Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (11): 2063-2073.doi: 10.12122/j.issn.1673-4254.2024.11.02
Miaozhu YIN1( ), Kuiyu CHEN1, Limin WU2,3, Pengyu JIANG1, Zhihui JI1, Nian ZHANG1, Huan ZHOU1, Hui HAN1(
), Kuiyu CHEN1, Limin WU2,3, Pengyu JIANG1, Zhihui JI1, Nian ZHANG1, Huan ZHOU1, Hui HAN1( )
)
Received:2024-05-06
Online:2024-11-20
Published:2024-11-29
Contact:
Hui HAN
E-mail:1492657304@qq.com;hanhuidoctor2022@163.com
Supported by:Miaozhu YIN, Kuiyu CHEN, Limin WU, Pengyu JIANG, Zhihui JI, Nian ZHANG, Huan ZHOU, Hui HAN. Gandou Bushen Decoction improves spermatogenesis and promotes spermatogenic cell proliferation in Wilson disease TX mice by activating testicular ERK signaling pathway[J]. Journal of Southern Medical University, 2024, 44(11): 2063-2073.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.11.02
| Group | Testicular copper level (μg/g) |
|---|---|
| Control | 2.772±0.306 |
| WD | 9.355±0.425* |
| Penicillamine | 4.129±0.550 # |
| GDBSD | 4.952±0.264 *# |
| GDBSD+U0126 | 5.597±0.678# |
Tab.1 Comparison of testicular copper levels among the 5 groups (μg/g, Mean±SD, n=5)
| Group | Testicular copper level (μg/g) |
|---|---|
| Control | 2.772±0.306 |
| WD | 9.355±0.425* |
| Penicillamine | 4.129±0.550 # |
| GDBSD | 4.952±0.264 *# |
| GDBSD+U0126 | 5.597±0.678# |
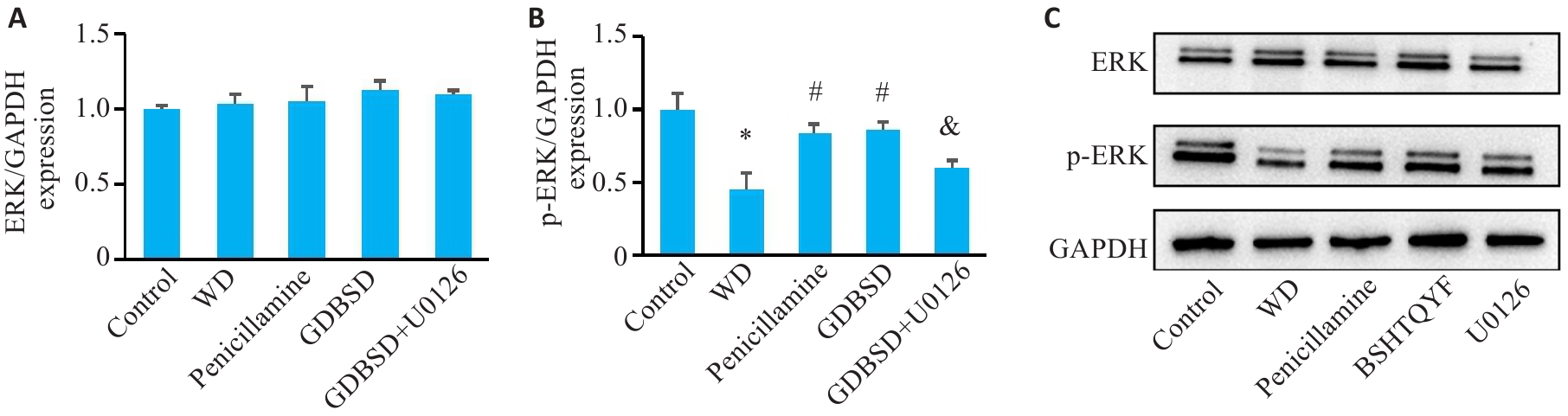
Fig.1 Western blotting for detecting expressions of ERK signaling pathway proteins in mouse testicular tissue. A: ERK. B: p-ERK (Mean±SD, n=5). C: Original protein bands. *P<0.05 vs Control group; #P<0.05 vs WD group; &P<0.05 vs GDBSD group.
