Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (7): 1370-1381.doi: 10.12122/j.issn.1673-4254.2024.07.17
Hongzhe WANG1( ), Haitang XIE1(
), Haitang XIE1( ), Wulan XU1, Ming Li2
), Wulan XU1, Ming Li2
Received:2024-04-16
Online:2024-07-20
Published:2024-07-25
Contact:
Haitang XIE
E-mail:wanghongzhe66@163.com;15104758818@163.com
Hongzhe WANG, Haitang XIE, Wulan XU, Ming Li. Urolithin A alleviates respiratory syncytial virus-induced lung infection in neonatal mice by activating miR-136-mediated Sirt1 signaling[J]. Journal of Southern Medical University, 2024, 44(7): 1370-1381.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.07.17
| Gene | Primer sequence(5′-3′) |
|---|---|
| mmu-miR-136 | Forward:5'-ACACTCCAGCTGGGACTCCATTTGTTTTGATGA-3' |
| Reverse:5'-CTCAACTGGTGTCGTGGA-3' | |
| hsa-miR-136 | Forward:5'-GCGCACTCCATTTGTTTTGAT-3' |
| Reverse:5'-GTGCAGGGTCCGAGGT-3' | |
| mmu-U6 | Forward:5'-CTCGCTTCGGCAGCACA-3' |
| Reverse:5'-AACGCTTCACGAATTTGCGT-3' | |
| hsa-U6 | Forward:5'-AAAGCAAATCATCGGACGACC-3' |
| Reverse:5'-GTACAACACATTGTTTCCTCGGA-3' | |
| mmu-Sirt1 | Forward:5'-GACGCTGTGGCAGATTGTTA-3' |
| Reverse:5'-GGAATCCCACAGGAGACAGA-3' | |
| hsa-Sirt1 | Forward:5'-TGCCGGAAACAATACCTCCA-3' |
| Reverse:5'-AGACACCCCAGCTCCAGTTA-3' | |
| mmu-GAPDH | Forward:5'-GGCCTCCAAGGAGTAAGAAA-3' |
| Reverse:5'-GCCCCTCCTGTTATTATGG-3' | |
| hsa-GAPDH | Forward:5'-TGTGGGCATCAATGGATTTGG-3' |
| Reverse:5'-ACACCATGTATTCCGGGTCAAT-3' |
Tab.1 Primer sequences for qRT-PCR
| Gene | Primer sequence(5′-3′) |
|---|---|
