoning ZHANG, Zhikang ZHOU, Li TONG, Jiangbei CAO. Activation of astrocytes in the dorsomedial hypothalamus accelerates sevoflurane anesthesia emergence in mice[J]. Journal of Southern Medical University, 2025, 45(4): 751-759.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.04.10
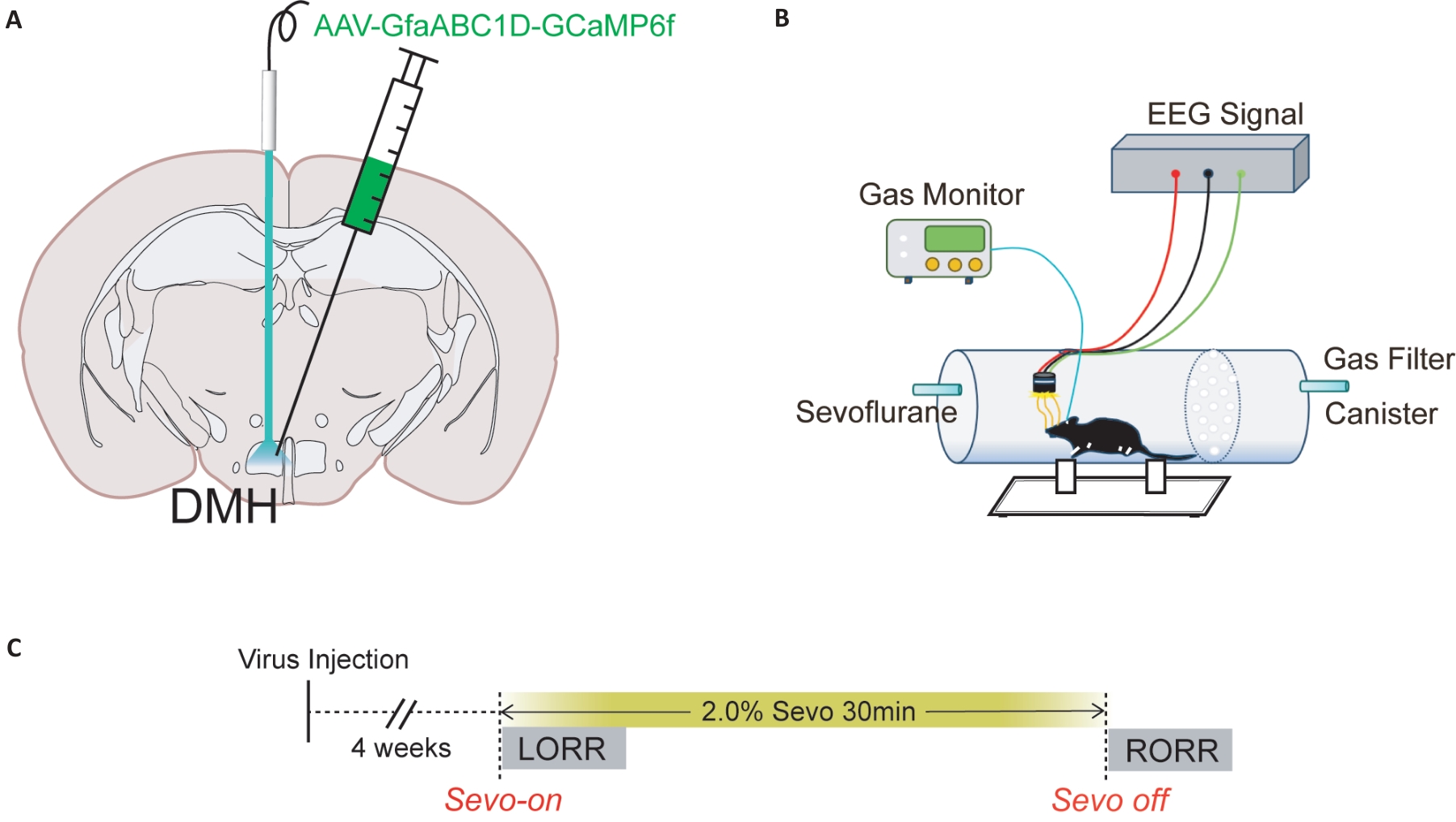
Fig.1 Diagram of virus injection into the brain and schematic diagram of behavioral experiment. A: Schematic diagram of calcium imaging virus injection into the DMH of a mouse. B: Schematic diagram of the calcium signal recording system C: Detailed flow chart of calcium imaging experiment, including the time of sevoflurane on and off, LORR and RORR.