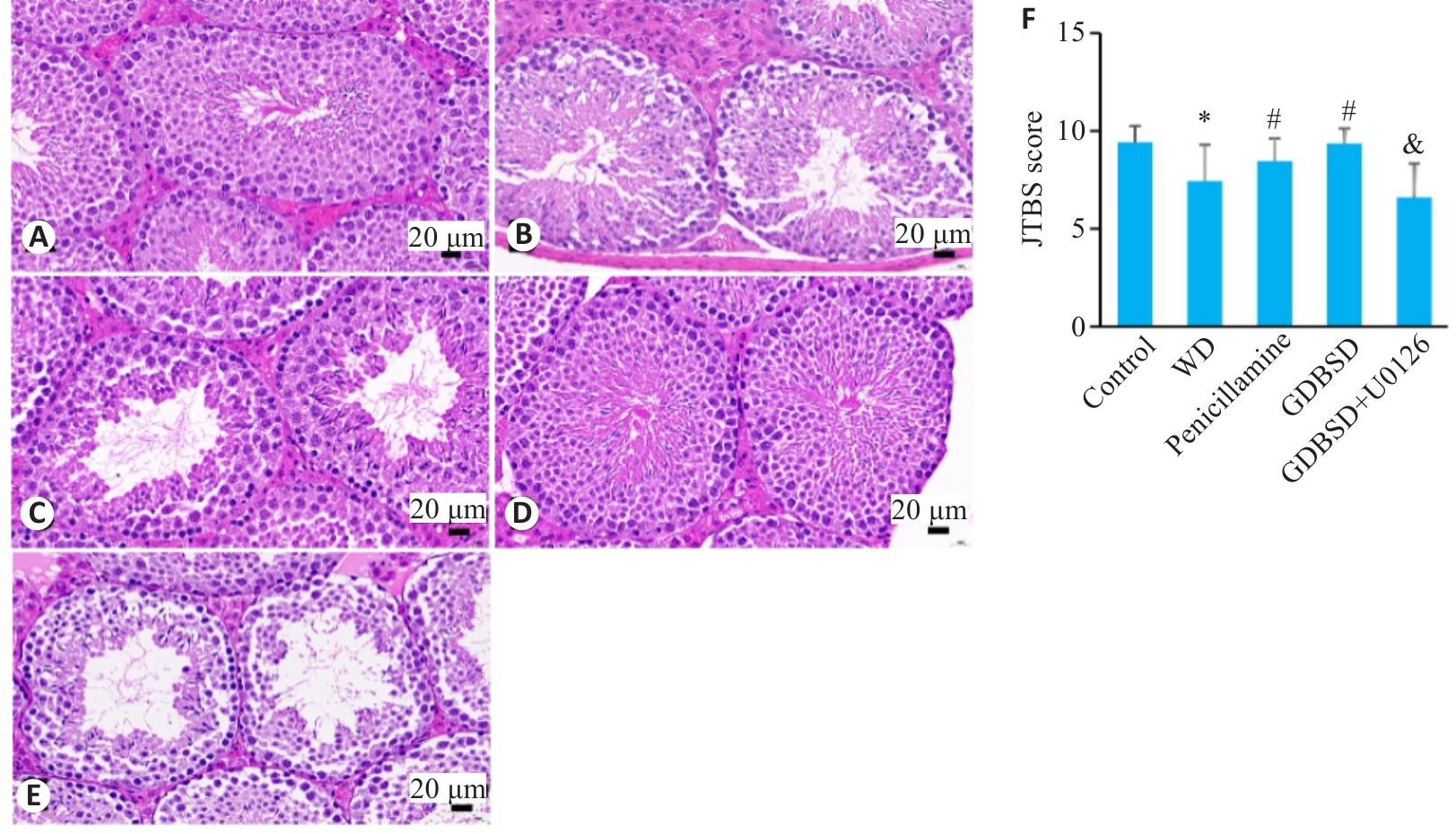
Fig.2 HE staining of testicular tissues of the mice. A: Control group. B: WD group. C: Penicillamine group. D: GDBSD group. E: GDBSD+U0126 group. F: Johnsen scores of the germinal tubules in the testicular tissue. *P<0.05 vs control group; #P<0.05 vs WD group; &P<0.05 vs GDBSD group.
| Group | Sperm density (107/mL) | Sperm survival rate (%) | Sperm malformation rate (%) |
|---|---|---|---|
| Control | 4.73±0.17 | 71.51±1.65 | 14.97±2.35 |
| WD | 2.20±0.22* | 20.59±1.81* | 79.62±4.44* |
| Penicillamine | 3.50±0.16*# | 61.72±1.12*# | 26.96±3.60*# |
| GDBSD | 3.43±0.50*# | 66.53±1.72*# | 24.84±1.67*# |
| GDBSD+U0126 | 2.67±0.12& | 52.50±2.25 & | 77.74±3.39 & |
Tab.2 Comparison of sperm quality of the mice (Mean±SD, n=5)
| Group | Sperm density (107/mL) | Sperm survival rate (%) | Sperm malformation rate (%) |
|---|---|---|---|
