Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (10): 1985-1994.doi: 10.12122/j.issn.1673-4254.2024.10.17
Siyi CHENG1,2,3,4( ), Zerui CHEN1,2,3,4, Changjiang YU1,2,3,4, Tucheng SUN1,2,3,4, Shuoji ZHU1,2,3,4(
), Zerui CHEN1,2,3,4, Changjiang YU1,2,3,4, Tucheng SUN1,2,3,4, Shuoji ZHU1,2,3,4( ), Nanbo LIU1,2,3,4(
), Nanbo LIU1,2,3,4( ), Ping ZHU1,2,3,4(
), Ping ZHU1,2,3,4( )
)
Received:2024-04-25
Online:2024-10-20
Published:2024-10-31
Contact:
Shuoji ZHU, Nanbo LIU, Ping ZHU
E-mail:debarah17@foxmail.com;zhushuoji@gmail.com;liu.nanbo@163.com;tanganqier@163.com
Supported by:Siyi CHENG, Zerui CHEN, Changjiang YU, Tucheng SUN, Shuoji ZHU, Nanbo LIU, Ping ZHU. Intrinsic steady-state pattern of mouse cardiac electrophysiology: analysis using a characterized quantitative electrocardiogram strategy[J]. Journal of Southern Medical University, 2024, 44(10): 1985-1994.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.10.17
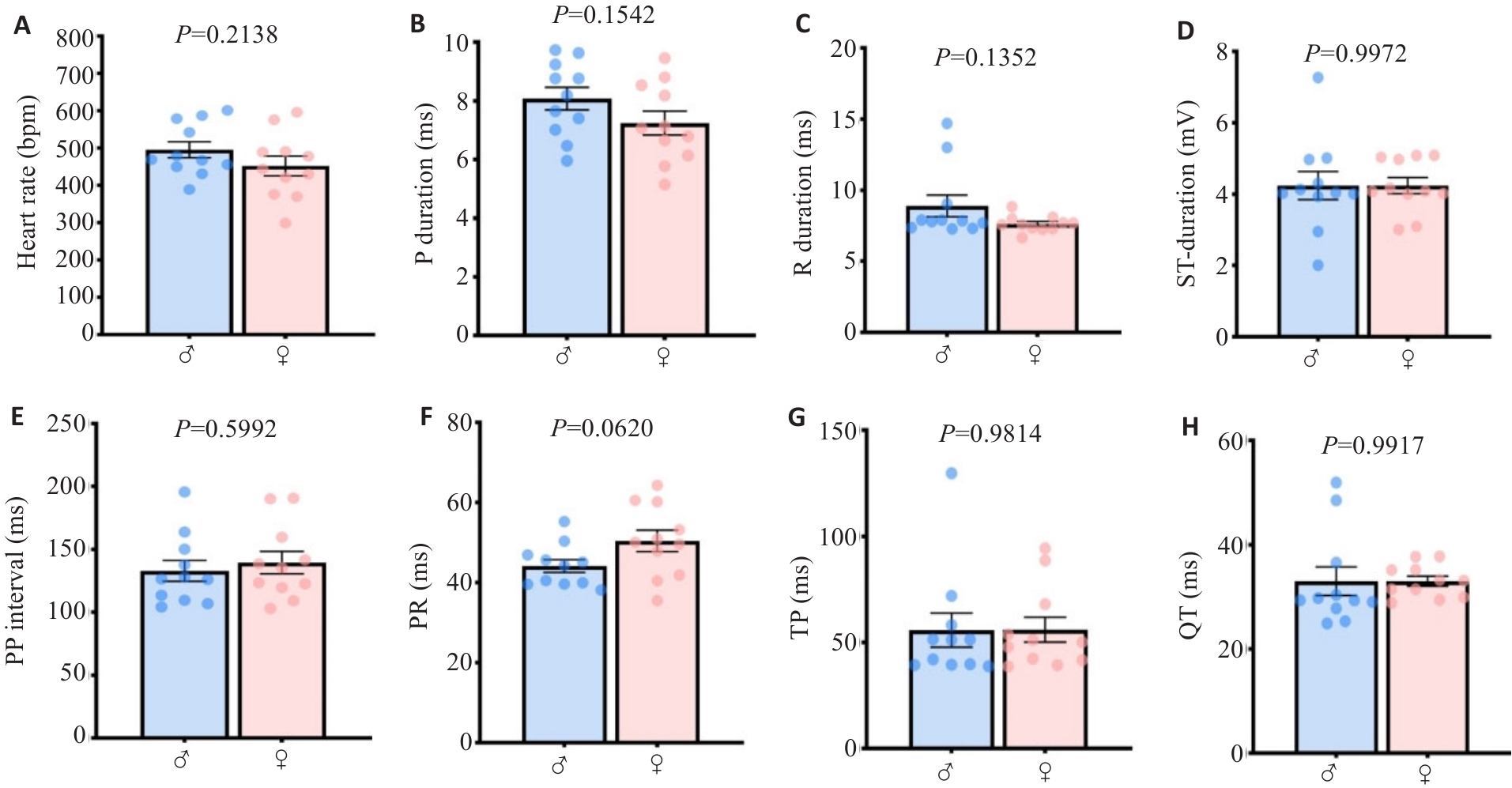
Fig. 2 The main quantified ECGsqa time course parameters in mice are conserved between genders. A: Heart rate. B: P wave rising segment duration. C: R wave rising segment duration. D: ST rising segment duration. E: PP interval. F: PR interval. G: P interval. H: QT interval.
