Journal of Southern Medical University ›› 2025, Vol. 45 ›› Issue (2): 245-253.doi: 10.12122/j.issn.1673-4254.2025.02.05
Yali ZHAO1( ), Jiayi LI1, Bianli GU1, Pan CHEN1, Li ZHANG1, Xiaoman ZHANG2, Pingjuan YANG1, Linlin SHI1(
), Jiayi LI1, Bianli GU1, Pan CHEN1, Li ZHANG1, Xiaoman ZHANG2, Pingjuan YANG1, Linlin SHI1( ), Shegan GAO1(
), Shegan GAO1( )
)
Received:2024-10-14
Online:2025-02-20
Published:2025-03-03
Contact:
Linlin SHI, Shegan GAO
E-mail:676305392@qq.com;celine_shih@ haust.edu.cn;gsg112258@163.com
Supported by:Yali ZHAO, Jiayi LI, Bianli GU, Pan CHEN, Li ZHANG, Xiaoman ZHANG, Pingjuan YANG, Linlin SHI, Shegan GAO. Ag2Se nanoparticles suppress growth of murine esophageal cancer allograft in mice by eliminating Porphyromonas gingivalis[J]. Journal of Southern Medical University, 2025, 45(2): 245-253.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2025.02.05
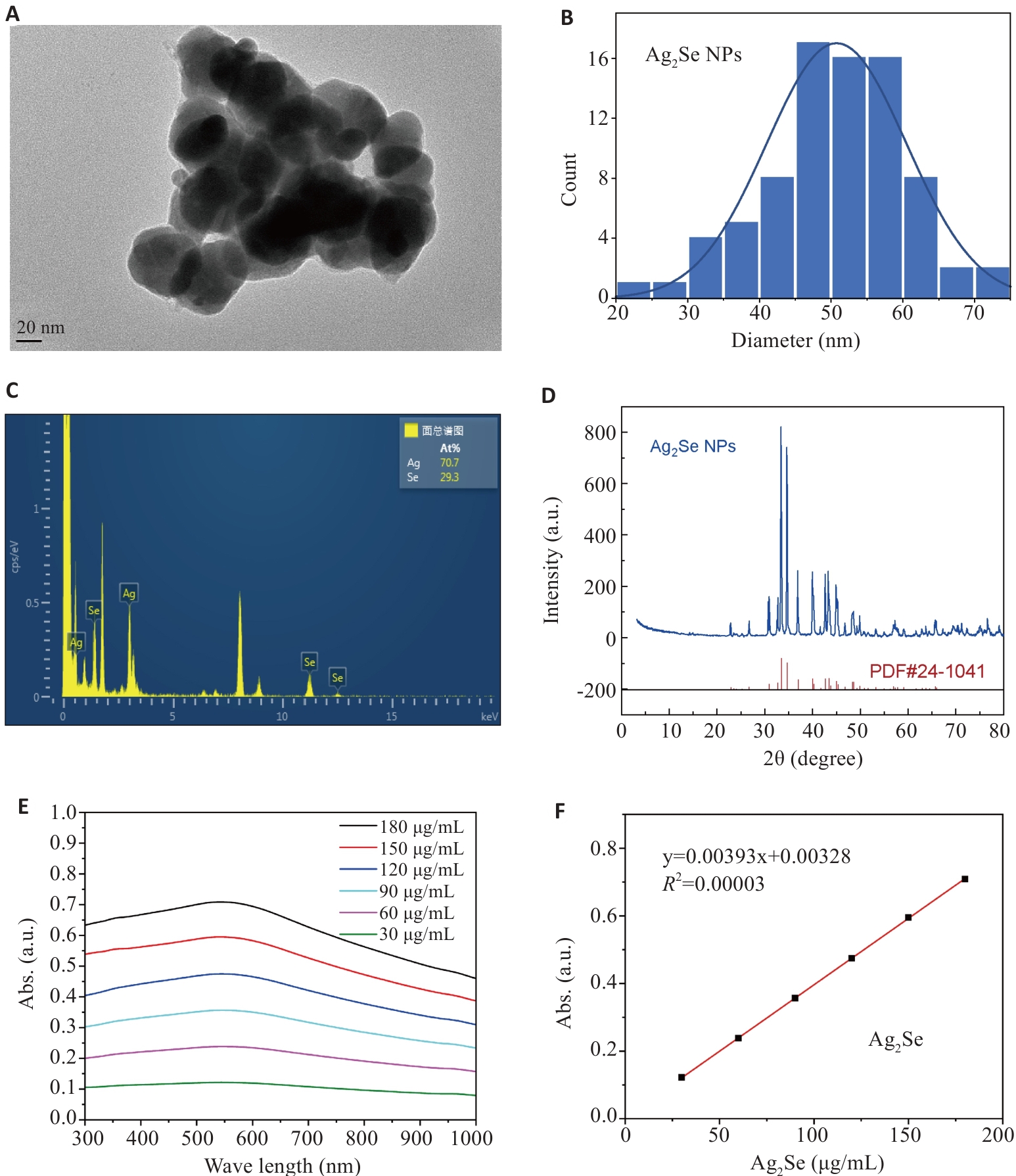
Fig.1 Characterization of Ag2Se nanoparticles. A, B: Transmission electron microscopy of Ag2Se nanoparticles and the particle size distribution. C: EDS spectrum of Ag2Se nanoparticles. D: XRD of patterns of Ag2Se nanoparticles. E: Ultraviolet absorption map of Ag2Se nanoparticles at different concentrations. F: Fitting equation of ultraviolet absorption of Ag2Se nanoparticles.
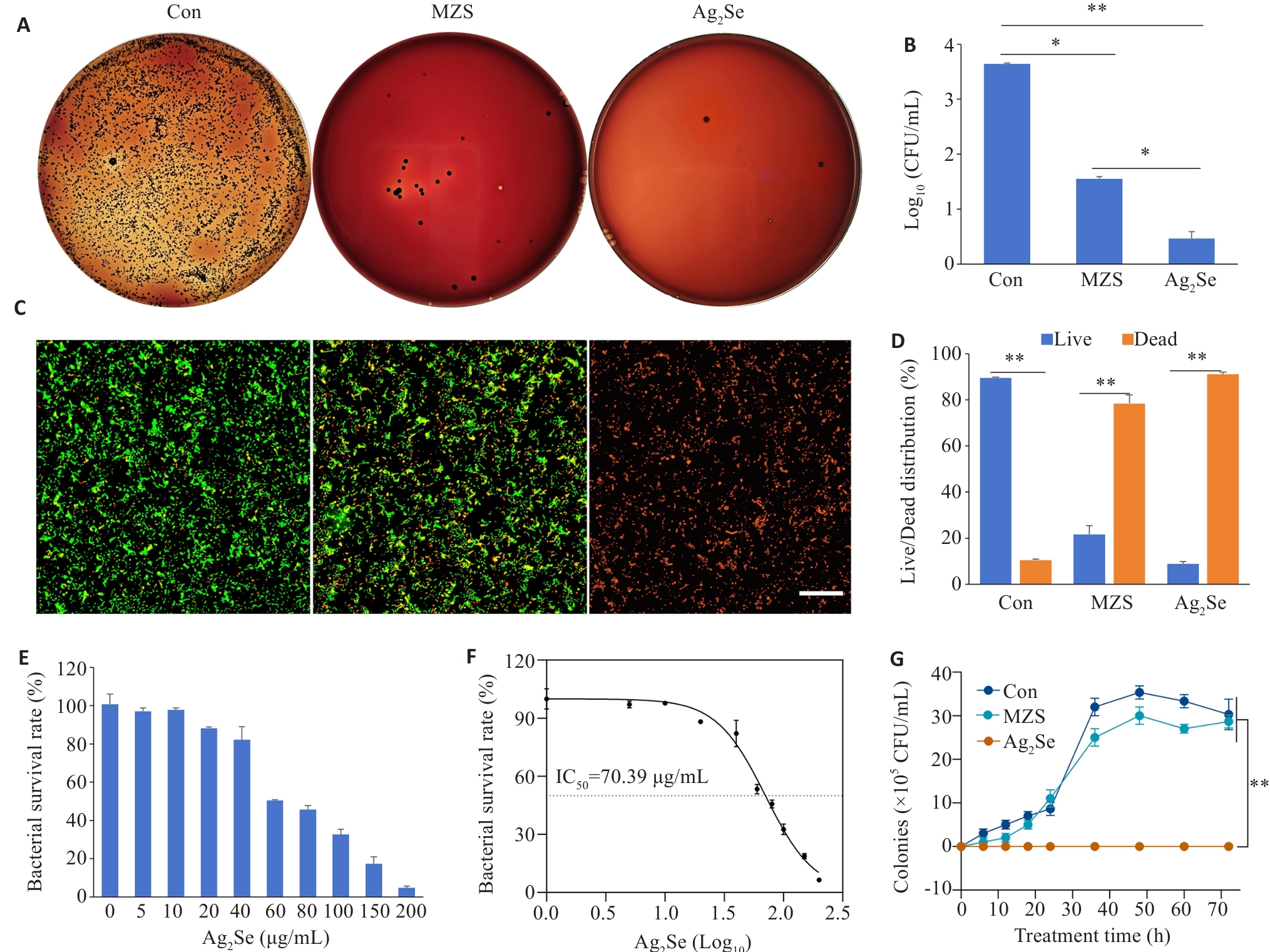