| mmu-miR-136 | Forward:5'-ACACTCCAGCTGGGACTCCATTTGTTTTGATGA-3' |
| Reverse:5'-CTCAACTGGTGTCGTGGA-3' | |
| hsa-miR-136 | Forward:5'-GCGCACTCCATTTGTTTTGAT-3' |
| Reverse:5'-GTGCAGGGTCCGAGGT-3' | |
| mmu-U6 | Forward:5'-CTCGCTTCGGCAGCACA-3' |
| Reverse:5'-AACGCTTCACGAATTTGCGT-3' | |
| hsa-U6 | Forward:5'-AAAGCAAATCATCGGACGACC-3' |
| Reverse:5'-GTACAACACATTGTTTCCTCGGA-3' | |
| mmu-Sirt1 | Forward:5'-GACGCTGTGGCAGATTGTTA-3' |
| Reverse:5'-GGAATCCCACAGGAGACAGA-3' | |
| hsa-Sirt1 | Forward:5'-TGCCGGAAACAATACCTCCA-3' |
| Reverse:5'-AGACACCCCAGCTCCAGTTA-3' | |
| mmu-GAPDH | Forward:5'-GGCCTCCAAGGAGTAAGAAA-3' |
| Reverse:5'-GCCCCTCCTGTTATTATGG-3' | |
| hsa-GAPDH | Forward:5'-TGTGGGCATCAATGGATTTGG-3' |
| Reverse:5'-ACACCATGTATTCCGGGTCAAT-3' |
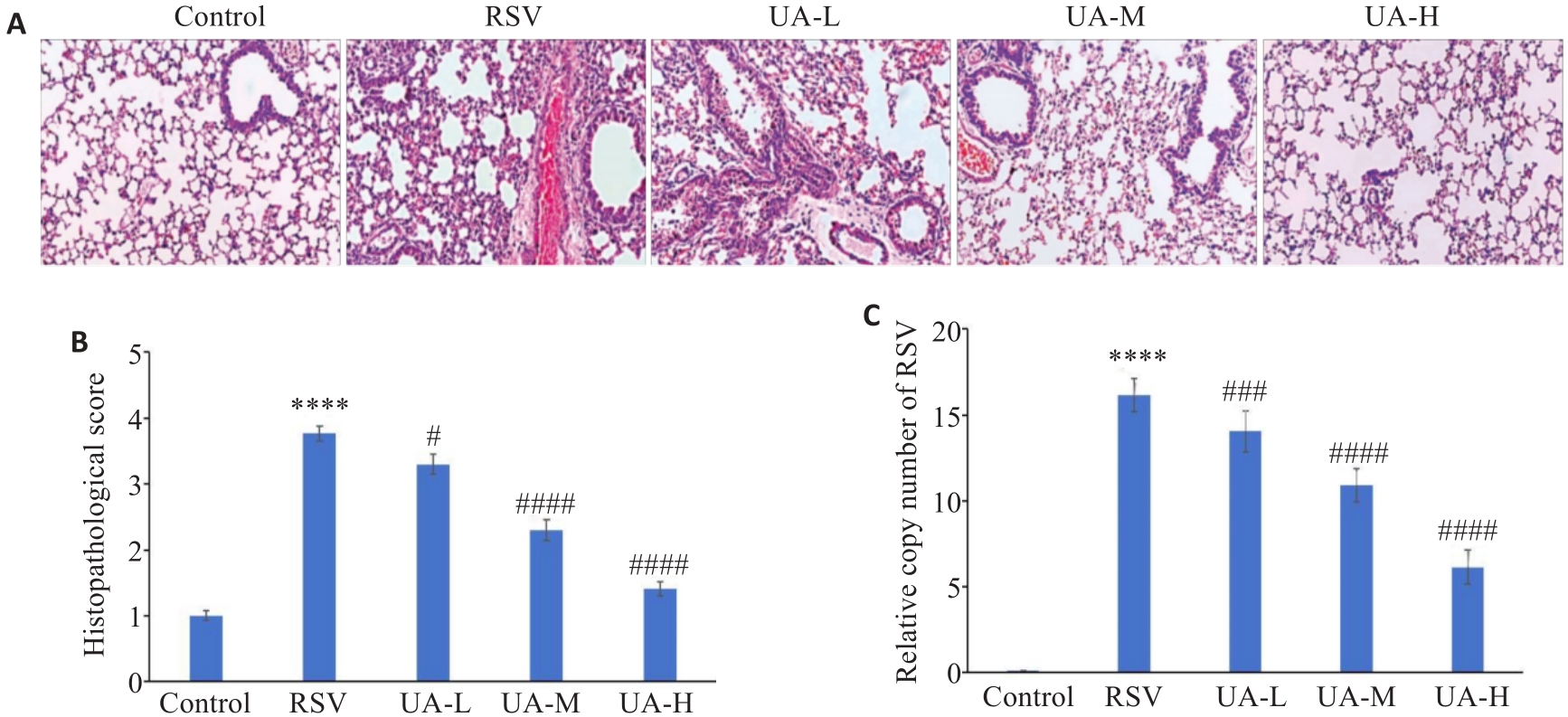
Fig.1 UA alleviates RSV-induced lung injury in neonatal mice. A: HE staining showing lung tissue injury in the mice (Original magnification: ×200). B: Pathological score based on HE staining results. C: Quantification of the relative copy number of RSV in mouse lung tissues. ****P<0.0001 vs Control group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

Fig.2 UA reduces RSV-induced lung inflammation in neonatal mice. A-D: Total cell (A), lymphocyte (B), macrophage (C) and neutrophil (D) counts in mouse BALF. E-L: Contents of TNF-α (E), IL-1β (F), IL-4 (G), IL-5 (H), IL-6 (I), IL-13 (J), IFN-γ (K) and CXCL1 (L) in lung tissues of mice detected by ELISA. ****P<0.0001 vs Control group; #P<0.05, ##P<0.01, ####P<0.0001 vs RSV group.

