Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (3): 577-586.doi: 10.12122/j.issn.1673-4254.2025.03.15
Zehan LI1( ), Meng LIANG2, Gencheng HAN2, Xuewu ZHANG1(
), Meng LIANG2, Gencheng HAN2, Xuewu ZHANG1( )
)
Received:2024-09-10
Online:2025-03-20
Published:2025-03-28
Contact:
Xuewu ZHANG
E-mail:13691216793@163.com;zhangxuewu@ybu.edu.cn
Supported by:Zehan LI, Meng LIANG, Gencheng HAN, Xuewu ZHANG. Therapeutic effects of inulin-type oligosaccharides of Morinda officinalis on Streptococcus pneumoniae meningitis in mice[J]. Journal of Southern Medical University, 2025, 45(3): 577-586.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.03.15
| Gene | Primer sequence (5'-3') |
|---|---|
| 18S | F:TTGACGGAAGGGCACCACCAG |
| R:GCACCACCACCACGGAATCG | |
| TNF-α | F:CCCTCACACTCAGATCATCTTCT |
| R:GCTACGACGTGGGCTACAG | |
| IL-6 | F:GATGGATGCTACCAAACTGGA |
| R:TCTGAAGGACTCTGGCTTTG | |
| IL-1β | F:TGAAGCAGCTATGGCAACTG |
| R:AGGTCAAAGGTTTGGAAGCA | |
| IL-18 | F:CCAAATCACTTCCTCTTGGC |
| R:GGCCAAAGTTGTCTGATTCC | |
| IFN-γ | F:AAGCGTCATTGAATCACACCTG |
| R:TGACCTCAAACTTGGCAATACTC | |
| iNOS | F:GTTCTCAGCCCAACAATACAAGA |
| R:GTGGACGGGTCGATGTCAC | |
| NLRP3 | F:ATTACCCGCCCGAGAAAGG |
| R:TCGCAGCAAAGATCCACACAG | |