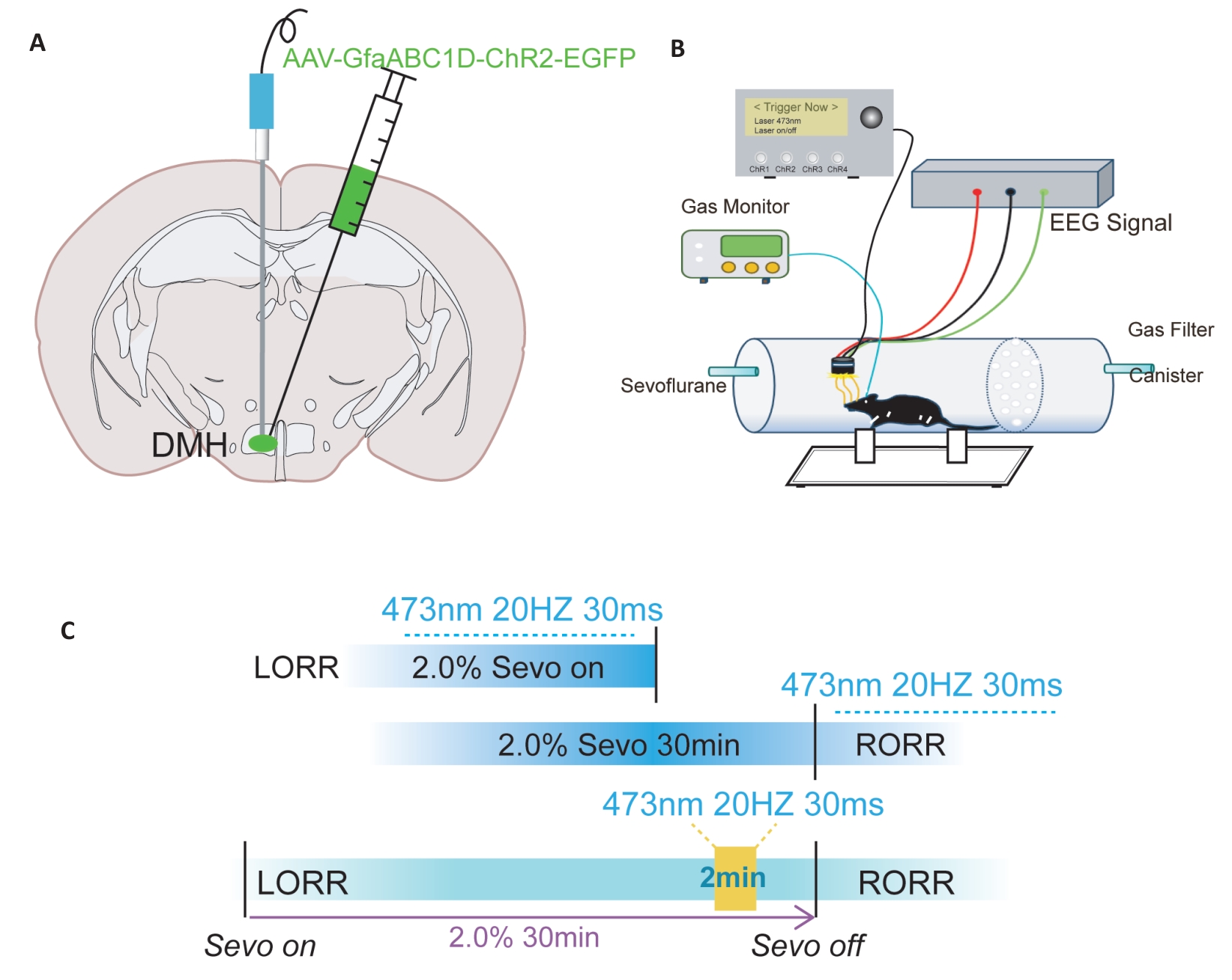
Fig.2 Schematic diagram of optogenetic virus injection and behavioral experiments. A: Illustration of a coronal brain slice showing the locations of virus injection and optic fiber implantation. B: Schematic diagram of the equipment used for behavioral observation, optic fiber stimulation, and EEG recording during anesthesia. C: Flowchart of optogenetic modulation of behavioral testing under general anesthesia.