Fig.3 UA treatment inhibits RSV-induced apoptosis in BEAS-2B cells. A: CCK-8 assay for assessing viability of BEAS-2B cells treated with 2.5, 5, 10, 20 and 40 µmol/L UA for 24 or 48 h. B: Flow cytometry for analyzing apoptosis of BEAS-2B cells infected with RSV for 2 h followed by treatment with 2.5, 5 or 10 µmol/L UA for 48 h. C: Quantification of the percentage of apoptotic cells. D: TUNEL assay for detecting cell apoptosis in the lung tissues of the mice (400×). E: Number of TUNEL-positive cells. *P<0.05, **P<0.01, ****P<0.0001 vs Control group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

Fig.4 UA promotes RSV infection-induced autophagy in BEAS-2B cells. A: Western blotting for detecting LC3-II/I, p62, and Beclin-1 expressions in lung tissues of mice. B-D: Quantification of LC3-II/I (B), p62 (C), and Beclin-1 (D) expressions. E, F: Immunofluorescence assay for detecting LC3B expression in BEAS-2B cells infected with RSV for 2 h followed by treatment with UA (2.5, 5 and 10 µmol/L) for 48 h (×400). ****P<0.0001 vs Control group; #P<0.05, ####P<0.0001 vs RSV group.

Fig.5 UA increases Sirt1 expression by inhibiting miR-136 in RSV-infected BEAS-2B cells. A: Expressions of miR-136 in lung tissues of mice and BEAS-2B cells detected by qRT-PCR. B, C: Sirt1 mRNA (B) and protein (C) levels analyzed by qRT-PCR and Western blotting. D: Prediction of the binding site of miR-136 to 3'-UTR of Sirt1 mRNA using miRanda, targetScan and RNAInter bioinformatics databases. E: Dual-luciferase reporter assay of BEAS-2B cells co-transfected with miR-136 (or miR-NC) and Sirt1-WT or Sirt1-MUT. F-H: Sirt1 mRNA and protein expressions determined by qRT-PCR (F) and Western blotting (G, H) analysis in BEAS-2B cells transfected with miR-136 or miR-NC. I: Western blotting for detecting protein expressions of Sirt1 in RSV (100TCID50)-infected BEAS-2B cells treated with 10 µmol/L UA, transfected with miR-136, or both. ***P<0.001, ****P<0.0001 vs Control or miR-NC group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

Fig.6 UA inhibits RSV-induced inflammation and apoptosis and promotes autophagy in BEAS-2B cells by activating miR-136-mediated Sirt1 signaling pathway. A-H: ELISA measurement of contents of TNF-α (A), IL-1β (B), IL-4 (C), IL-5 (D), IL-6 (E), IL-13 (F), IFN-γ (G) and CXCL1 (H) in RSV (100TCID50)-infected BEAS-2B cells treated with 10 µmol/L UA, UA+miR-136, UA+Sirt1 inhibitor (Ex527), or UA+miR-136+Ex527. I: Cell apoptosis assessed by flow cytometry. J: Percentage of apoptotic cells. K: Immunofluorescence staining for detecting expression of LC3B (×400). L: Quantification of LC3B-positive cells. **P<0.01, ****P<0.0001 vs Control group; ####P<0.0001 vs RSV group; &P<0.05, &&P<0.01, &&&P<0.001, &&&&P<0.0001 vs RSV+UA group; $P<0.05, $$P<0.01, $$$P<0.001, $$$$P<0.0001 vs RSV+UA+miR-136 or RSV+UA+Ex527 group.