| Control | 4.73±0.17 | 71.51±1.65 | 14.97±2.35 |
| WD | 2.20±0.22* | 20.59±1.81* | 79.62±4.44* |
| Penicillamine | 3.50±0.16*# | 61.72±1.12*# | 26.96±3.60*# |
| GDBSD | 3.43±0.50*# | 66.53±1.72*# | 24.84±1.67*# |
| GDBSD+U0126 | 2.67±0.12& | 52.50±2.25 & | 77.74±3.39 & |
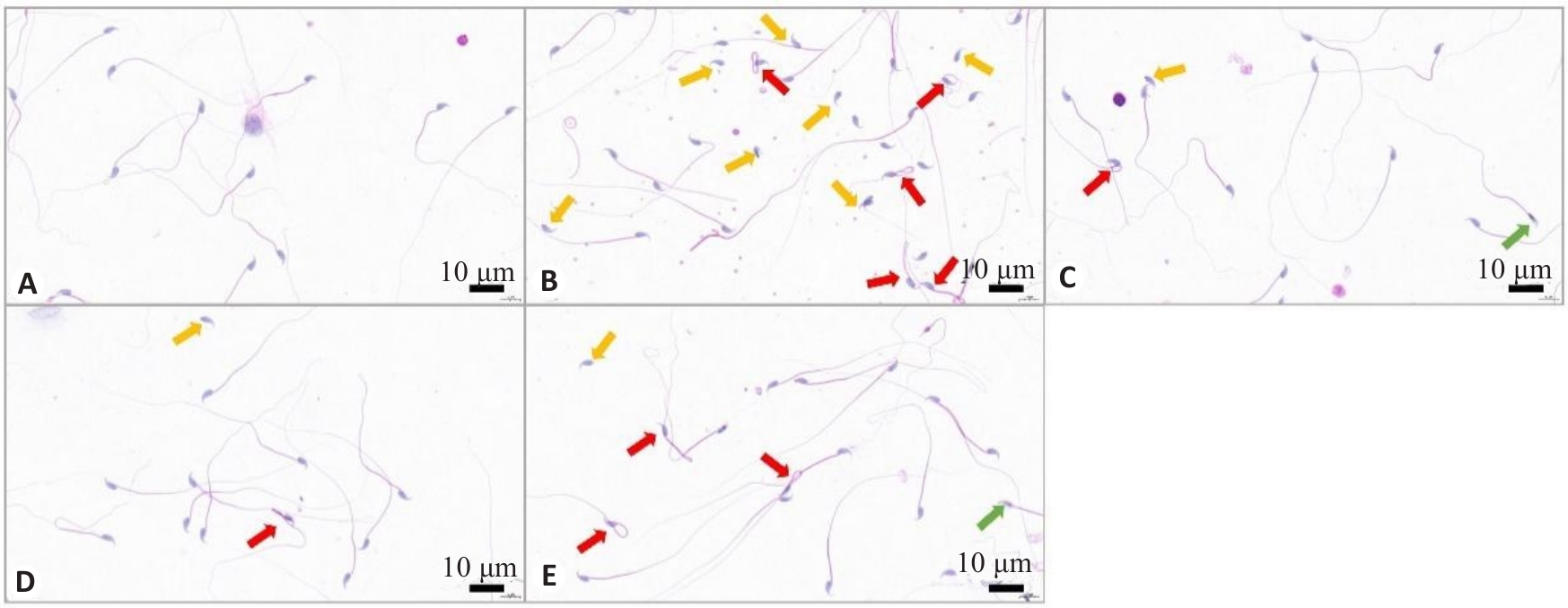
Fig.4 Representative images of HE staining of mouse spermatozoa. A: Control group. B: WD group. C: penicillamine group. D: GDBSD group. E: GDBSD+U0126 group. Red arrows: Sperms with tail curl; Orange arrows: Sperms with a head without a tail; Green arrows: Sperms with head deformity.
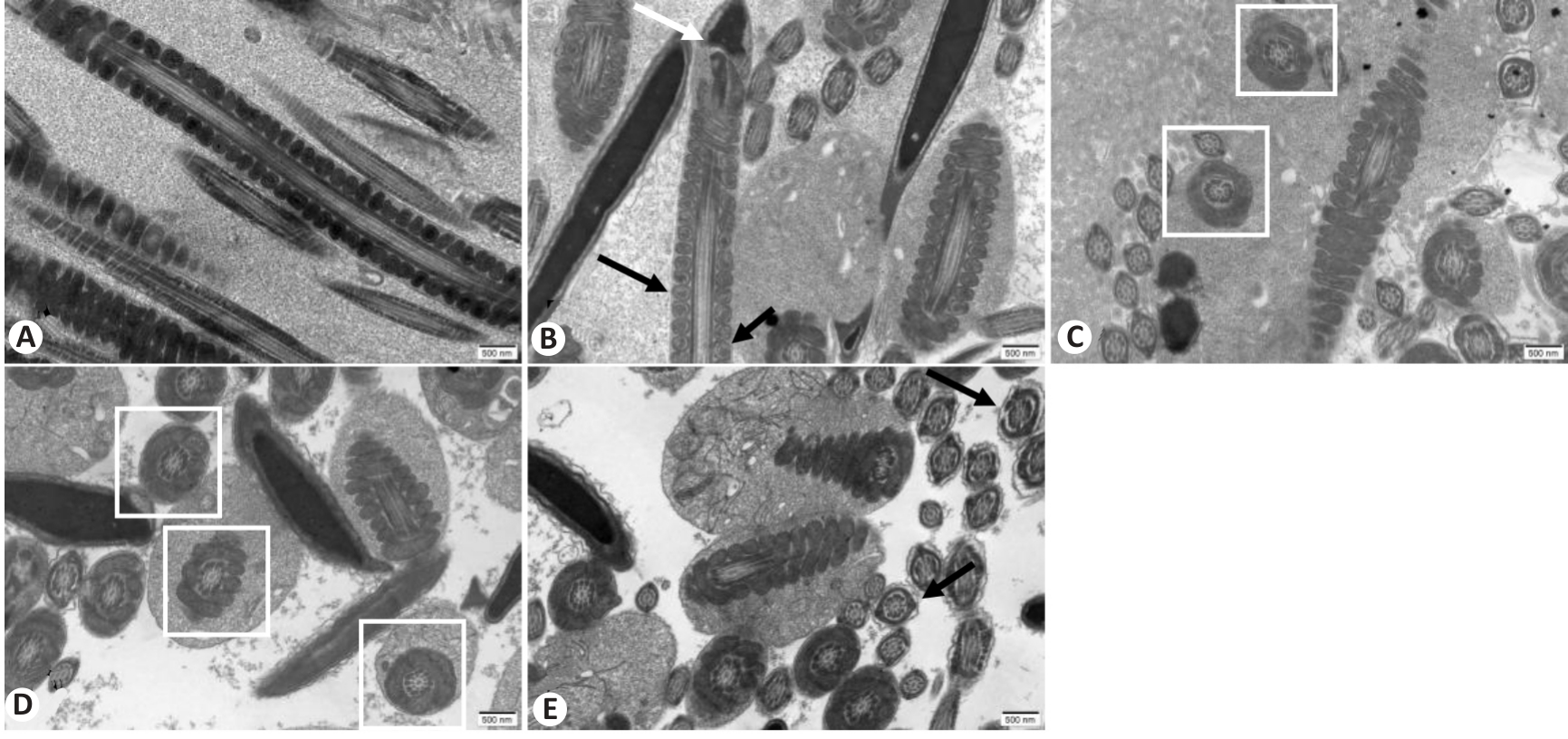
Fig. 5 Ultrastructural changes of mouse spermatozoa in each group observed with transmission electron microscope (scale bar=500 nm). A: Control group. B: WD group. C: Penicillamine group. D: GDBSD group. E: GDBSD+U0126 group.