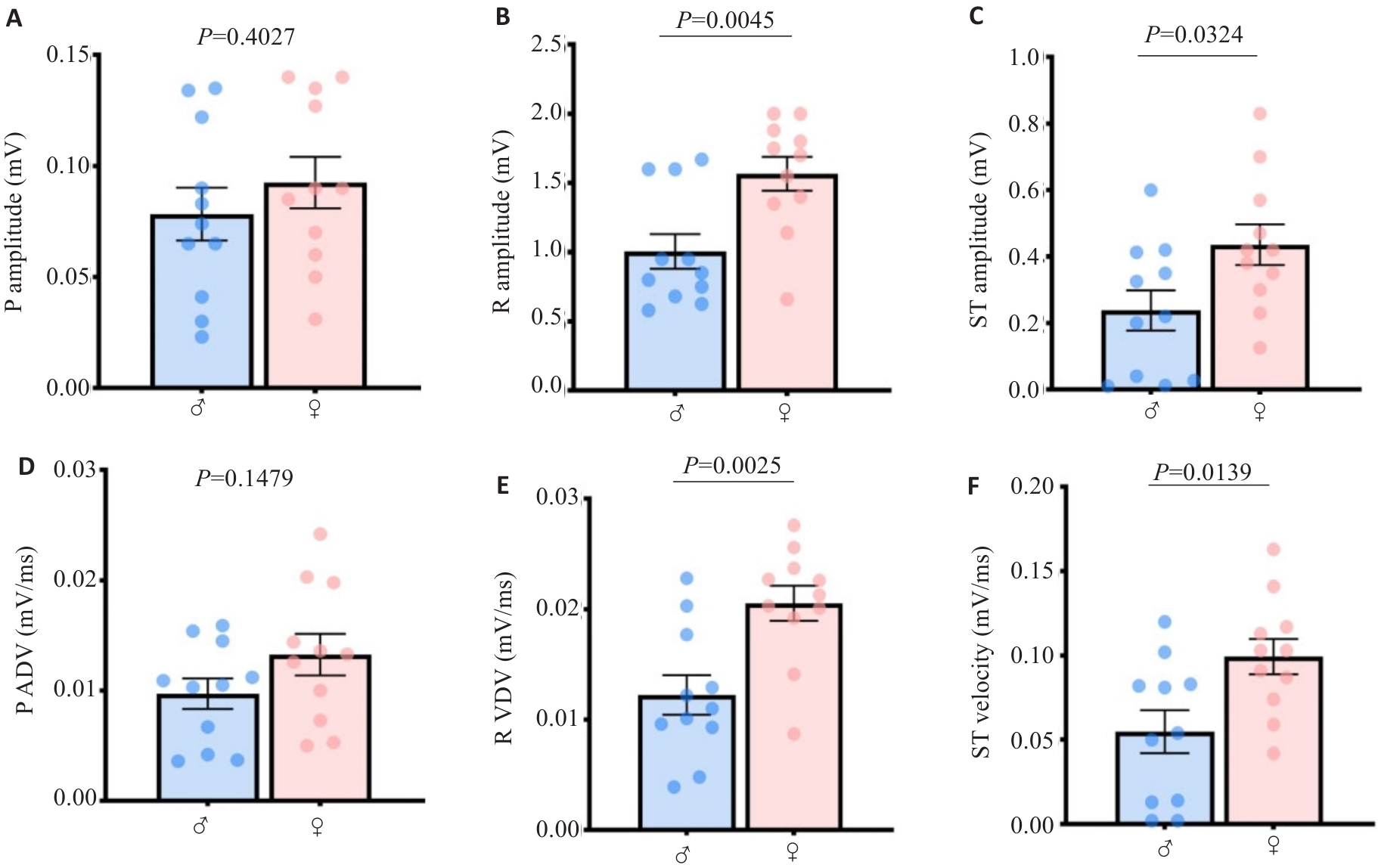
Fig.3 Quantitative values of atrioventricular ECGsqa amplitude and velocity in mice harbor mathematical characteristics of gender differences. A: P wave amplitude. B: R wave amplitude. C: ST segment amplitude. D: P wave velocity. E: R wave velocity. F: ST segment velocity.
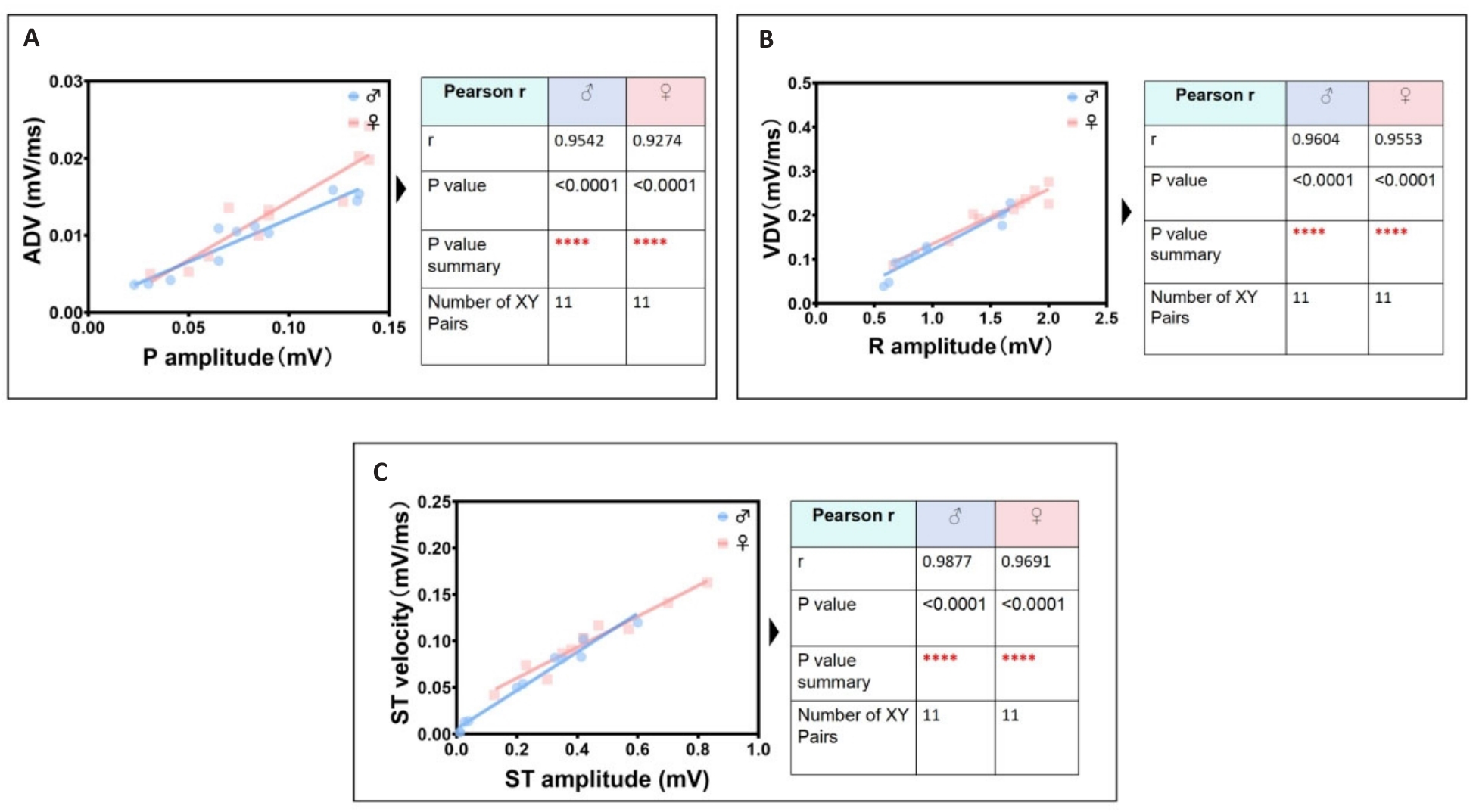
Fig.4 Identification group of the most significant linear correlation parameters in ECGsqa of both male and female mice. A: P wave amplitude is positively correlated with atrial depolarization rate (ADV). B: R wave amplitude is positively correlated with ventricular depolarization rate (VDV). C: ST segment amplitude is positively correlated with ventricular ST segment velocity.