| 1 | Jain H, Schweitzer JW, Justice NA. Respiratory syncytial virus infection in children[M]. StatPearls, 2023. |
| 2 | Toivonen L, Karppinen S, Schuez-Havupalo L, et al. Respiratory syncytial virus infections in children 0-24 months of age in the community[J]. J Infect, 2020, 80(1): 69-75. |
| 3 | Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children[J]. J Allergy Clin Immunol, 2017, 140(4): 895-906. |
| 4 | Jartti T, Bønnelykke K, Elenius V, et al. Role of viruses in asthma[J]. Semin Immunopathol, 2020, 42(1): 61-74. |
| 5 | Kwon YS, Park SH, Kim MA, et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults[J]. BMC Infect Dis, 2017, 17(1): 785. |
| 6 | Kuo CH, Tsai ML, Li CH, et al. Altered pattern of macrophage polarization as a biomarker for severity of childhood asthma[J]. J Inflamm Res, 2021, 14: 6011-23. |
| 7 | Vasconcelos LHC, Ferreira SRD, Silva MDCC, et al. Uncovering the role of oxidative imbalance in the development and progression of bronchial asthma[J]. Oxid Med Cell Longev, 2021, 2021: 6692110. |
| 8 | Carande EJ, Pollard AJ, Drysdale SB. Management of respiratory syncytial virus bronchiolitis: 2015 survey of members of the European society for paediatric infectious diseases[J]. J Can Des Mal Infect De La Microbiol Med, 2016, 2016: 9139537. |
| 9 | Cerdá B, Periago P, Espín JC, et al. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds[J]. J Agric Food Chem, 2005, 53(14): 5571-6. |
| 10 | D'Amico D, Andreux PA, Valdés P, et al. Impact of the natural compound urolithin A on health, disease, and aging[J]. Trends Mol Med, 2021, 27(7): 687-99. |
| 11 | Lou LJ, Wang M, He JJ, et al. Urolithin A (UA) attenuates ferroptosis in LPS-induced acute lung injury in mice by upregulating Keap1-Nrf2/HO-1 signaling pathway[J]. Front Pharmacol, 2023, 14: 1067402. |
| 12 | Cao XL, Wan H, Wan H. Urolithin A induces protective autophagy to alleviate inflammation, oxidative stress, and endoplasmic reticulum stress in pediatric pneumonia[J]. Allergol Immunopathol, 2022, 50(6): 147-53. |
| 13 | Wang SY, Qiao JH, Chen YP, et al. Urolithin A inhibits enterovirus 71 replication and promotes autophagy and apoptosis of infected cells in vitro [J]. Arch Virol, 2022, 167(10): 1989-97. |
| 14 | Felekkis K, Touvana E, Stefanou CH, et al. MicroRNAs: a newly described class of encoded molecules that play a role in health and disease[J]. Hippokratia, 2010, 14(4): 236-40. |
| 15 | Liu Z, Fan PP, Chen M, et al. miRNAs and leukotrienes in respiratory syncytial virus infection[J]. Front Pediatr, 2021, 9: 602195. |
| 16 | Zhang X, Huang F, Yang DY, et al. Identification of miRNA-mRNA crosstalk in respiratory syncytial virus- (RSV-) associated pediatric pneumonia through integrated miRNAome and transcriptome analysis[J]. Mediators Inflamm, 2020, 2020: 8919534. |
| 17 | Atherton LJ, Jorquera PA, Bakre AA, et al. Determining immune and miRNA biomarkers related to respiratory syncytial virus (RSV) vaccine types[J]. Front Immunol, 2019, 10: 2323. |
| 18 | Eilam-Frenkel B, Naaman H, Brkic G, et al. MicroRNA 146-5p, miR-let-7c-5p, miR-221 and miR-345-5p are differentially expressed in Respiratory Syncytial Virus (RSV) persistently infected HEp-2 cells[J]. Virus Res, 2018, 251: 34-9. |
| 19 | Zhuang SH, Tang QY, Chen P, et al. Bivalirudin exerts antiviral activity against respiratory syncytial virus-induced lung infections in neonatal mice[J]. Acta Pharm, 2022, 72(3): 415-25. |