Fig.2 Antibacterial performance evaluation of Ag2Se nanoparticles in vitro. A: P. gingivalis cultures with different treatments for 6 h and inoculated on solid Columbia blood agar plates at 37 ℃ in a anaerobic chamber for 7 days. B: Quantitative map of colony formation. C: CLSM images of P. gingivalis stained with MycoLight™ Green and PI nucleic acid dye (green and red fluorescence represent live and dead bacteria, respectively), at Ag2Se concentration of 80 μg/mL (scale bar=30 µm). D: Quantitative map of live and dead bacterium. E: Bacterial survival after treatment at different drug concentrations (0, 5, 10, 20, 40, 60, 80, 100, 150, 200 μg/mL). F: Effects of different drug concentrations, expressed as logarithmic values (Log10), on bacterial survival. The IC50 value was 70.39 μg/mL. G: Growth curves of P.gingivalis with different treatments. Data are presented as Mean±SD (n=3). *P<0.05, **P<0.01.
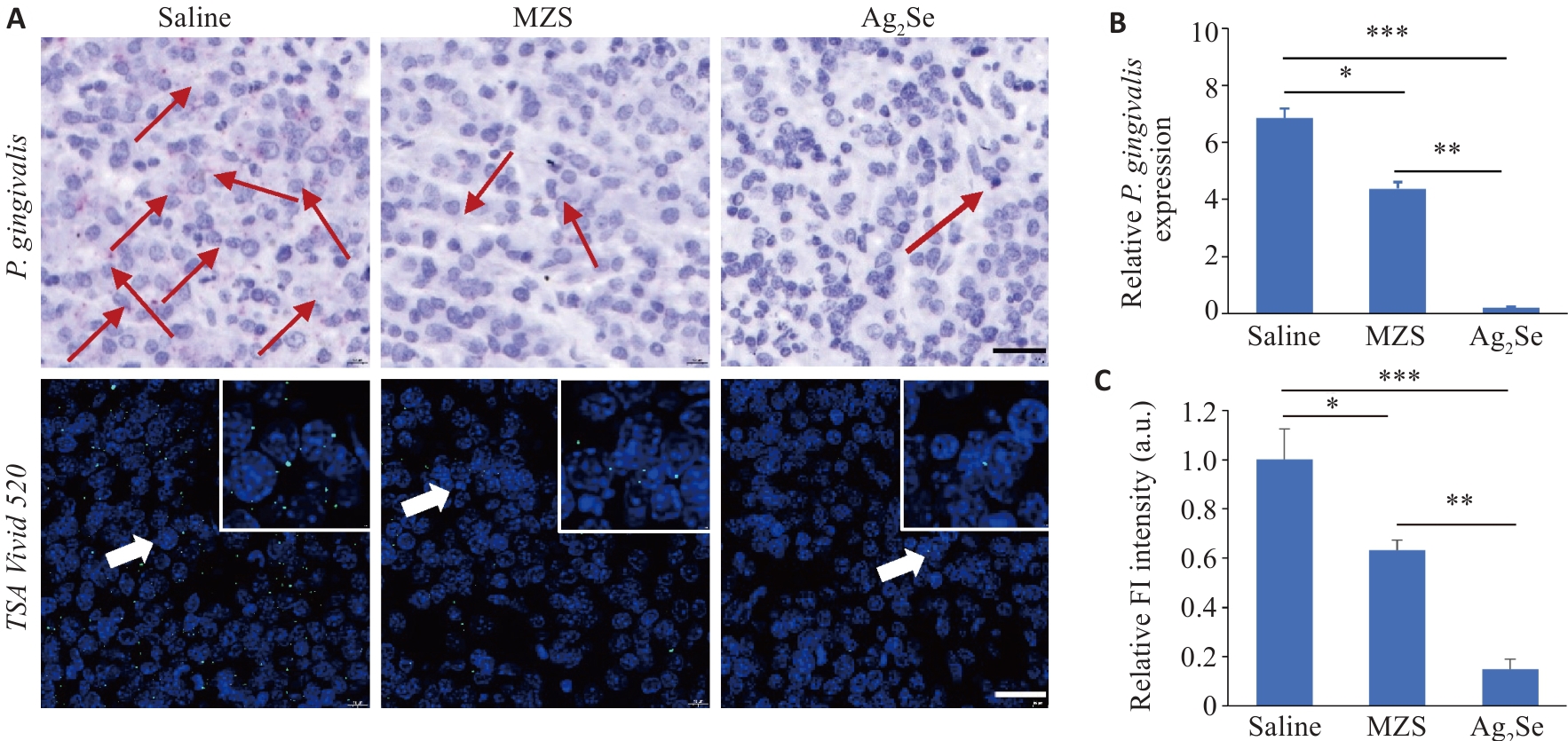
Fig.3 Antibacterial evaluation of Ag2Se nanoparticles in tumor tissues in tumor-bearing mice. A: RNAscope in situ hybridization to detect the abundance of P. gingivalis in the tumor tissues (scale bar=25 μm). Red and green fluorescence signals both indicate P. gingivalis. B: Quantification of P. gingivalis DNA in the tumor tissue from different treatment groups using qPCR detection. C: Quantitative analysis of fluorescence intensity. Data are presented as Mean±SD (n=5). *P<0.05, **P<0.01, ***P<0.001.