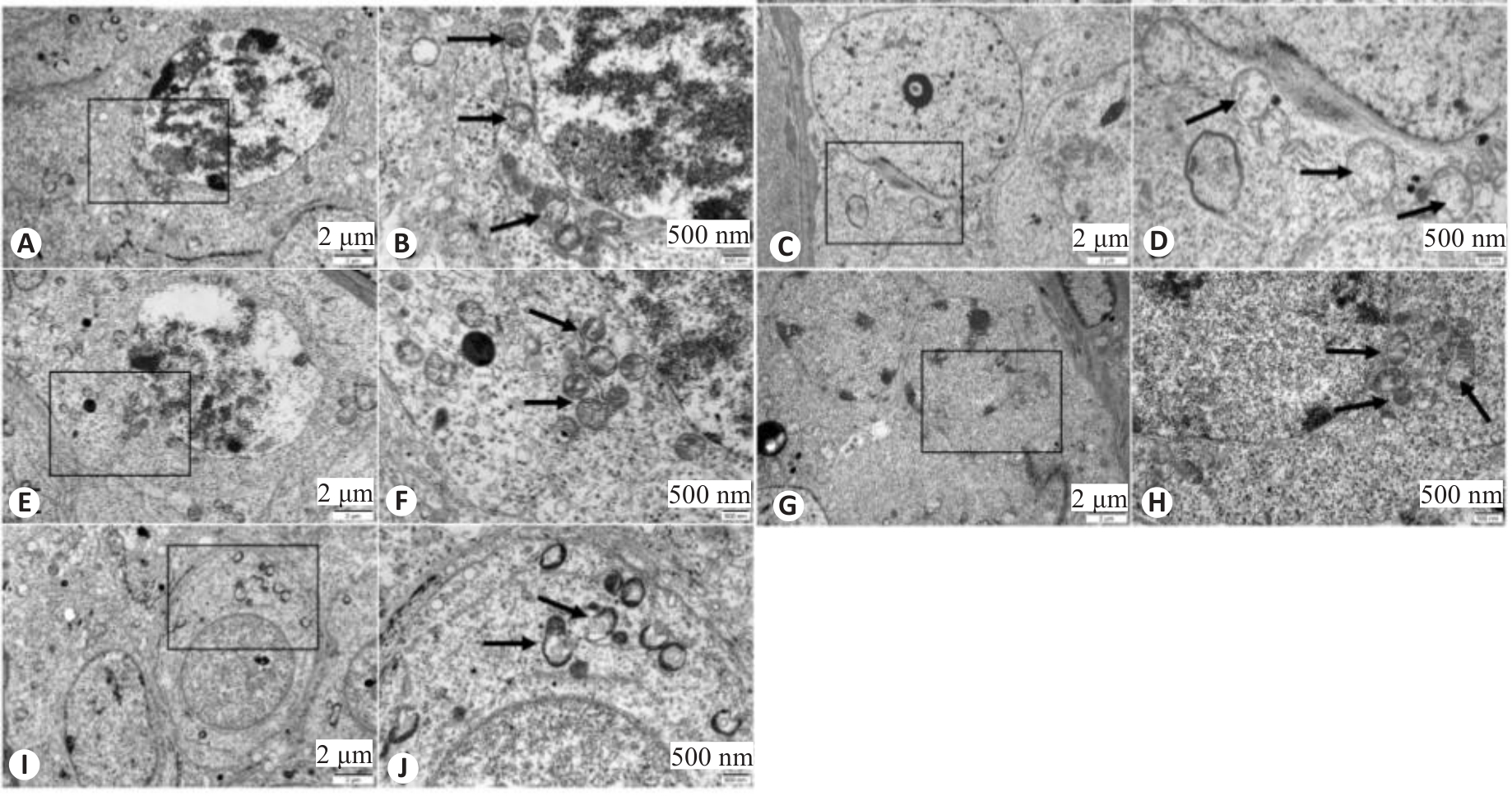
Fig.6 Ultrastructure of mouse spermatocytes in each group observed with transmission electron microscope. A, B: Control group. C, D: WD group. E, F: Penicillamine group. G, H: GDBSD group. I, J: GDBSD+U0126 group. Black arrows indicate mitochondrial structures.