| ASC | F:CTTGTCAGGGGATGAACTCAAAA |
| R:GCCATACGACTCCAGATAGTAGC | |
| Caspase-1 | F:ACAAGGCACGGGACCTATG |
| R:TCCCAGTCAGTCCTGGAAATG | |
| GSDMD | F:CCATCGGCCTTTGAGAAAGTG |
| R:ACACATGAATAACGGGGTTTCC |
Tab.1 Primer sequence used in qRT-PCR
| Gene | Primer sequence (5'-3') |
|---|---|
| 18S | F:TTGACGGAAGGGCACCACCAG |
| R:GCACCACCACCACGGAATCG | |
| TNF-α | F:CCCTCACACTCAGATCATCTTCT |
| R:GCTACGACGTGGGCTACAG | |
| IL-6 | F:GATGGATGCTACCAAACTGGA |
| R:TCTGAAGGACTCTGGCTTTG | |
| IL-1β | F:TGAAGCAGCTATGGCAACTG |
| R:AGGTCAAAGGTTTGGAAGCA | |
| IL-18 | F:CCAAATCACTTCCTCTTGGC |
| R:GGCCAAAGTTGTCTGATTCC | |
| IFN-γ | F:AAGCGTCATTGAATCACACCTG |
| R:TGACCTCAAACTTGGCAATACTC | |
| iNOS | F:GTTCTCAGCCCAACAATACAAGA |
| R:GTGGACGGGTCGATGTCAC | |
| NLRP3 | F:ATTACCCGCCCGAGAAAGG |
| R:TCGCAGCAAAGATCCACACAG | |
| ASC | F:CTTGTCAGGGGATGAACTCAAAA |
| R:GCCATACGACTCCAGATAGTAGC | |
| Caspase-1 | F:ACAAGGCACGGGACCTATG |
| R:TCCCAGTCAGTCCTGGAAATG | |
| GSDMD | F:CCATCGGCCTTTGAGAAAGTG |
| R:ACACATGAATAACGGGGTTTCC |
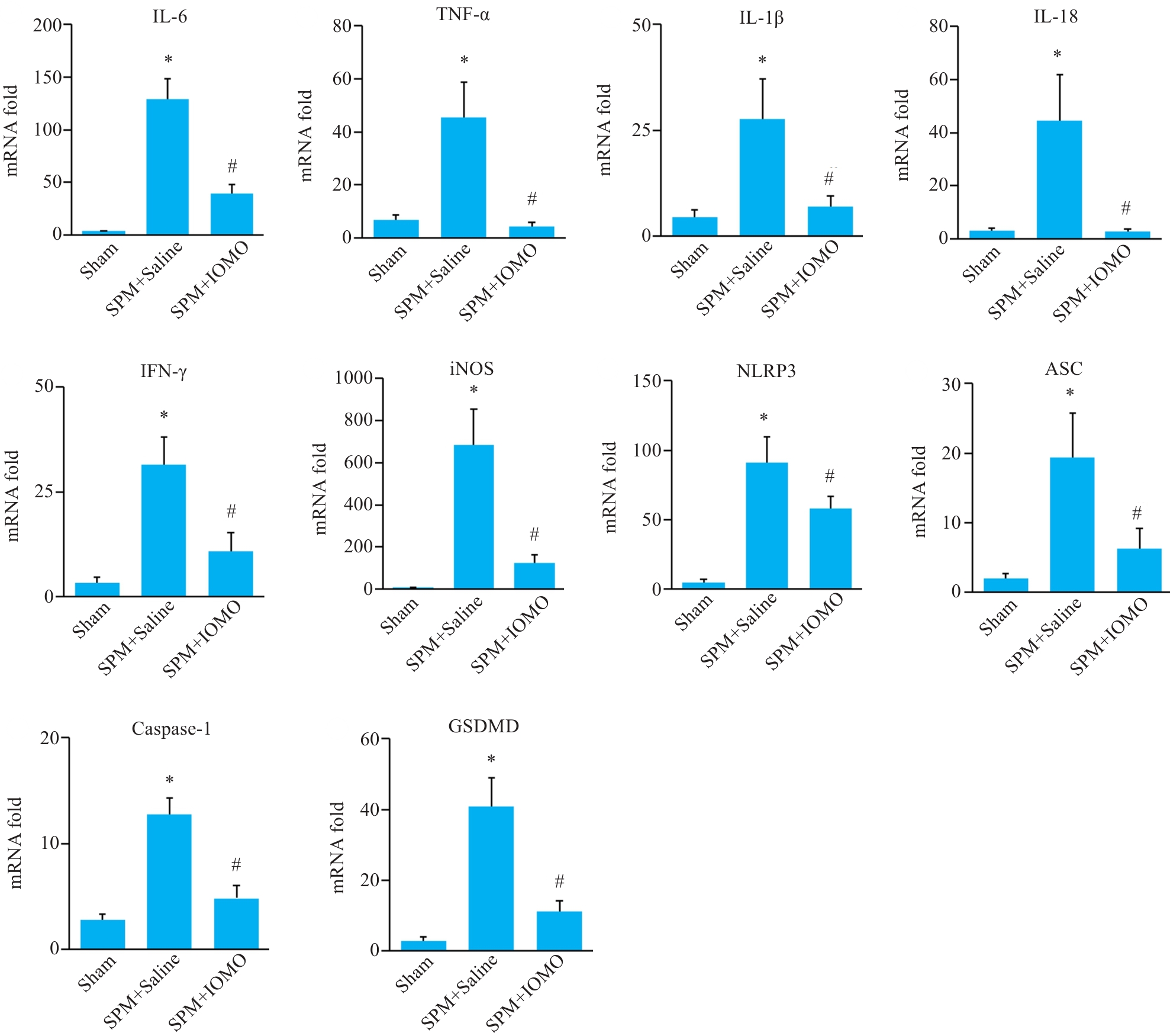
Fig.5 Effects of IOMO on mRNA levels of inflammation-related molecules mRNA levels in the cerebral cortex of SPM mice (Mean±SD, n=4). *P<0.05 vs Sham group, #P<0.05 vs SPM+Saline group.