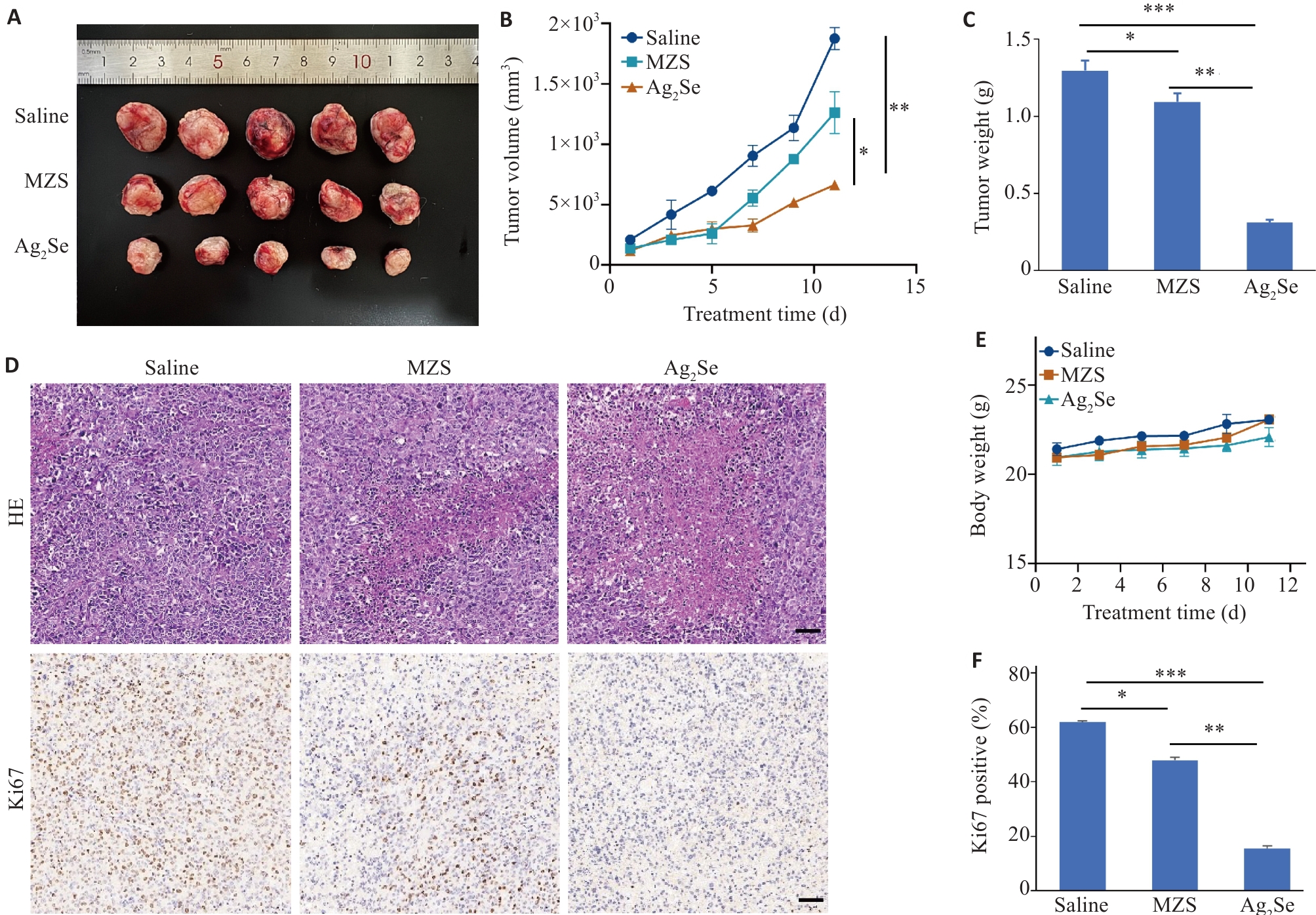
Fig.4 Effect of Ag2Se nanoparticles on growth of esophageal cancer cells in mice. A: Volume of the dissected tumors in each group. B: Tumor volume curves of the mice with different treatments. C: Tumor weight of the mice treated with saline, MZS and Ag2Se nanoparticles. D: HE staining and Ki67 immunohistochemistry of the tumor sections in each group (scale bar=50 μm). E: Body weight curves of C57 mice with different treatments. F: Quantitative analysis of Ki67 staining in different groups. Data are presented as Mean±SD (n=5). *P<0.05, **P<0.01, ***P<0.001.