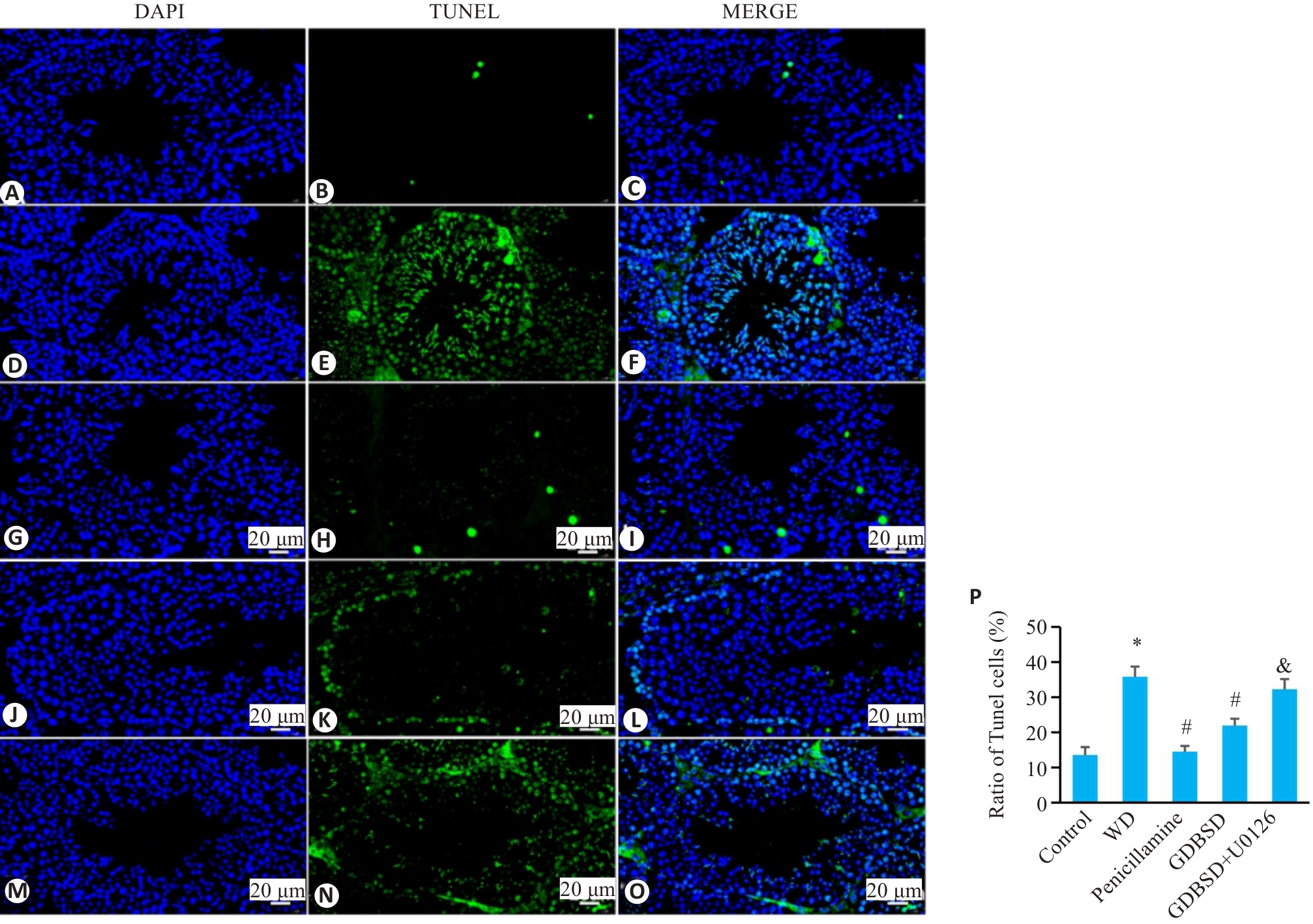
Fig. 7 TUNEL staining of mouse testicular tissue. A-C: Control group. D-F: WD group. G-I: Penicillamine group. J-L: GDBSD group. M-O: GDBSD+U0126 group. P: Apoptosis rate of testicular tissue in each group. Apoptotic cells are stained green and nuclei are stained blue (Mean±SD,n=5). *P<0.05 vs Control group; #P<0.05 vs WD group; &P<0.05 vs GDBSD group.