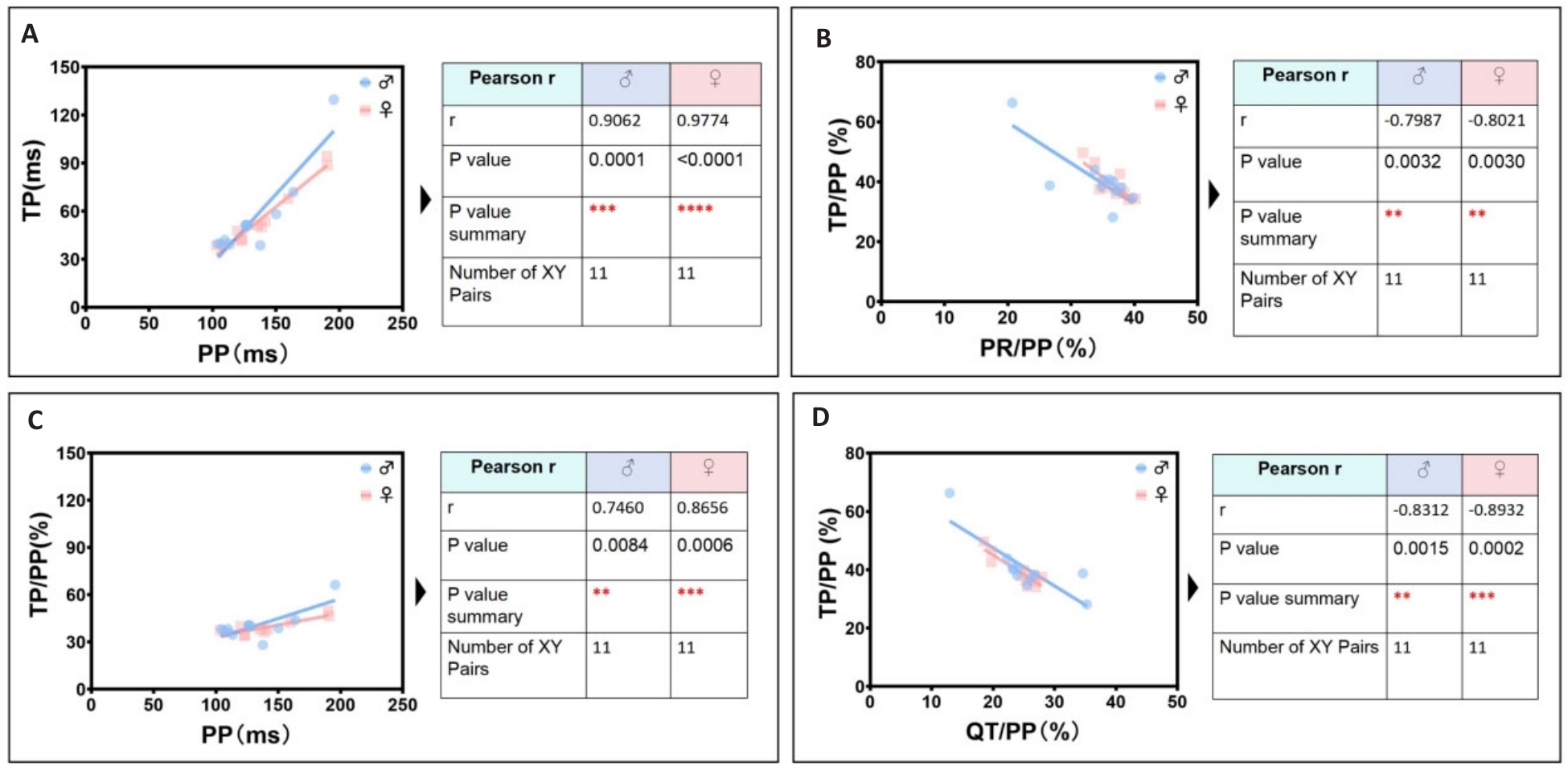
Fig.5 Identification group of secondary significant linear correlation parameters in ECGsqa of both male and female mice. A: PP interval is positively correlated with TP interval. B: PR/PP ratio is negatively correlated with TP/PP ratio. C: PP interval is positively correlated with TP/PP ratio. D: QT/PP ratio is negatively correlated with TP/ PP ratio.