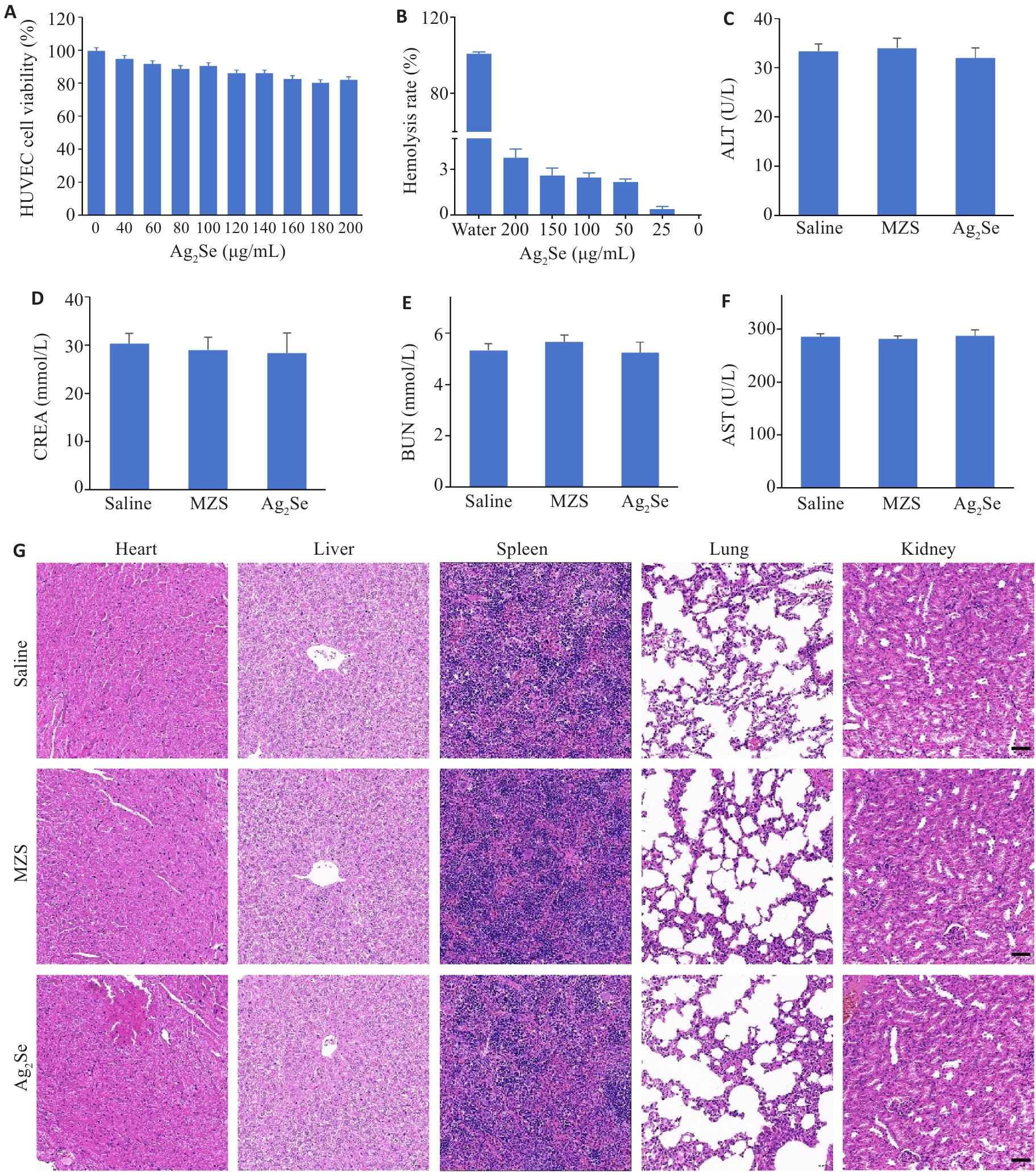
Fig.5 Evaluation of biocompatibility of Ag2Se nanoparticles. A: HUVEC viability after incubation with different concentrations of Ag2Se nanoparticles for 24 h. B: Hemolysis assay after different treatments and the hemolysis ratio detected at 540 nm. C-F: ALT, AST, BUN, and CREA of the mice after different treatments. G: HE staining of the heart, liver, spleen, lung, and kidney tissues in different groups (scale bar=50 μm). Data are presented as Mean±SD (n=3).