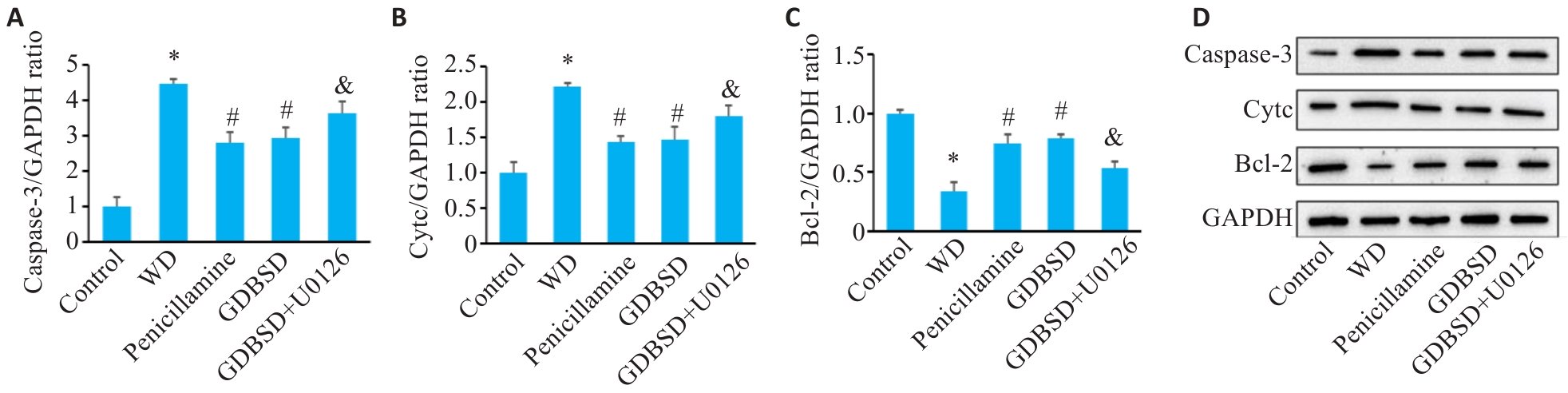
Fig. 8 Western blotting for detecting expressions of apoptosis-related proteins in mouse testis. A: Caspase-3. B: Cytc. C: Bcl-2. D: Original protein bands. *P<0.05 vs Control group, #P<0.05 vs WD group, &P<0.05 vs GDBSD group.
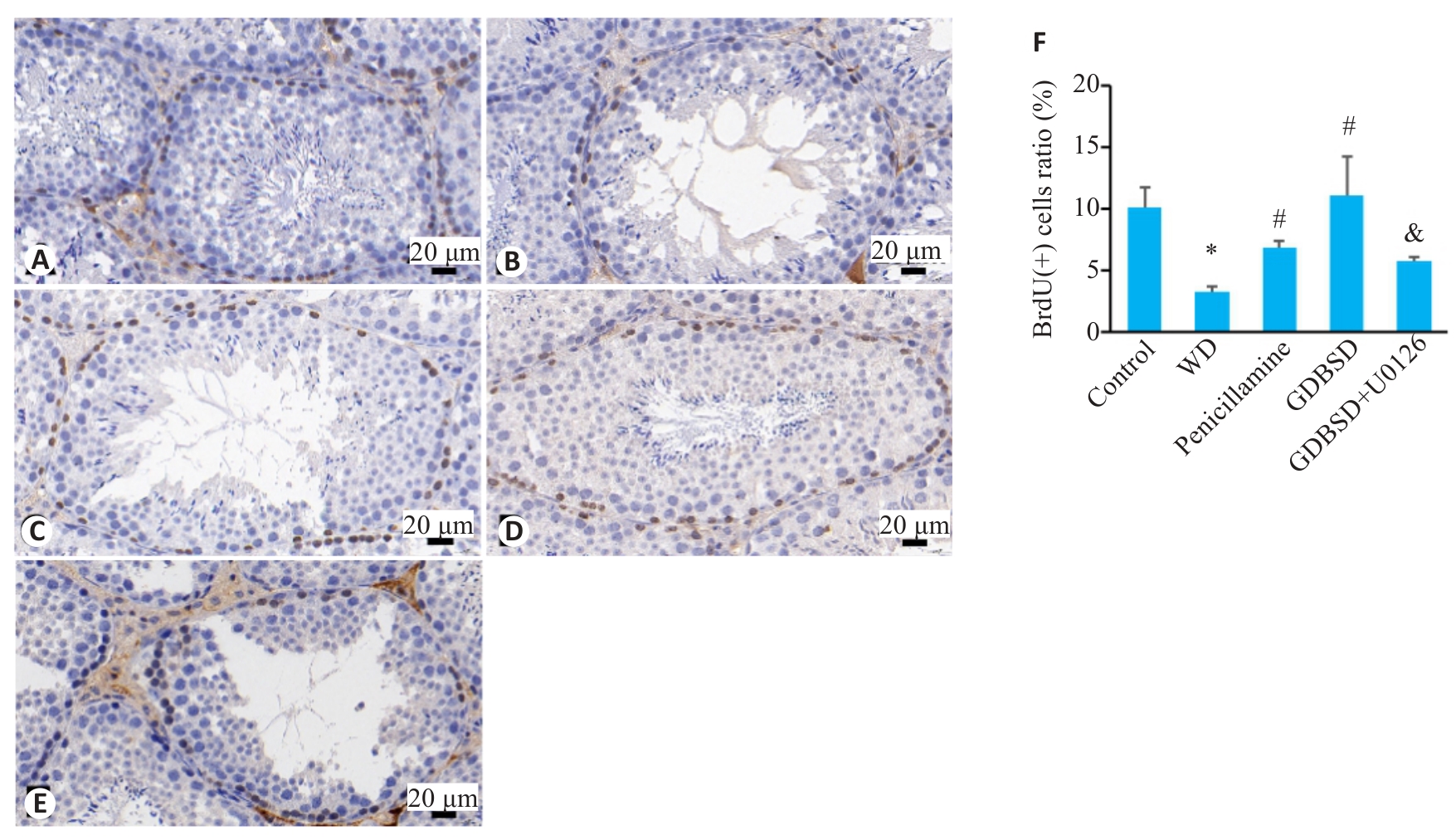
Fig.9 Immunohistochemically labeled BrdU-positive cells in mouse testicular tissue. A: Control group. B: WD group. C: Penicillamine group. D: GDBSD group. E: GDBSD+U0126 group. F: Percentages of BrdU-positive cells in each group. *P<0.05 vs Control group; #P<0.05 vs WD group; &P<0.05 vs GDBSD group.