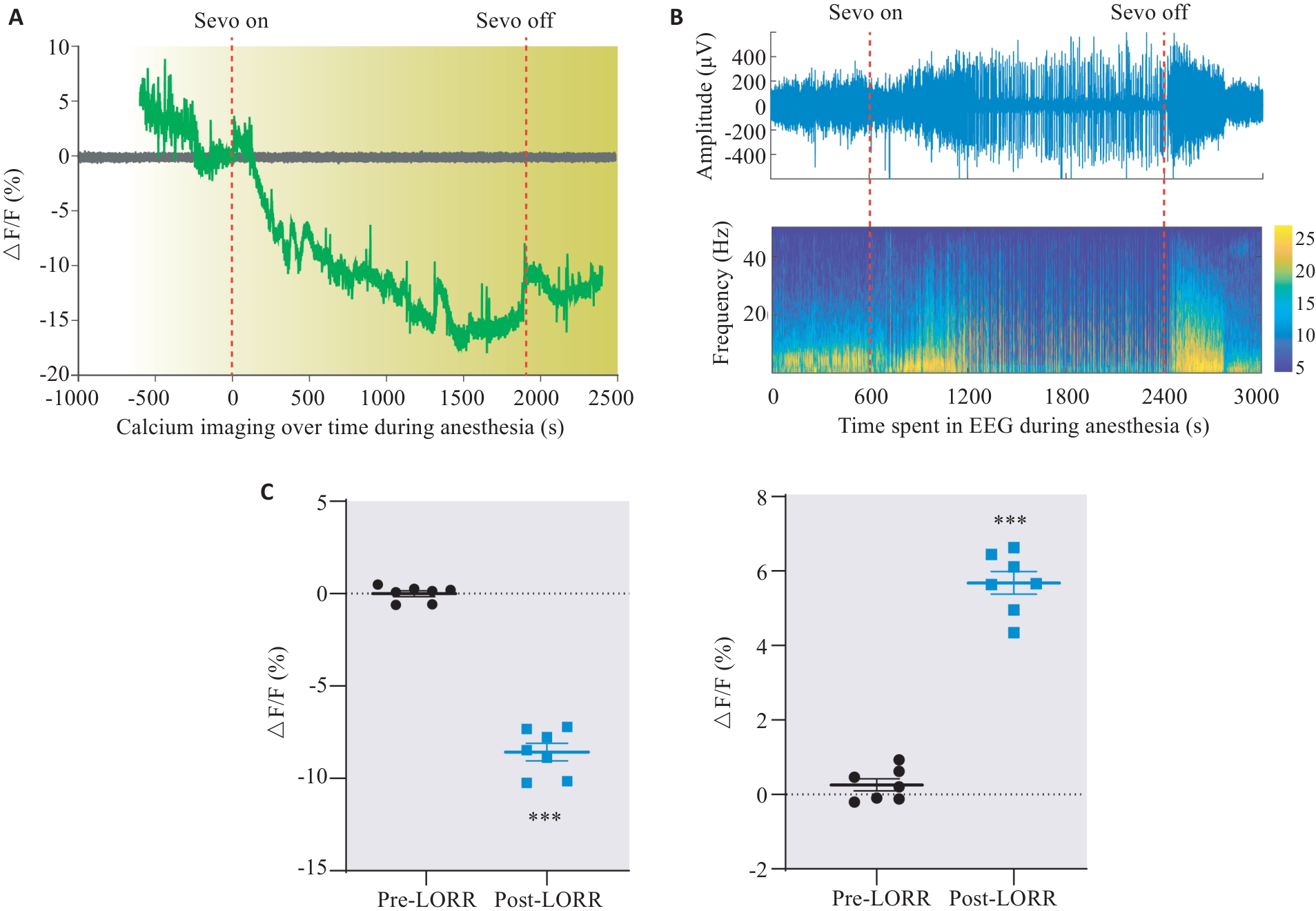
Fig.4 Changes of calcium activity in DMH astrocytes of rats during sevoflurane anesthesia emergence. A: Dynamic monitoring of changes in astrocyte activity during sevoflurane anesthesia. B: Changes in EEG activity during sevoflurane anesthesia corresponding to Fig.A. C: Changes of ΔF/F value of astrocytes in the DMH region during anesthesia induction and awakening. ***P<0.001 vs EGFP group.