| 1 | Fu AK, Yao BQ, Dong TT, et al. Emerging roles of intratumor microbiota in cancer metastasis[J]. Trends Cell Biol, 2023, 33(7): 583-93. |
| 2 | Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond[J]. Nat Rev Cancer, 2010, 10(6): 403-14. |
| 3 | Griffith DM, Li HY, Werrett MV, et al. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles[J]. Chem Soc Rev, 2021, 50(21): 12037-69. |
| 4 | Zhang SK, Xu HF, Zhang LY, et al. Cervical cancer: Epidemiology, risk factors and screening[J]. Chin J Cancer Res, 2020, 32(6): 720-8. |
| 5 | Ou SW, Chen HP, Wang HF, et al. Fusobacterium nucleatum upregulates MMP7 to promote metastasis-related characteristics of colorectal cancer cell via activating MAPK(JNK)-AP1 axis[J]. J Transl Med, 2023, 21(1): 704. |
| 6 | Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location[J]. Clin Transl Gastroenterol, 2016, 7(11): e200. |
| 7 | Fu Y, Li J, Cai WY, et al. The emerging tumor microbe microenvironment: from delineation to multidisciplinary approach-based interventions[J]. Acta Pharm Sin B, 2024, 14(4): 1560-91. |
| 8 | Jiang HX, Li L, Bao YX, et al. Microbiota in tumors: new factor influencing cancer development[J]. Cancer Gene Ther, 2024, 31(12): 1773-85. |
| 9 | Gao SG, Liu YW, Duan XX, et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy[J]. Br J Cancer, 2021, 125(3): 433-44. |
| 10 | Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. |
| 11 | Zitvogel L, Ayyoub M, Routy B, et al. Microbiome and anticancer immunosurveillance[J]. Cell, 2016, 165(2): 276-87. |
| 12 | Zitvogel L, Daillère R, Roberti MP, et al. Anticancer effects of the microbiome and its products[J]. Nat Rev Microbiol, 2017, 15(8): 465-78. |
| 13 | Nanayakkara AK, Boucher HW, Fowler VG Jr, et al. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward[J]. CA Cancer J Clin, 2021, 71(6): 488-504. |
| 14 | Petrelli F, Ghidini M, Ghidini A, et al. Use of antibiotics and risk of cancer: a systematic review and meta-analysis of observational studies[J]. Cancers, 2019, 11(8): 1174. |
| 15 | Chambers LM, Esakov Rhoades EL, Bharti R, et al. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer[J]. Cancer Res, 2022, 82(24): 4654-69. |
| 16 | Ghanem S, Kim CJ, Dutta D, et al. Antimicrobial therapy during cancer treatment: beyond antibacterial effects[J]. J Intern Med, 2021, 290(1): 40-56. |
| 17 | Liu ZY, Ma YG, Ye JX, et al. Drug delivery systems for enhanced tumour treatment by eliminating intra-tumoral bacteria[J]. J Mater Chem B, 2024, 12(5): 1194-207. |
| 18 | Shi JJ, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities[J]. Nat Rev Cancer, 2017, 17(1): 20-37. |
| 19 | Liu JY, Wang ZY, Liu FD, et al. Chemical transformations of nanosilver in biological environments[J]. ACS Nano, 2012, 6(11): 9887-99. |
| 20 | Li W, Ding QH, Li MQ, et al. Stimuli-responsive and targeted nanomaterials: Revolutionizing the treatment of bacterial infections[J]. J Control Release, 2025, 377: 495-523. |
| 21 | Subhan MA, Yalamarty SSK, Filipczak N, et al. Recent advances in tumor targeting via EPR effect for cancer treatment[J]. J Pers Med, 2021, 11(6): 571. |