| Group | Fertility rate (%) | Pups per litter |
|---|---|---|
| Control | 80.00 | 10.11±1.73 |
| WD | 37.50 | 4.00±0.82* |
| Penicillamine | 62.50 | 7.40±1.04# |
| GDBSD | 66.67 | 8.14±1.55# |
| GDBSD+U0126 | 42.86 | 5.00±0.81& |
Tab.3 Comparison of fertility of male mice among the 5 groups (Mean±SD, n=5)
| Group | Fertility rate (%) | Pups per litter |
|---|---|---|
| Control | 80.00 | 10.11±1.73 |
| WD | 37.50 | 4.00±0.82* |
| Penicillamine | 62.50 | 7.40±1.04# |
| GDBSD | 66.67 | 8.14±1.55# |
| GDBSD+U0126 | 42.86 | 5.00±0.81& |
| 1 | Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase[J]. J Biolog Chem, 1998, 273(29): 18623-32. |
| 2 | Ala A, Walker AP, Ashkan K, et al. Wilson's disease[J]. Lancet, 2007, 369(9559): 397-408. |
| 3 | Sandahl TD, Laursen TL, Munk DE, et al. The prevalence of Wilson's disease: an update[J]. Hepatology, 2020, 71(2): 722-32. |
| 4 | Litwin T, Bembenek J, Antos A, et al. The maternal and fetal outcomes of pregnancy in Wilson's disease: a systematic literature review and meta-analysis[J]. Biomedicines, 2022, 10(9): 2072. |
| 5 | Pfeiffenberger J, Beinhardt S, Gotthardt DN, et al. Pregnancy in Wilson's disease: management and outcome[J]. Hepatology, 2018, 67(4): 1261-9. |
| 6 | Tarnacka B, Rodo M, Cichy S, et al. Procreation ability in Wilson's disease[J]. Acta Neurol Scand, 2000, 101(6): 395-8. |
| 7 | Zhang H, Zhang WW, Mo CY, et al. Production of functional sperm from in vitro-cultured premeiotic spermatogonia in a marine fish[J]. Zool Res, 2022, 43(4): 537-51. |
| 8 | 韩 辉, 郑明翠, 吴丽敏, 等. 补肾化痰祛瘀法治疗肝豆状核变性少弱精子症临床研究[J]. 时珍国医国药, 2018, 29(10): 2421-3. |
| 9 | Herman S, Lipiński P, Ogórek M, et al. Molecular regulation of copper homeostasis in the male gonad during the process of spermatogenesis[J]. Int J Mol Sci, 2020, 21(23): 9053. |
| 10 | Chen HL, Wang YY, Luo J, et al. Autophagy and apoptosis mediated nano-copper-induced testicular damage[J]. Ecotoxicol Environ Saf, 2022, 229: 113039. |
| 11 | Guo HR, Ouyang YJ, Yin H, et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder[J]. Redox Biol, 2022, 49: 102227. |
| 12 | Litwin T, Dušek P, Członkowska A. Symptomatic treatment of neurologic symptoms in Wilson disease[J]. Handb Clin Neurol, 2017, 142: 211-23. |
| 13 | Lee EJ, Woo MH, Moon JS, et al. Efficacy and safety of D-penicillamine, trientine, and zinc in pediatric Wilson disease patients[J]. Orphanet J Rare Dis, 2024, 19(1): 261. |
| 14 | Weiss KH, Stremmel W. Clinical considerations for an effective medical therapy in Wilson's disease[J]. Ann N Y Acad Sci, 2014, 1315: 81-5. |
| 15 | European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease[J]. J Hepatol, 2012, 56(3): 671-85. |
| 16 | 韩 辉, 杨文明, 张 娟, 等. 肝豆状核变性的中医证候特征[J]. 中医药临床杂志, 2014, 26(1): 16-9. |
| 17 | Dong T, Wu MC, Tang LL, et al. GanDouLing promotes proliferation and differentiation of neural stem cells in the mouse model of Wilson's disease[J]. Biosci Rep, 2021, 41(1): BSR20202717. |
| 18 | 何望生, 杨文明, 汪 瀚, 等. 肝豆灵汤改善痰瘀互结型Wilson病患者肝脏功能的临床观察[J]. 中国实验方剂学杂志, 2020, 26(8): 105-11. |
| 19 | Cheng CL, Wang Q, Huang YR, et al. Gandouling inhibits hepatic fibrosis in Wilson's disease through Wnt-1/β‑catenin signaling pathway[J]. J Ethnopharmacol, 2023, 311: 116445. |
| 20 | 奚亚明, 韩 辉, 吴丽敏, 等. 男性肝豆状核变性合并生殖功能损害的中医证候特征[J]. 中医药通报, 2018, 17(5): 56-8. |
| 21 | 陈秋莹. 补肾化痰祛瘀方对女性Wilson病合并生殖系统损害患者的临床观察及对雌性TX小鼠下丘脑-垂体-卵巢轴的作用研究[D]. 安徽中医药大学, 2023. |
| 22 | 王婷婷. 补肾化痰祛瘀方对男性Wilson病伴生殖系统损害的临床观察及对下丘脑-垂体-睾丸轴的实验研究[D]. 合肥: 安徽中医药大学, 2022. |
| 23 | 奚亚明. 男性肝豆状核变性合并生殖损害的中医证候特征及中医药治疗临床研究[D]. 安徽中医药大学, 2020. |
| 24 | 王路瑶, 韩 辉, 赵 丹, 等. 韩辉运用肝豆补肾汤辨治男性肝豆状核变性合并生殖系统损害经验[J]. 中医药通报, 2024, 23(2): 16-9. |