| 20 | Yang YS, Liu Y, Wang YW, et al. Regulation of SIRT1 and its roles in inflammation[J]. Front Immunol, 2022, 13: 831168. |
| 21 | Fukuda Y, Akimoto K, Homma T, et al. Virus-induced asthma exacerbations: SIRT1 targeted approach[J]. J Clin Med, 2020, 9(8): 2623. |
| 22 | Owczarczyk AB, Schaller MA, Reed M, et al. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus-induced immune responses[J]. J Immunol, 2015, 195(4): 1637-46. |
| 23 | Elesela S, Morris SB, Narayanan S, et al. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells[J]. PLoS Pathog, 2020, 16(2): e1008319. |
| 24 | Li HB, Niu XX, Shi HJ, et al. circHECTD1 attenuates apoptosis of alveolar epithelial cells in acute lung injury[J]. Lab Invest, 2022, 102(9): 945-56. |
| 25 | Martínez I, Lombardía L, Herranz C, et al. Cultures of HEp-2 cells persistently infected by human respiratory syncytial virus differ in chemokine expression and resistance to apoptosis as compared to lytic infections of the same cell type[J]. Virology, 2009, 388(1): 31-41. |
| 26 | Sun Y, López CB. Respiratory syncytial virus infection in mice and detection of viral genomes in the lung using RT-qPCR[J]. Bio-protocol, 2016, 6(10): e1819. |
| 27 | Ruiz-Galiana J, Cantón R, De Lucas Ramos P, et al. Respiratory syncytial virus: a new era[J]. Rev Esp Quimioter, 2024, 37(2): 134-48. |
| 28 | Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis[J]. Lancet, 2022, 399(10340): 2047-64. |
| 29 | Chatterjee A, Mavunda K, Krilov LR. Current state of respiratory syncytial virus disease and management[J]. Infect Dis Ther, 2021, 10(): 5-16. |
| 30 | Zhang XL, Zhang X, Hua W, et al. Expert consensus on the diagnosis, treatment, and prevention of respiratory syncytial virus infections in children[J]. World J Pediatr, 2024, 20(1): 11-25. |
| 31 | Zou G, Cao SS, Gao Z, et al. Current state and challenges in respiratory syncytial virus drug discovery and development[J]. Antiviral Res, 2024, 221: 105791. |
| 32 | Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults[J]. N Engl J Med, 2023, 388(7): 595-608. |
| 33 | Larrosa M, González-Sarrías A, García-Conesa MT, et al. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities[J]. J Agric Food Chem, 2006, 54(5): 1611-20. |
| 34 | Espín JC, Larrosa M, García-Conesa MT, et al. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far[J]. Evid Based Complement Alternat Med, 2013, 2013: 270418. |
| 35 | Kujawska M, Jodynis-Liebert J. Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection[J]. World J Gastroenterol, 2020, 26(23): 3170-81. |
| 36 | Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health[J]. Food Res Int, 2011, 44(5): 1150-60. |
| 37 | Jiao PF, Wang YR, Ren GF, et al. Urolithin A exerts a protective effect on lipopolysaccharide-induced acute lung injury by regulating HMGB1-mediated MAPK and NF-κB signaling pathways[J]. Naunyn Schmiedebergs Arch Pharmacol, 2024, [Online ahead of print]. |
| 38 | Kim S, Kim J, Song Y, et al. Unripe Rubus occidentalis, ellagic acid, and urolithin A attenuate inflammatory responses in IL-1β-stimulated A549 cells and PMA-stimulated differentiated HL-60 cells[J]. Nutrients, 2023, 15(15): 3364. |
| 39 | Carvajal JJ, Avellaneda AM, Salazar-Ardiles C, et al. Host components contributing to respiratory syncytial virus pathogenesis[J]. Front Immunol, 2019, 10: 2152. |