| 22 | Bruna T, Maldonado-Bravo F, Jara P, et al. Silver nanoparticles and their antibacterial applications[J]. Int J Mol Sci, 2021, 22(13): 7202. |
| 23 | Ren QW, Wang Y, Qian J, et al. Biosynthesis of Ag2Se nanoparticles as a broad-spectrum antimicrobial agent with excellent bioco-mpatibility[J]. J Hazard Mater, 2024, 465: 133201. |
| 24 | Ozdal OG. Green synthesis of Ag, Se, and Ag2Se nanoparticles by Pseudomonas aeruginosa: characterization and their biological and photocatalytic applications[J]. Folia Microbiol, 2024, 69(3): 625-38. |
| 25 | Koul B, Poonia AK, Yadav D, et al. Microbe-mediated biosynthesis of nanoparticles: applications and future prospects[J]. Biomolecules, 2021, 11(6): 886. |
| 26 | Dhanker R, Hussain T, Tyagi P, et al. The emerging trend of bio-engineering approaches for microbial nanomaterial synthesis and its applications[J]. Front Microbiol, 2021, 12: 638003. |
| 27 | El-Khawaga AM, Zidan A, El-Mageed AIAA. Preparation methods of different nanomaterials for various potential applications: a review[J]. J Mol Struct, 2023, 1281: 135148. |
| 28 | Yang XJ, Wang C, Zhang XX, et al. Photothermal and adsorption effects of silver selenide nanoparticles modified by different surfactants in nursing care of cancer patients[J]. Sci Technol Adv Mater, 2020, 21(1): 584-92. |
| 29 | Sepich-Poore GD, Zitvogel L, Straussman R, et al. The microbiome and human cancer[J]. Science, 2021, 371(6536): eabc4552. |
| 30 | Chen MF, Lu MS, Hsieh CC, et al. Porphyromonas gingivalis promotes tumor progression in esophageal squamous cell carcinoma[J]. Cell Oncol, 2021, 44(2): 373-84. |
| 31 | Li RY, Huang B, Tian H, et al. Immune evasion in esophageal squamous cell cancer: from the perspective of tumor microenvironment[J]. Front Oncol, 2022, 12: 1096717. |
| 32 | Kolenbrander PE, Palmer RJ Jr, Rickard AH, et al. Bacterial interactions and successions during plaque development[J]. Periodontol 2000, 2006, 42: 47-79. |
| 33 | Rams TE, Sautter JD, van Winkelhoff AJ. Emergence of antibiotic-resistant Porphyromonas gingivalis in United States periodontitis patients[J]. Antibiotics, 2023, 12(11): 1584. |
| 34 | Chen SR, Zou SJ. Porphyromonas gingivalis HmuY and its possible effect on the pathogenesis of periodontitis[J]. Sichuan Da Xue Xue Bao Yi Xue Ban, 2022, 53(6): 1104-9. |
| 35 | Ndayishimiye J, Kumeria T, Popat A, et al. Nanomaterials: the new antimicrobial magic bullet[J]. ACS Infect Dis, 2022, 8(4): 693-712. |
| 36 | Tang SH, Zheng J. Antibacterial activity of silver nanoparticles: structural effects[J]. Adv Healthc Mater, 2018, 7(13): e1701503. |
| 37 | Valdez-Salas B, Beltrán-Partida E, Zlatev R, et al. Structure-activity relationship of diameter controlled Ag@Cu nanoparticles in broad-spectrum antibacterial mechanism[J]. Mater Sci Eng C Mater Biol Appl, 2021, 119: 111501. |
| 38 | Tang H, Yang ST, Yang YF, et al. Blood clearance, distribution, transformation, excretion, and toxicity of near-infrared quantum dots Ag2Se in mice[J]. ACS Appl Mater Interfaces, 2016, 8(28): 17859-69. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||