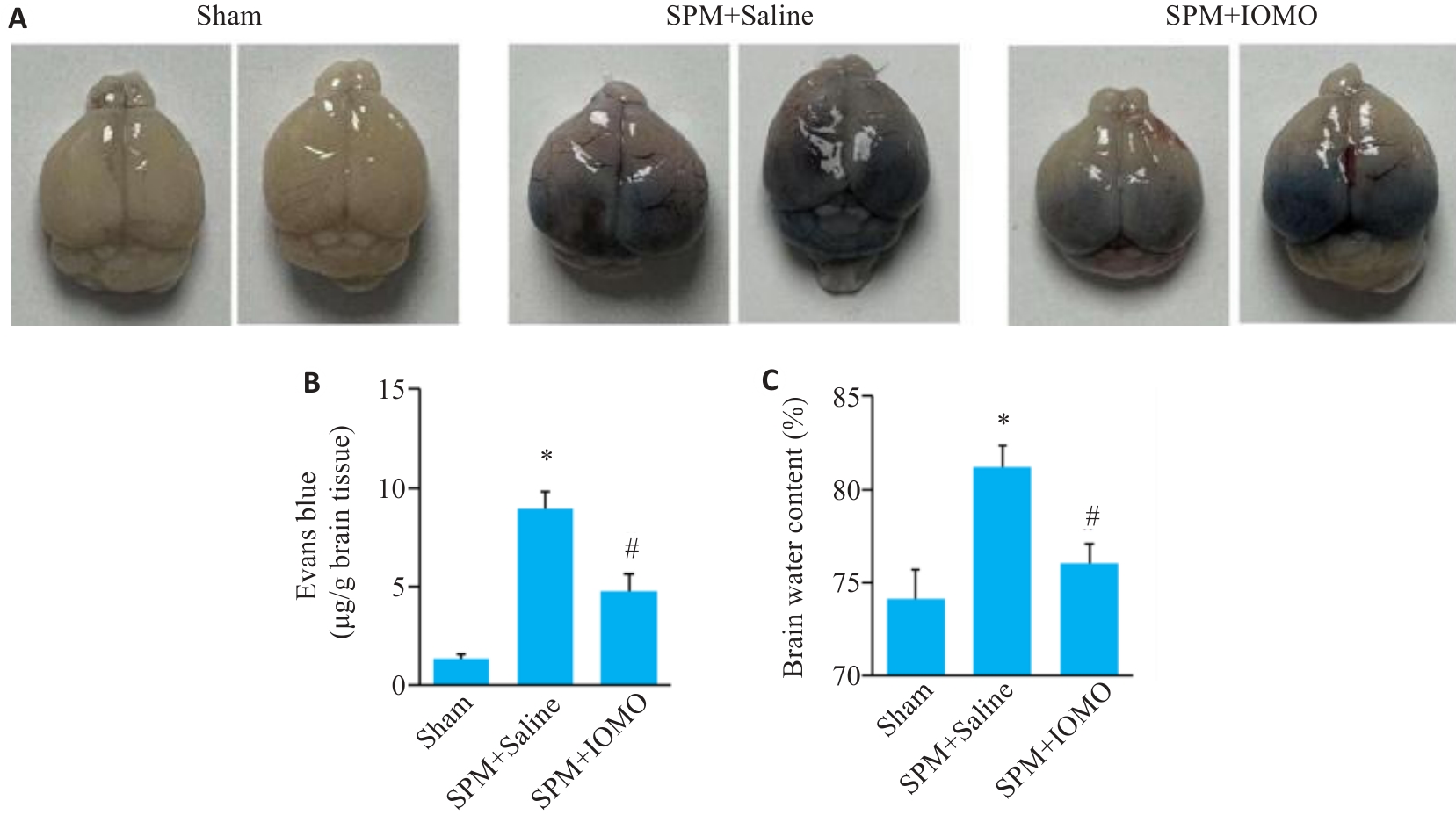
Fig.6 Effects of IOMO on brain water content and BBB permeability of SPM mice (Mean±SD, n=6). A: Evans blue (EB) staining. B: Statistics of the results of EB staining. C: Statistics of brain water content. *P<0.05 vs Sham group,#P<0.05 vs SPM+Saline group.