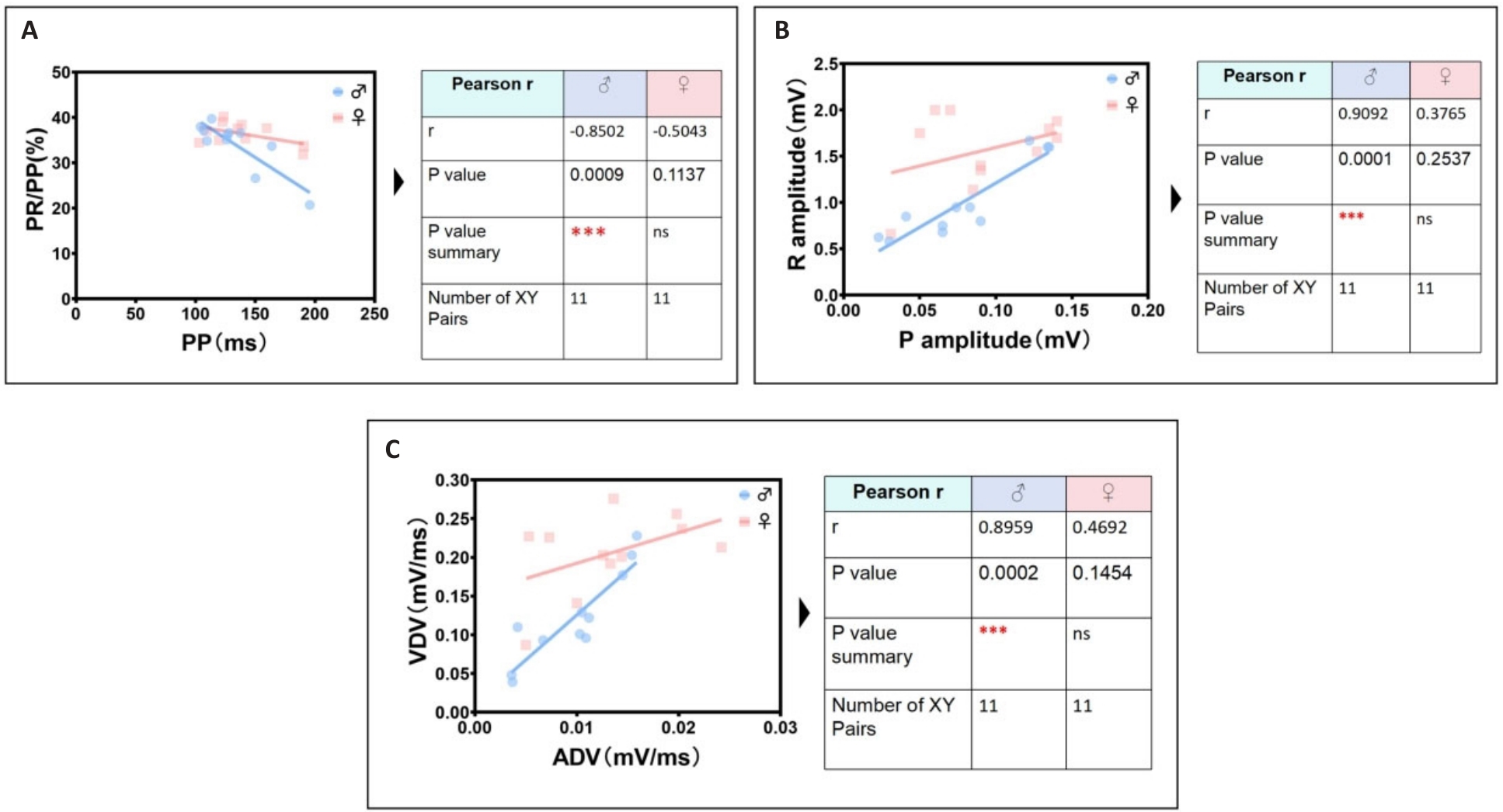
Fig.6 The most significant unique identification group of ECG linear parameters related to male mice. A: Male PP interval is negatively correlated with male PR/PP ratio. B: Male P wave amplitude is positively correlated with male R wave amplitude. C: Male ADV is positively correlated with male VDV.
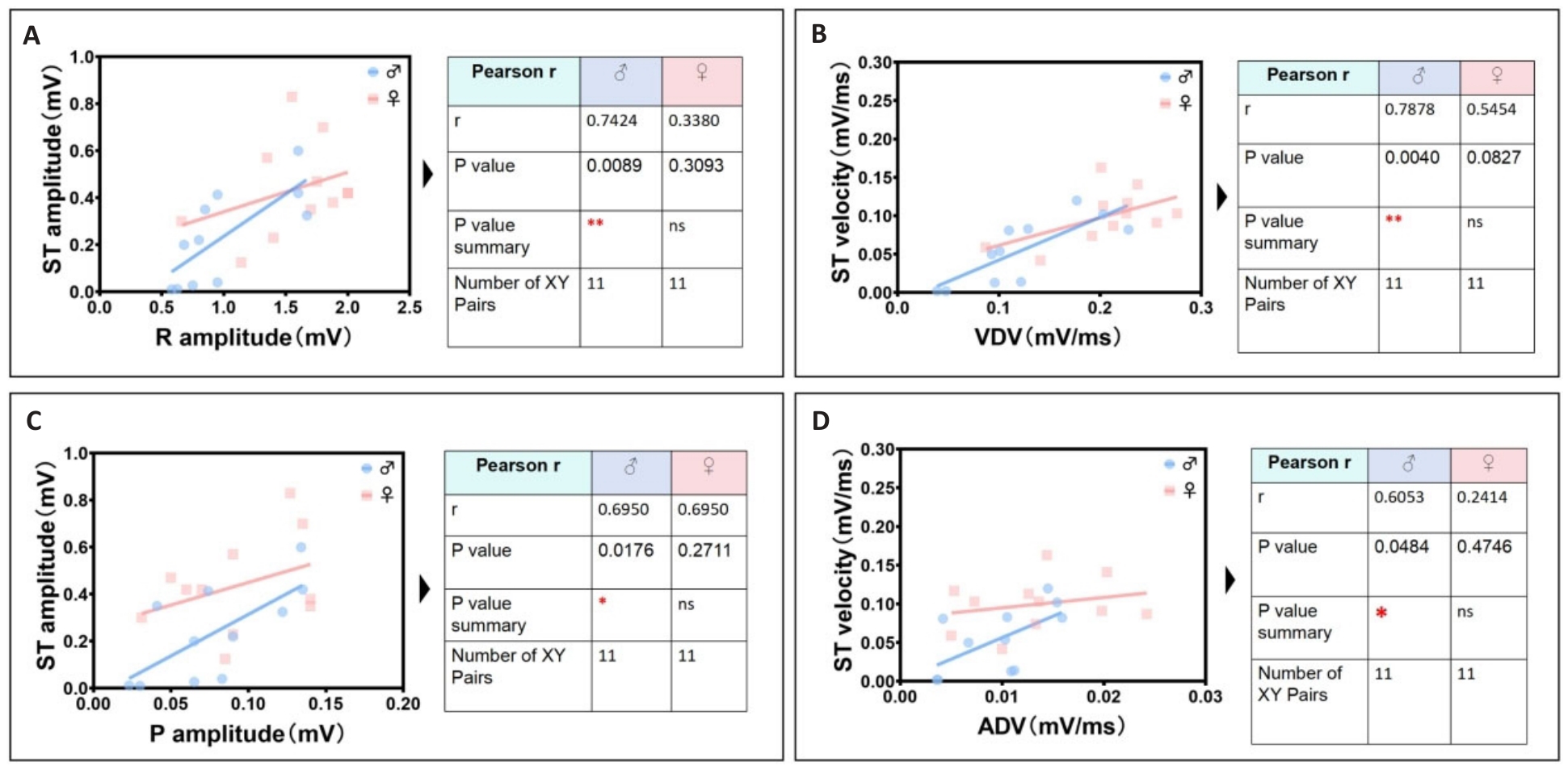
Fig.7 Identification group of unique cardiac electrical linearity-related parameters in male mice. A: Male R wave amplitude is positively correlated with male ST segment amplitude. B: Male VDV is positively correlated with