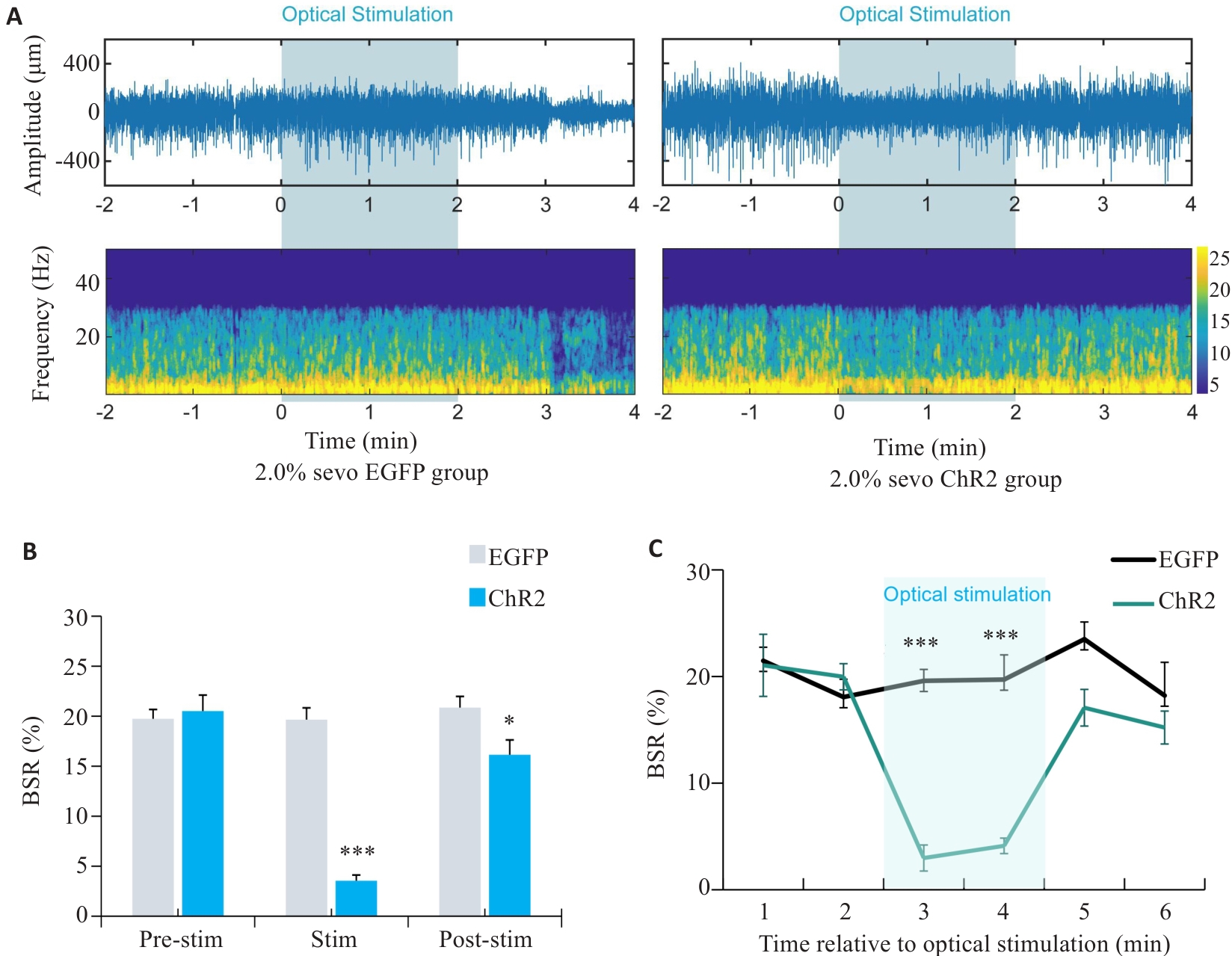
Fig.7 EEG of rats with optogenetically activated DMH astrocytes under 2.0% sevoflurane anesthesia. A: Comparison of representative EEG spectra between EGFP group and ChR2 group under 2.0% sevoflurane anesthesia. B: Comparison of EEG burst suppression rate between EGFP group and ChR2 group after 2 min of light stimulation at 25th min of sevoflurane anesthesia. C: Changes in the brain spontaneous rhythm (BSR%) curve per minute before and after light stimulation. *P<0.05, ***P<0.001 vs EGFP group.
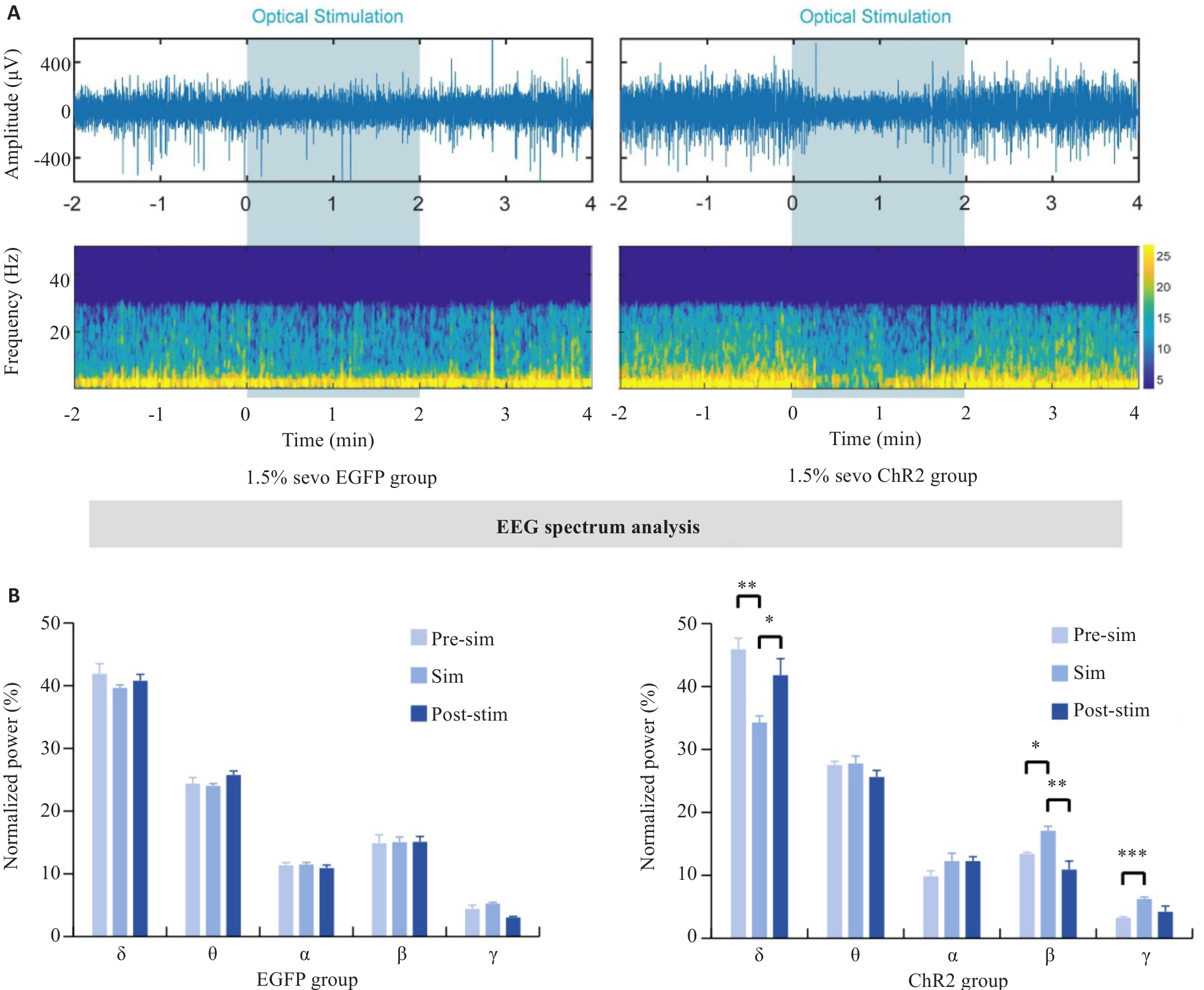
Fig.8 EEG of rats with optogenetically activated DMH astrocytes under 1.5% sevoflurane anesthesia. A: Comparison of representative EEG spectra between EGFP group and ChR2 group under 1.5% sevoflurane anesthesia. B: Quantification of EEG power percentage during optogenetic activation in EGFP and ChR2 mice. *P<0.05, **P<0.01, ***P<0.001.
| 1 | Palanca BA, Avidan MS, Mashour GA. Human neural correlates of sevoflurane-induced unconsciousness[J]. Br J Anaesth, 2017, 119(4): 573-82. |
| 2 | Xu W, Wang L, Yuan XS, et al. Sevoflurane depresses neurons in the medial parabrachial nucleus by potentiating postsynaptic GABAA receptors and background potassium channels[J]. Neuropharm-acology, 2020, 181: 108249. |
| 3 | Yi TT, Wang N, Huang J, et al. A sleep-specific midbrain target for sevoflurane anesthesia[J]. Adv Sci, 2023, 10(15): 2300189. |
| 4 | Alhadidi QM, Bahader GA, Arvola O, et al. Astrocytes in functional recovery following central nervous system injuries[J]. J Physiol, 2024, 602(13): 3069-96. |
| 5 | He QQ, Ji LW, Wang YY, et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption[J]. Cell Metab, 2024, 36(9): 1998-2014.e15. |
| 6 | Cai P, Huang SN, Lin ZH, et al. Regulation of wakefulness by astrocytes in the lateral hypothalamus[J]. Neuropharmacology, 2022, 221: 109275. |