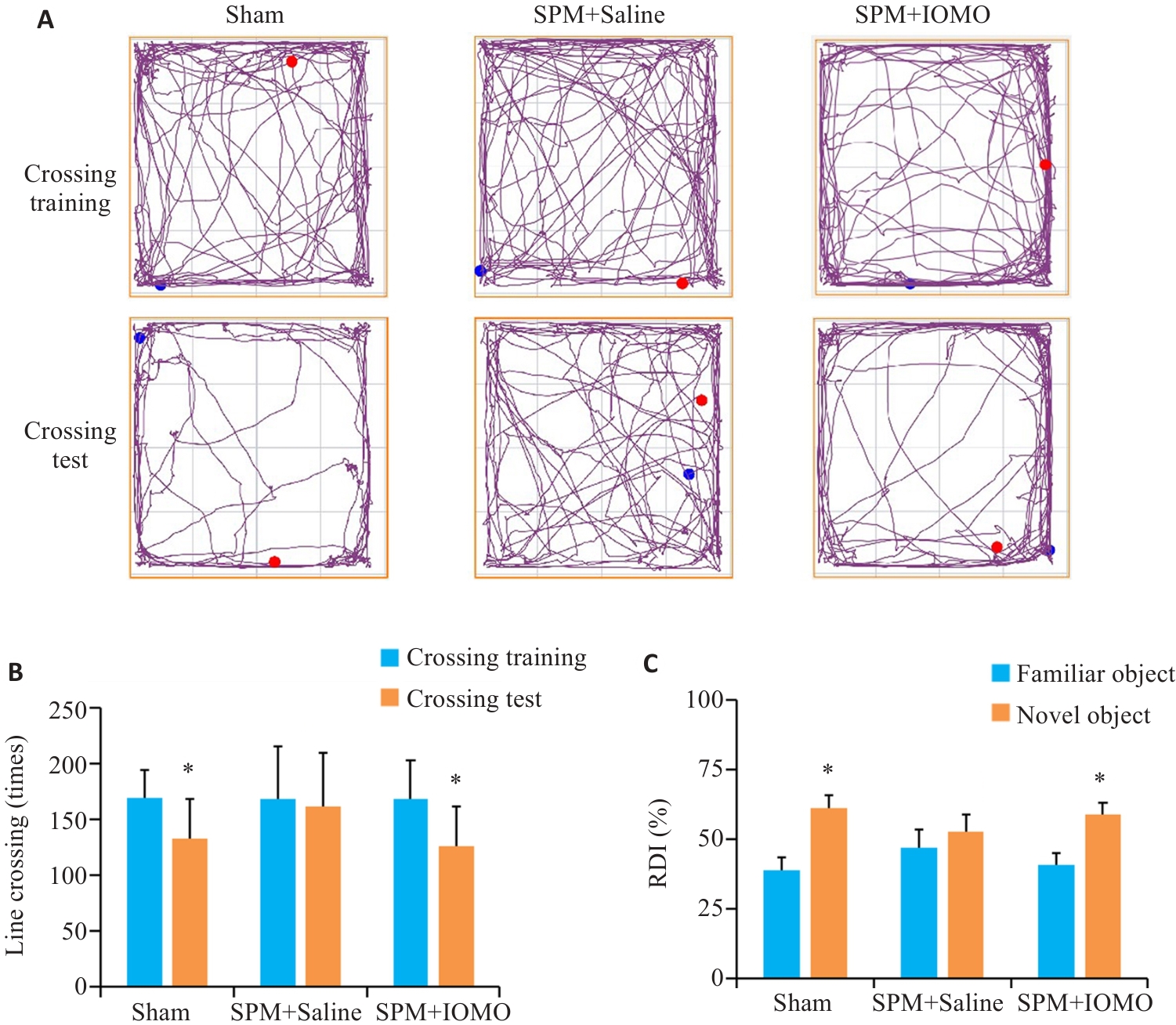
Fig.9 IOMO alleviates SPM-induced learning and memory dysfunction in mice (Mean±SD, n=8). A, B: Traveling traces and statistics in open field test (*P<0.05 vs Crossing Training). C: Statistics of the results of new object recognition test (*P<0.05 vs familiar group).