male ST segment velocity. C: Male P wave amplitude is positively correlated with male ST segment amplitude. D: Male ADV is positively correlated with male ST segment velocity.
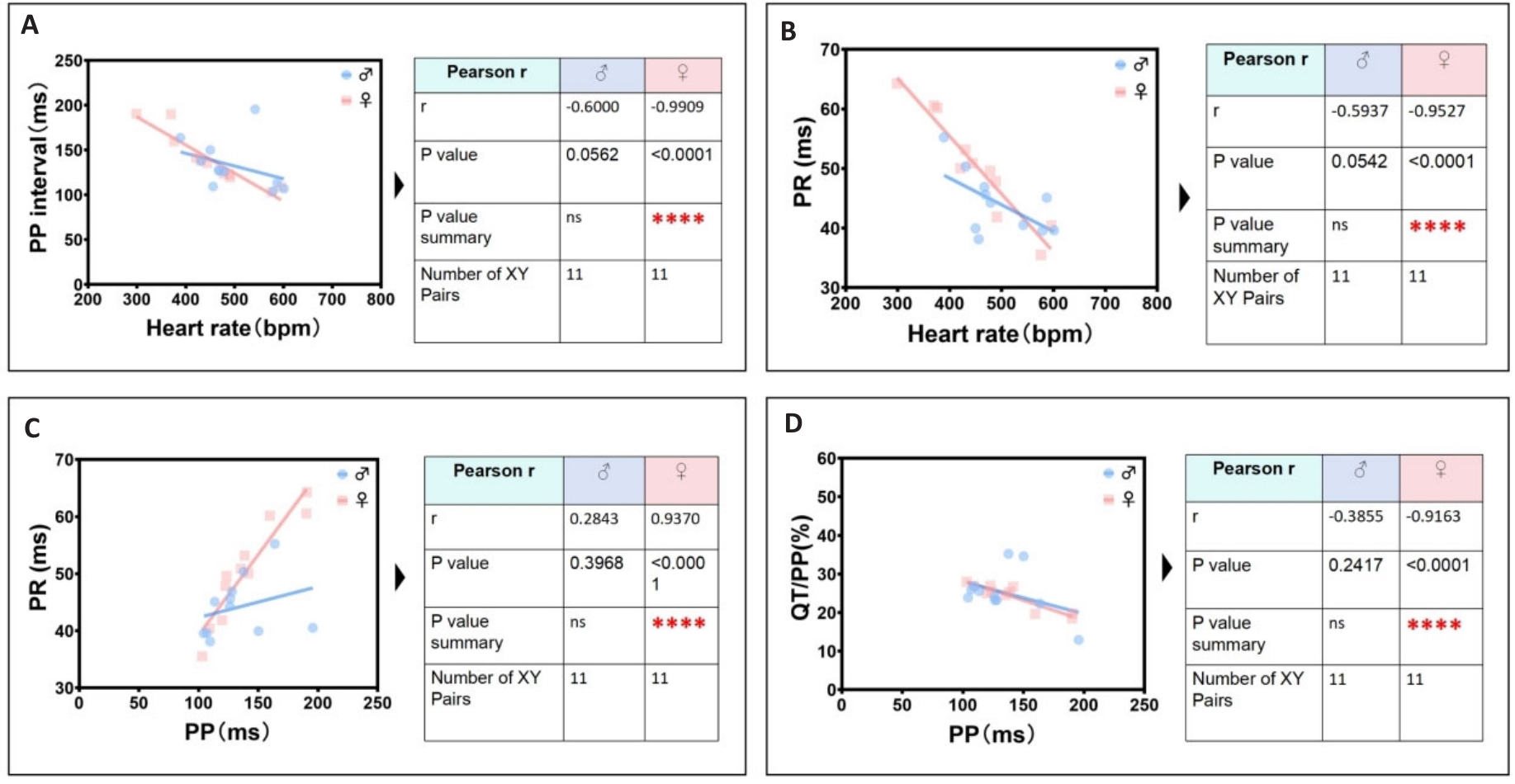
Fig.8 The most significant unique identification group of ECG linearity-related parameters in female mice. A: Female heart rate is negatively correlated with female PP interval. B: Female heart rate is negatively correlated with female PR interval. C: Female PP interval is positively correlated with female PR interval. D: Female PP interval is negatively correlated with female QT/PP ratio.