| 25 | 赵 丹, 韩 辉, 房新如, 等. 肝豆补肾汤治疗男性肝豆状核变性合并生殖损害的理想点法综合疗效评价研究[J]. 中医药临床杂志, 2024, 36(4): 720-6. |
| 26 | Chen J, Aguilera G. Vasopressin protects hippocampal neurones in culture against nutrient deprivation or glutamate-induced apoptosis[J]. J Neuroendocrinol, 2010, 22(10): 1072-81. |
| 27 | Pan HC, Jiang Q, Yu Y, et al. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells[J]. Neurochem Int, 2015, 80: 60-71. |
| 28 | Chen ZH, Liu MJ, Hu JH, et al. Substance P restores spermatogenesis in busulfan-treated mice: a new strategy for male infertility therapy[J]. Biomedecine Pharmacother, 2021, 133: 110868. |
| 29 | Xu BF, Washington AM, Hinton BT. PTEN signaling through RAF1 proto-oncogene serine/threonine kinase (RAF1)/ERK in the epididymis is essential for male fertility[J]. Proc Natl Acad Sci USA, 2014, 111(52): 18643-8. |
| 30 | Zhang ZW, Zhi-GangTan, Qiao N, et al. Copper-induced spermatozoa head malformation is related to oxidative damage to testes in CD-1 mice[J]. Biol Trace Elem Res, 2016, 173(2): 427-32. |
| 31 | Wang TT, Wu LM, Chen QY, et al. Copper deposition in Wilson's disease causes male fertility decline by impairing reproductive hormone release through inducing apoptosis and inhibiting ERK signal in hypothalamic-pituitary of mice[J]. Front Endocrinol, 2022, 13: 961748. |
| 32 | Klee JG. Undiagnosed Wilson's disease as cause of unexplained miscarriage[J]. Lancet, 1979, 2(8139): 423. |
| 33 | 奚亚明, 韩 辉. 男性肝豆状核变性生殖激素水平临床观察[J]. 中医药临床杂志, 2019, 31(2): 208-10. |
| 34 | Aydemir B, Kiziler AR, Onaran I, et al. Impact of Cu and Fe concentrations on oxidative damage in male infertility[J]. Biol Trace Elem Res, 2006, 112(3): 193-203. |
| 35 | Sharma P, Kaushal N, Saleth LR, et al. Oxidative stress-induced apoptosis and autophagy: balancing the contrary forces in spermatogenesis[J]. Biochim Biophys Acta Mol Basis Dis, 2023, 1869(6): 166742. |
| 36 | Almog T, Lazar S, Reiss N, et al. Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality[J]. J Biol Chem, 2008, 283(21): 14479-89. |
| 37 | Brown JL, Xie JJ, Brieño-Enriquez MA, et al. Sex- and age-specific impact of ERK loss within the pituitary gonadotrope in mice[J]. Endocrinology, 2018, 159(3): 1264-76. |
| 38 | Xu A, Li X, Li K, et al. Linoleic acid promotes testosterone production by activating Leydig cell GPR120/ERK pathway and restores BPA-impaired testicular toxicity[J]. Steroids, 2020, 163: 108677. |
| 39 | Wu PK, Becker A, Park JI. Growth inhibitory signaling of the raf/MEK/ERK pathway[J]. Int J Mol Sci, 2020, 21(15): 5436. |
| 40 | Suzuki C, Tanigawa M, Tanaka H, et al. Effect of D-serine on spermatogenesis and extracellular signal-regulated protein kinase (ERK) phosphorylation in the testis of the silkworm, Bombyx mori [J]. J Insect Physiol, 2014, 67: 97-104. |
| 41 | Sun PB, Wang YY, Gao T, et al. Hsp90 modulates human sperm capacitation via the Erk1/2 and p38 MAPK signaling pathways[J]. Reprod Biol Endocrinol, 2021, 19(1): 39. |
| 42 | Grimaldi P, Orlando P, Di Siena S, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis[J]. Proc Natl Acad Sci USA, 2009, 106(27): 11131-6. |
| 43 | Zhang CH, Wang Y, Sun QQ, et al. Copper nanoparticles show obvious in vitro and in vivo reproductive toxicity via ERK mediated signaling pathway in female mice[J]. Int J Biol Sci, 2018, 14(13): 1834-44. |
| 44 | Yang Y, Zhang XJ, Cui HY, et al. Apelin-13 protects the brain against ischemia/reperfusion injury through activating PI3K/Akt and ERK1/2 signaling pathways[J]. Neurosci Lett, 2014, 568: 44-9. |
| 45 | 徐乐文, 董健健, 高曼莉, 等. 肝豆汤联合青霉胺对Wilson病模型肝脏铜死亡抑制作用[J]. 中国中西医结合杂志, 2024, 44(3): 331-8. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||