| 1 | Glimåker M, Naucler P, Sjölin J. Etiology, clinical presentation, outcome and the effect of initial management in immuno-compromised patients with community acquired bacterial meningitis[J]. J Infect, 2020, 80(3): 291-7. |
| 2 | Mook-Kanamori BB, Geldhoff M, van der Poll T, et al. Pathogenesis and pathophysiology of pneumococcal meningitis[J]. Clin Microbiol Rev, 2011, 24(3): 557-91. |
| 3 | Gil E, Wall E, Noursadeghi M, et al. Streptococcus pneumoniae meningitis and the CNS barriers[J]. Front Cell Infect Microbiol, 2023, 12: 1106596. |
| 4 | Costerus JM, Brouwer MC, Bijlsma MW, et al. Community-acquired bacterial meningitis[J]. Curr Opin Infect Dis, 2017, 30(1): 135-41. |
| 5 | Gao ZQ, Song YJ, Hsiao TY, et al. Machine-learning-assisted microfluidic nanoplasmonic digital immunoassay for cytokine storm profiling in COVID-19 patients[J]. ACS Nano, 2021, 15(11): 18023-36. |
| 6 | Huang K, Zhang P, Zhang ZH, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms[J]. Pharmacol Ther, 2021, 225: 107843. |
| 7 | Zhang JH, Xin HL, Xu YM, et al. Morinda officinalis How.-A comprehensive review of traditional uses, phytochemistry and pharmacology[J]. J Ethnopharmacol, 2018, 213: 230-55. |
| 8 | Wang YH, Yin YY, Yao JQ, et al. Behavioral evaluation of anti-post-traumatic stress disorder effect of inulin-type oligosaccharides of Morinda officinalis[J]. Chin J Pharmacol Toxicol. 2021, 35(1): 27-35. |
| 9 | Zhang HL, Li J, Xia JM, et al. Antioxidant activity and physicochemical properties of an acidic polysaccharide from Morinda officinalis [J]. Int J Biol Macromol, 2013, 58: 7-12. |
| 10 | Shin JS, Yun KJ, Chung KS, et al. Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-κB inactivation[J]. Food Chem Toxicol, 2013, 53: 263-71. |
| 11 | Li ZF, Xu HX, Xu Y, et al. Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation[J]. CNS Neurosci Ther, 2021, 27(12): 1570-86. |
| 12 | Liang J, Liang JW, Hao HR, et al. The extracts of Morinda officinalis and its hairy roots attenuate dextran sodium sulfate-induced chronic ulcerative colitis in mice by regulating inflammation and lymphocyte apoptosis[J]. Front Immunol, 2017, 8: 905. |
| 13 | Yau B, Mitchell AJ, Too LK, et al. Interferon-γ-induced nitric oxide synthase-2 contributes to blood/brain barrier dysfunction and acute mortality in experimental Streptococcus pneumoniae meningitis[J]. J Interferon Cytokine Res, 2016, 36(2): 86-99. |
| 14 | Koopmans MM, Engelen-Lee J, Brouwer MC, et al. Characterization of a Listeria monocytogenes meningitis mouse model[J]. J Neuroinflammation, 2018, 15(1): 257. |
| 15 | Li G, Tang LL, Hou CM, et al. Peripheral injection of tim-3 antibody attenuates VSV encephalitis by enhancing MHC-I presentation[J]. Front Immunol, 2021, 12: 667478. |
| 16 | Waltl I, Käufer C, Gerhauser I, et al. Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage[J]. Brain Behav Immun, 2018, 74: 186-204. |
| 17 | Gao ZF, Xie S, Wang LY, et al. Hypidone hydrochloride (YL-0919) protects mice from meningitis via Sigma1R-STAT1-NLRP3-GSDMD pathway[J]. Int Immunopharmacol, 2024, 128: 111524. |
| 18 | Yau B, Hunt NH, Mitchell AJ, et al. Blood-brain barrier pathology and CNS outcomes in Streptococcus pneumoniae meningitis[J]. Int J Mol Sci, 2018, 19(11): 3555. |
| 19 | van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis[J]. Clin Microbiol Infect, 2016, 22(): S37-62. |
| 20 | Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis[J]. Cochrane Database Syst Rev, 2015, 2015(9): CD004405. |
| 21 | 钟 亮, 林如琴, 龙北国, 等. 鼠李糖乳杆菌抑制阪崎克罗诺杆菌致脑膜炎的体内外实验[J]. 南方医科大学学报, 2015, 35(8): 1079-83. DOI: 10.3969/j.issn.1673-4254.2015.08.001 |
| 22 | Tunkel AR, Michael Scheld W. Corticosteroids for everyone with meningitis[J]? N Engl J Med, 2002, 347(20): 1613-5. |