| 7 | Wang YF, Song YP, Tong L, et al. GABAergic neurons in the dorsomedial hypothalamus regulate states of consciousness in sevoflurane anesthesia[J]. iScience, 2023, 26(1): 105913. |
| 8 | Zhong HX, Tong L, Gu N, et al. Endocannabinoid signaling in hypothalamic circuits regulates arousal from general anesthesia in mice[J]. J Clin Invest, 2017, 127(6): 2295-309. |
| 9 | Yoshimoto A, Morikawa S, Kato E, et al. Top-down brain circuits for operant bradycardia[J]. Science, 2024, 384(6702): 1361-8. |
| 10 | Wang D, Bao C, Wu HM, et al. A hypothalamus-lateral periaqueductal gray GABAergic neural projection facilitates arousal following sevoflurane anesthesia in mice[J]. CNS Neurosci Ther, 2024, 30(9): e70047. |
| 11 | Gompf HS, Aston-Jones G. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity[J]. Brain Res, 2008, 1224: 43-52. |
| 12 | Brazhe A, Verisokin A, Verveyko D, et al. Astrocytes: new evidence, new models, new roles[J]. Biophys Rev, 2023, 15(5): 1303-33. |
| 13 | Zhu YW, Ma JL, Li YL, et al. Adenosine-dependent arousal induced by astrocytes in a brainstem circuit[J]. Adv Sci, 2024, 11(48): 2407706. |
| 14 | Kumar VJ, Scheffler K, Grodd W. The structural connectivity mapping of the intralaminar thalamic nuclei[J]. Sci Rep, 2023, 13(1): 11938. |
| 15 | Zhang Z, Huang YC, Chen XY, et al. State-specific regulation of electrical stimulation in the intralaminar thalamus of macaque monkeys: network and transcriptional insights into arousal[J]. Adv Sci, 2024, 11(33): e2402718. |
| 16 | Liu Q, Bell BJ, Kim DW, et al. A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus[J]. Nat Commun, 2023, 14(1): 6381. |
| 17 | Tsuji S, Brace CS, Yao RQ, et al. Sleep-wake patterns are altered with age, Prdm13 signaling in the DMH, and diet restriction in mice[J]. Life Sci Alliance, 2023, 6(6): e202301992. |