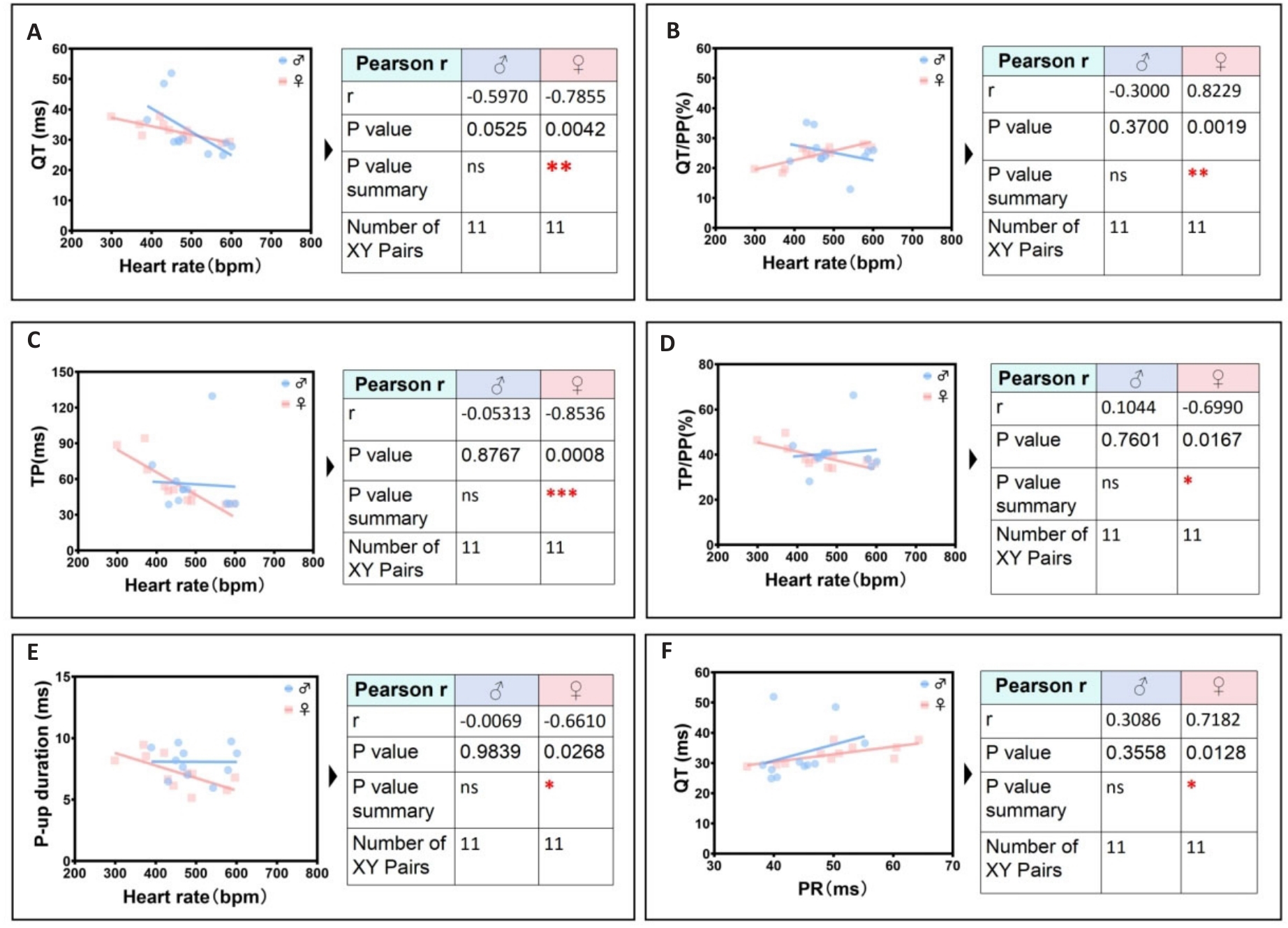
Fig.9 Secondary significant unique identification group of ECG linearity-related parameters in female mice. A: Female heart rate is negatively correlated with female QT interval. B: Female heart rate is positively correlated with female QT/PP ratio. C: Female heart rate is negatively correlated with female TP ratio. D: Female heart rate is negatively correlated with female TP/PP ratio. E: Heart rate is negatively correlated the peak time of rising P wave in female. F: Female PR interval is positively correlated with female QT interval.
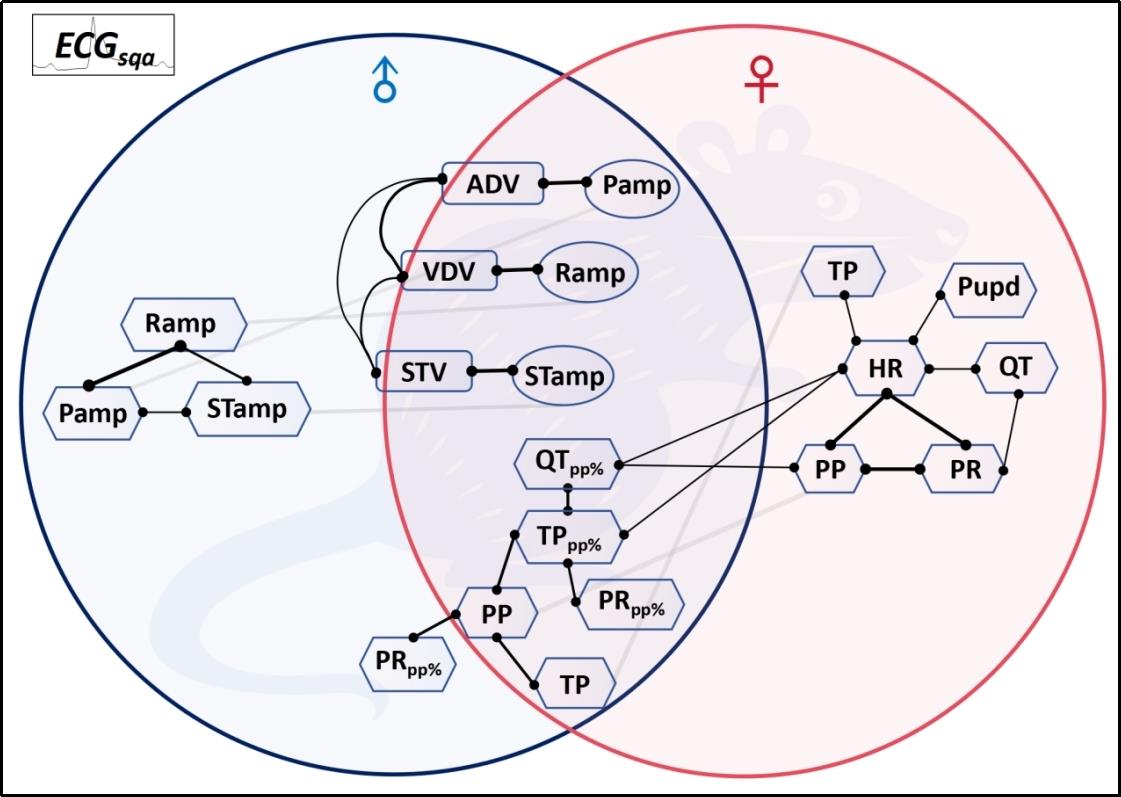
Fig.10 Quantitative ECG correlation parameter network characterizes the intrinsic physiological homeostasis pattern of the mouse cardiac electrical conduction system.