| 23 | Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy[J]. Trends Neurosci, 2002, 25(1): 38-45. |
| 24 | 王 轶, 郭婷婷, 尹勇玉, 等. 菊淀粉型巴戟天寡糖对慢性应激模型小鼠抑郁、焦虑样行为的改善及对调节性T细胞数量的调节作用[J]. 中国药理学与毒理学杂志, 2022, 36(6): 426-34. DOI: 10.3867/j.issn.1000-3002.2022.06.004 |
| 25 | Yang X, Chen DL, Yang J, et al. Effects of oligosaccharides from Morinda officinalis on gut microbiota and metabolome of APP/PS1 transgenic mice[J]. Front Neurol, 2018, 9: 412. |
| 26 | 孔庆梅, 舒 良, 张鸿燕, 等. 巴戟天寡糖胶囊治疗抑郁症的临床疗效与安全性[J]. 中国临床药理学杂志, 2011, 27(3): 170-3. |
| 27 | 呼亚玲. 巴戟天寡糖胶囊治疗轻中度抑郁症急性发作期的临床研究[J]. 中西医结合心脑血管病杂志, 2018, 16(7): 970-1, 992. |
| 28 | 李囿松, 呼亚玲, 申 静, 等. 巴戟天寡糖胶囊治疗广泛人群下急性发作期轻中度抑郁症的Ⅳ期临床研究[J]. 中药药理与临床, 2022, 38(4): 136-9. |
| 29 | Sharief MK, Ciardi M, Thompson EJ. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-alpha but not interleukin-1 beta[J]. J Infect Dis, 1992, 166(2): 350-8. |
| 30 | Barichello T, Generoso JS, Simões LR, et al. Interleukin-1β receptor antagonism prevents cognitive impairment following experimental bacterial meningitis[J]. Curr Neurovasc Res, 2015, 12(3): 253-61. |
| 31 | Klein RS, Hunter CA. Protective and pathological immunity during central nervous system infections[J]. Immunity, 2017, 46(6): 891-909. |
| 32 | Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis[J]. J Biol Chem, 2005, 280(14): 13906-12. |
| 33 | Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease[J]. Pharmacol Rev, 2005, 57(2): 173-85. |
| 34 | Barichello T, Generoso JS, Simões LR, et al. Role of oxidative stress in the pathophysiology of pneumococcal meningitis[J]. Oxid Med Cell Longev, 2013, 2013: 371465. |
| 35 | Meli DN, Christen S, Leib SL. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway[J]. J Infect Dis, 2003, 187(9): 1411-5. |
| 36 | Braun JS, Hoffmann O, Schickhaus M, et al. Pneumolysin causes neuronal cell death through mitochondrial damage[J]. Infect Immun, 2007, 75(9): 4245-54. |
| 37 | Hupp S, Heimeroth V, Wippel C, et al. Astrocytic tissue remodeling by the meningitis neurotoxin pneumolysin facilitates pathogen tissue penetration and produces interstitial brain edema[J]. Glia, 2012, 60(1): 137-46. |
| 38 | Coutinho LG, Grandgirard D, Leib SL, et al. Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis[J]. BMC Infect Dis, 2013, 13: 326. |
| 39 | Hoegen T, Tremel N, Klein M, et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release[J]. J Immunol, 2011, 187(10): 5440-51. |
| 40 | Häusler KG, Prinz M, Nolte C, et al. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages[J]. Eur J Neurosci, 2002, 16(11): 2113-22. |
| 41 | Too LK, Ball HJ, McGregor IS, et al. The pro-inflammatory cytokine interferon-gamma is an important driver of neuropathology and behavioural sequelae in experimental pneumococcal meningitis[J]. Brain Behav Immun, 2014, 40: 252-68. |
| 42 | Liu CY, Zhou F, Pan DY, et al. Study on effect of melatonin on protection of brains of mice with Cryptococcus neoformans meningitis[J]. Chin J Nosocomiol, 2020, 30(7): 961-966. |
| 43 | Winkler F, Koedel U, Kastenbauer S, et al. Differential expression of nitric oxide synthases in bacterial meningitis: role of the inducible isoform for blood-brain barrier breakdown[J]. J Infect Dis, 2001, 183(12): 1749-59. |
| 44 | Chi LD, Chen LX, Zhang JW, et al. Development and application of bio-sample quantification to evaluate stability and pharmacokinetics of inulin-type fructo-oligosaccharides from Morinda Officinalis[J]. J Pharm Biomed Anal, 2018, 156: 125-32. |
| 45 | Zhang ZW, Gao CS, Zhang H, et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota[J]. Acta Pharm Sin B, 2022, 12(8): 3298-312. |
| 46 | 李新翼, 刘玉杰, 邓克崇, 等. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-10. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||