| 18 | Li L, Zhang MQ, Sun X, et al. Role of dorsomedial hypothalamus GABAergic neurons in sleep-wake states in response to changes in ambient temperature in mice[J]. Int J Mol Sci, 2022, 23(3): 1270. |
| 19 | Fuente-Martín E, García-Cáceres C, Argente-Arizón P, et al. Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes[J]. Sci Rep, 2016, 6: 23673. |
| 20 | García-Marín V, García-López P, Freire M. Cajal's contributions to Glia research[J]. Trends Neurosci, 2007, 30(9): 479-87. |
| 21 | Ingiosi AM, Hayworth CR, Frank MG. Activation of basal forebrain astrocytes induces wakefulness without compensatory changes in sleep drive[J]. J Neurosci, 2023, 43(32): 5792-809. |
| 22 | Srinivasan R. Calcium signals in astrocytes of the fly brain promote sleep[J]. Cell Calcium, 2021, 94: 102341. |
| 23 | Garofalo S, Picard K, Limatola C, et al. Role of Glia in the regulation of sleep in health and disease[J]. Compr Physiol, 2020, 10(2): 687-712. |
| 24 | Chao DHM, Kirchner MK, Pham C, et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance[J]. Cell Metab, 2022, 34(10): 1532-47.e6. |
| 25 | Chen CR, Zhong YH, Jiang S, et al. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice[J]. eLife, 2021, 10: e69909. |
| 26 | Peng WL, Wu ZF, Song K, et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons[J]. Science, 2020, 369(6508): eabb0556. |
| 27 | Liu PC, Yao W, Chen XY, et al. Parabrachial nucleus astrocytes regulate wakefulness and isoflurane anesthesia in mice[J]. Front Pharmacol, 2023, 13: 991238. |
| 28 | Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information[J]. Trends Neurosci, 2009, 32(8): 421-31. |
| 29 | Péter M, Héja L. High-frequency imaging reveals synchronised delta- and Theta-band Ca2+ oscillations in the astrocytic soma in vivo [J]. Int J Mol Sci, 2024, 25(16): 8911. |
| 30 | Kuga N, Sasaki T, Takahara Y, et al. Large-scale calcium waves traveling through astrocytic NetworksIn vivo[J]. J Neurosci, 2011, 31(7): 2607-14. |