| 1 | Martin SS, Aday AW, Almarzooq ZI, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American heart association[J]. Circulation, 2024, 149(8): e347-60. |
| 2 | Goette A, Auricchio A, Boriani G, et al. EHRA White Paper: knowledge gaps in arrhythmia management-status 2019[J]. Europace, 2019, 21(7): 993-4. |
| 3 | Thomas RJ. Cardiac rehabilitation-challenges, advances, and the road ahead[J]. N Engl J Med, 2024, 390(9): 830-41. |
| 4 | Kowey PR, Naccarelli GV. Antiarrhythmic drug therapy: where do we go from here?[J]. Circulation, 2024, 149(11): 801-3. |
| 5 | Nogami A, Kurita T, Abe H, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias[J]. J Arrhythm, 2021, 37(4): 709-870. |
| 6 | Kingma J, Simard C, Drolet B. Overview of cardiac arrhythmias and treatment strategies[J]. Pharmaceuticals, 2023, 16(6): 844. |
| 7 | Fye WB. A history of the origin, evolution, and impact of electrocardiography [J]. Am J Cardiol, 1994, 73(13):937-49. |
| 8 | Jahmunah V, Oh SL, Wei JKE, et al. Computer-aided diagnosis of congestive heart failure using ECG signals‑A review[J]. Phys Med, 2019, 62: 95-104. |
| 9 | Oestereicher MA, Wotton JM, Ayabe S, et al. Comprehensive ECG reference intervals in C57BL/6N substrains provide a generalizable guide for cardiac electrophysiology studies in mice[J]. Mamm Genome, 2023, 34(2): 180-99. |
| 10 | Obergassel J, O'Reilly M, Sommerfeld LC, et al. Effects of genetic background, sex, and age on murine atrial electrophysiology[J]. Europace, 2021, 23(6): 958-69. |
| 11 | Haq KT, Cooper BL, Berk F, et al. The effect of sex and age on ex vivo cardiac electrophysiology: insight from a guinea pig model[J]. Am J Physiol Heart Circ Physiol, 2023, 324(1): H141-54. |
| 12 | Ahmadi P, Afzalian A, Jalali A, et al. Age and gender differences of basic electrocardiographic values and abnormalities in the general adult population; Tehran Cohort Study[J]. BMC Cardiovasc Disord, 2023, 23(1): 303. |
| 13 | Xie M, Zhu SJ, Liu G, et al. A novel quantitative electrocardiography strategy reveals the electroinhibitory effect of tamoxifen on the mouse heart[J]. J Cardiovasc Transl Res, 2023, 16(5): 1232-48. |
| 14 | Jia BZ, Qi YT, Wong-Campos JD, et al. A bioelectrical phase transition patterns the first vertebrate heartbeats[J]. Nature, 2023, 622(7981): 149-55. |
| 15 | Levin M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer[J]. Cell, 2021, 184(8): 1971-89. |
| 16 | Gajendragadkar PR, von Ende A, Ibrahim M, et al. Assessment of the causal relevance of ECG parameters for risk of atrial fibrillation: a Mendelian randomisation study[J]. PLoS Med, 2021, 18(5): e1003572. |
| 17 | Amuzescu B, Airini R, Epureanu FB, et al. Evolution of mathematical models of cardiomyocyte electrophysiology[J]. Math Biosci, 2021, 334: 108567. |
| 18 | Lawson BAJ, Drovandi CC, Cusimano N, et al. Unlocking data sets by calibrating populations of models to data density: a study in atrial electrophysiology[J]. Sci Adv, 2018, 4(1): e1701676. |
| 19 | Morotti S, Liu C, Hegyi B, et al. Quantitative cross-species translators of cardiac myocyte electrophysiology: model training, experimental validation, and applications[J]. Sci Adv, 2021, 7(47): eabg0927. |
| 20 | Mazhar F, Bartolucci C, Regazzoni F, et al. A detailed mathematical model of the human atrial cardiomyocyte: integration of electrophysiology and cardiomechanics[J]. J Physiol, 2024, 602(18): 4543-83. |
| 21 | Ntalla I, Weng LC, Cartwright JH, et al. Multi-ancestry GWAS of the electrocardiographic PR interval identifies 202 loci underlying cardiac conduction[J]. Nat Commun, 2020, 11(1): 2542. |
| 22 | van Duijvenboden S, Ramírez J, Young WJ, et al. Genomic and pleiotropic analyses of resting QT interval identifies novel loci and overlap with atrial electrical disorders[J]. Hum Mol Genet, 2021, 30(24): 2513-23. |
| 23 | Liu G, Iden JB, Kovithavongs K, et al. In vivo temporal and spatial distribution of depolarization and repolarization and the illusive murine T wave[J]. J Physiol, 2004, 555(Pt 1): 267-79. |
| 24 | Rodrigues JC, McIntyre B, Dastidar AG, et al. The effect of obesity on electrocardiographic detection of hypertensive left ventricular hypertrophy: recalibration against cardiac magnetic resonance[J]. J Hum Hypertens, 2016, 30(3): 197-203. |
| 25 | de Coster M, Demolder A, de Meyer V, et al. Diagnostic accuracy of R-wave detection by insertable cardiac monitors[J]. Pacing Clin Electrophysiol, 2020, 43(5): 511-7. |
| 26 | Ramírez J, van Duijvenboden S, Young WJ, et al. Common genetic variants modulate the electrocardiographic tpeak-to-tend interval[J]. Am J Hum Genet, 2020, 106(6): 764-78. |