| 31 | Dong R, Han YQ, Jiang LH, et al. Connexin 43 gap junction-mediated astrocytic network reconstruction attenuates isoflurane-induced cognitive dysfunction in mice[J]. J Neuroinflammation, 2022, 19(1): 64. |
| 32 | Nuriya M, Yasui D, Yamada T, et al. Direct posttranslational modification of astrocytic connexin 43 proteins by the general anesthetic propofol in the cerebral cortex[J]. Biochem Biophys Res Commun, 2018, 497(2): 734-41. |
| 33 | Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits[J]. Neuron, 2016, 91(2): 260-92. |
| [1] | Shafa Amir, Montasery Mohammad, Shahhosseini Sedighe, Keivanfar Majid, Mehr Asieh Maghami, Babaei Mahtab Ebrahim, Jafari Mohammad. Original ArticleRespiratory complications of propofol, sevoflurane, and dexmedetomidine anesthesia for fiberoptic bronchoscopy in children aged 1 month to 3 years: a randomized trial [J]. Journal of Southern Medical University, 2024, 44(9): 1631-1636. |
| [2] | Jie CHEN, Chenxu LIU, Chun WANG, Li LI, Weiting TAO, Jingru XUN, Honghui TANG, Li HUANG. Exogenous leptin improves cerebral ischemia-reperfusion-induced glutamate excitotoxic injury in mice by up-regulating GLT-1 and GLAST expression in astrocytes [J]. Journal of Southern Medical University, 2024, 44(6): 1079-1087. |
| [3] | MAN Hao, WANG Jianwei, WU Mao, SHAO Yang, YANG Junfeng, LI Shaoshuo, LÜ Jinye, ZHOU Yue. Jisuikang formula promotes spinal cord injury repair in rats by activating the YAP/PKM2 signaling axis in astrocytes [J]. Journal of Southern Medical University, 2024, 44(4): 636-643. |
| [4] | CAO Fuyang, GUO Yongxin, GUO Shuting, ZHOU Zhikang, CAO Jiangbei, TONG Li, MI Weidong. Activation of GABAergic neurons in the zona incerta accelerates anesthesia induction with sevoflurane and propofol without affecting anesthesia maintenance or awakening in mice [J]. Journal of Southern Medical University, 2023, 43(5): 718-726. |
| [5] | WANG Wenfa, YANG Yong, WANG LI, GUO Xin, TIAN Lingfang, WANG He, HU Yuzhen, LIU Rui. Sevoflurane alleviates ventilator-induced lung injury in rats by down-regulating the TRPV4/C-PLA2 signaling pathway [J]. Journal of Southern Medical University, 2023, 43(11): 1886-1891. |
| [6] | . Application of sevoflurane and laryngeal mask in cesarean section in women with heart disease [J]. Journal of Southern Medical University, 2018, 38(02): 229-. |
| [7] | . Src kinase inhibitor PP2 protects rat astrocytes from hypoxia/reoxygenation injury in vitro [J]. Journal of Southern Medical University, 2015, 35(02): 239-. |
| [8] |
.
Effects of sevoflurane preconditioning on cardiomyocyte apoptosis and myocardial inflammation in rats with sepsis [J]. Journal of Southern Medical University, 2014, 34(11): 1680-. |
| [9] |
.
Impact of topographic features of electrospun polymethylmethacrylate nanofibers on growth pattern of rat primary astrocytes [J]. Journal of Southern Medical University, 2014, 34(11): 1569-. |
| [10] | . 从COX2和5-LOX途径探讨七氟醚抗单肺通气致急性肺损伤的作用机制 [J]. Journal of Southern Medical University, 2013, 33(05): 625-. |
| [11] | . [J]. Journal of Southern Medical University, 2013, 33(04): 469-. |
| [12] | . [J]. Journal of Southern Medical University, 2013, 33(04): 463-. |
| [13] | . Expression of connexin43and functional modulation of gap junction in neonatal rat astrocytesin vitro [J]. Journal of Southern Medical University, 2012, 32(10): 1423-. |
| [14] | YANG Zhi-jun1, RAO Zhi-ren2, XU Ru-xiang1, WEI Ling3, WANG Xiao-bin2, JIANG Xiao-dan1. Three-dimensional structural reconstruction of the neurons and astrocytes in rat hippocampal CA3 area [J]. Journal of Southern Medical University, 2004, 24(04): 426-429. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||