| 27 | Yogasundaram H, Zheng YG, Ly E, et al. Relationship between baseline electrocardiographic measurements and outcomes in patients with high-risk heart failure: insights from the VerICiguaT Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial[J]. Eur J Heart Fail, 2023, 25(10): 1822-30. |
| 28 | Mayourian J, la Cava WG, Vaid A, et al. Pediatric ECG-based deep learning to predict left ventricular dysfunction and remodeling[J]. Circulation, 2024, 149(12): 917-31. |
| 29 | Ardissino M, Patel KHK, Rayes B, et al. Multiple anthropometric measures and proarrhythmic 12-lead ECG indices: a Mendelian randomization study[J]. PLoS Med, 2023, 20(8): e1004275. |
| 30 | Chen LY, Ribeiro ALP, Platonov PG, et al. P wave parameters and indices: a critical appraisal of clinical utility, challenges, and future research-a consensus document endorsed by the international so-ciety of electrocardiology and the international society for holter and noninvasive electrocardiology[J]. Circ Arrhythm Electrophysiol, 2022, 15(4): e010435. |
| 31 | Young WJ, Lahrouchi N, Isaacs A, et al. Genetic analyses of the electrocardiographic QT interval and its components identify additional loci and pathways[J]. Nat Commun, 2022, 13(1): 5144. |
| 32 | Broman MT, Nadadur RD, Perez-Cervantes C, et al. A genomic link from heart failure to atrial fibrillation risk: FOG2 modulates a TBX5/GATA4-dependent atrial gene regulatory network[J]. Circulation, 2024, 149(15): 1205-30. |
| 33 | Frimodt-M ller EK, Soliman EZ, Kizer JR, et al. Lifestyle habits associated with cardiac conduction disease[J]. Eur Heart J, 2023, 44(12): 1058-66. |
| 34 | Gottlieb LA, Larsen K, Halade GV, et al. Prolonged QT intervals in mice with cardiomyocyte-specific deficiency of the molecular clock[J]. Acta Physiol, 2021, 233(1): e13707. |
| 35 | Calò L, Crescenzi C, Martino A, et al. The diagnostic value of the 12-LeadECGin arrhythmogenic LeftVentricularCardiomyopathy: novel ECG signs[J]. JACC Clin Electrophysiol, 2023, 9(12): 2615-27. |
| 36 | Nam JM, Lim JE, Ha TW, et al. Cardiac-specific inactivation of Prdm16 effects cardiac conduction abnormalities and cardiomyopathy-associated phenotypes[J]. Am J Physiol Heart Circ Physiol, 2020, 318(4): H764-77. |
| 37 | Karakayali M, Artac I, Omar T, et al. Assessment of the efficacy of the electrocardiographic P-wave peak time in predicting atrial high rate episode in patients with cardiac implantable electronic devices[J]. J Electrocardiol, 2023, 80: 40-4. |
| 38 | Hennis K, Rötzer RD, Rilling J, et al. In vivo and ex vivo electrophysiological study of the mouse heart to characterize the cardiac conduction system, including atrial and ventricular vulnerability[J]. Nat Protoc, 2022, 17(5): 1189-222. |
| 39 | Litviňuková M, Talavera-López C, Maatz H, et al. Cells of the adult human heart[J]. Nature, 2020, 588(7838): 466-72. |
| 40 | Li Q, Lin ZW, Liu R, et al. Multimodal charting of molecular and functional cell states via in situ electro-sequencing[J]. Cell, 2023, 186(9): 2002-17. e21. |
| 41 | Jagannatha GNP, Antara IMPS, Kosasih AM, et al. P-wave peak time and P-wave dispersion in surface electrocardiography as initial predictors of new-onset atrial fibrillation in early-onset hypertension[J]. Hypertens Res, 2024, 47(1): 137-48. |
| 42 | Feeny AK, Rickard J, Trulock KM, et al. Machine learning of 12-lead QRS waveforms to identify cardiac resynchronization therapy patients with differential outcomes[J]. Circ Arrhythm Electrophysiol, 2020, 13(7): e008210. |
| 43 | Hnatkova K, Andršová I, Novotný T, et al. QRS micro-fragmentation as a mortality predictor[J]. Eur Heart J, 2022, 43(40): 4177-91. |
| 44 | Chen N, Wang L, Jiao JC, et al. RV1+RV3 index to differentiate idiopathic ventricular arrhythmias arising from right ventricular outflow tract and aortic sinus of Valsalva: a multicenter study[J]. J Am Heart Assoc, 2024, 13(7): e033779. |
| 45 | Wallet J, Kimura Y, Blom NA, et al. The R″ wave in V1 and the negative terminal QRS vector in aVF combine to a novel 12-lead ECG algorithm to identify slow conducting anatomical isthmus 3 in patients with tetralogy of Fallot[J]. Europace, 2023, 25(6): euad139. |
| [1] | Na YE, Chenwen WU, Jialin JIANG. A lung sound classification model with a spatial and channel reconstruction convolutional module [J]. Journal of Southern Medical University, 2024, 44(9): 1720-1728. |
| [2] | . Value of ultrasound shear wave elasticity imaging in diagnosis of Hashimoto’s thyroiditis [J]. Journal of Southern Medical University, 2017, 37(05): 683-. |
| [3] | . Assessment of plantar fasciitis using shear wave elastography [J]. Journal of Southern Medical University, 2014, 34(02): 206-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||