| 40 | Schmidt ME, Varga SM. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection[J]. Cytokine, 2020, 133: 154481. |
| 41 | Clementi N, Ghosh S, De Santis M, et al. Viral respiratory pathogens and lung injury[J]. Clin Microbiol Rev, 2021, 34(3): e00103-20. |
| 42 | O'Donnell DR, Milligan L, Stark JM. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection[J]. Virology, 1999, 257(1): 198-207. |
| 43 | Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective[J]. Cell, 2019, 176(1/2): 11-42. |
| 44 | Li JJ, Liu ML, Lv JN, et al. Polysaccharides from Platycodonis Radix ameliorated respiratory syncytial virus-induced epithelial cell apoptosis and inflammation through activation of miR-181a-mediated Hippo and SIRT1 pathways[J]. Int Immunopharmacol, 2022, 104: 108510. |
| 45 | Zhang Y, Shao LY. Decreased microRNA-140-5p contributes to respiratory syncytial virus disease through targeting Toll-like receptor 4[J]. Exp Ther Med, 2018, 16(2): 993-9. |
| 46 | Huang Z, Liu XX, Wu X, et al. MiR-146a alleviates lung injury caused by RSV infection in young rats by targeting TRAF-6 and regulating JNK/ERKMAPK signaling pathways[J]. Sci Rep, 2022, 12(1): 3481. |
| 47 | Wang SS, Ling YT, Yao YY, et al. Luteolin inhibits respiratory syncytial virus replication by regulating the miR-155/SOCS1/STAT1 signaling pathway[J]. Virol J, 2020, 17(1): 187. |
| 48 | Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation[J]. Inflammation, 2020, 43(5): 1589-98. |
| 49 | DiNicolantonio JJ, McCarty MF, O'Keefe JH. Nutraceutical activation of Sirt1: a review[J]. Open Heart, 2022, 9(2): e002171. |
| 50 | Peng XP, Li XH, Li Y, et al. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF‑κB acetylation[J]. Int Immunopharmacol, 2019, 70: 520-9. |
| 51 | Liu XL, Shao KQ, Sun TY. SIRT1 regulates the human alveolar epithelial A549 cell apoptosis induced by Pseudomonas aeruginosa lipopolysaccharide[J]. Cell Physiol Biochem, 2013, 31(1): 92-101. |
| 52 | Liu ZH, Meng YL, Miao Y, et al. Artesunate reduces sepsis-mediated acute lung injury in a SIRT1-dependent manner[J]. Bioimpacts, 2023, 13(3): 219-28. |
| 53 | Liu JY, Lv XJ, Dong WJ, et al. The role of SIRT1 in autophagy in lipopolysaccharide-induced mouse type II alveolar epithelial cells[J]. Inflammation, 2018, 41(6): 2222-35. |
| 54 | Velagapudi R, Lepiarz I, El-Bakoush A, et al. Induction of autophagy and activation of SIRT-1 deacetylation mechanisms mediate neuroprotection by the pomegranate metabolite urolithin A in BV2 microglia and differentiated 3D human neural progenitor cells[J]. Mol Nutr Food Res, 2019, 63(10): e1801237. |
| [1] | ZHOU Fengmin, GUO Yanju, CHEN Ning. Exercise promotes irisin expression to ameliorate renal injury in type 2 diabetic rats [J]. Journal of Southern Medical University, 2024, 44(4): 675-681. |
| [2] | YE Hongwei, ZHANG Yuming, YUN Qi, DU Ruoli, LI Lu, LI Yuping, GAO Qin. Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression [J]. Journal of Southern Medical University, 2024, 44(1): 45-51. |
| [3] | XIN Chen, WANG Xiaoying, LI Xiang, CHEN Yu, WANG Xue, NING Jiaxi, YANG Shi, WANG Zhongqiong. LncRNA SOX2OT enhances 5-fluorouracil resistance of cholangiocarcinoma cells by promoting autophagy via up-regulating SIRT1 expression [J]. Journal of Southern Medical University, 2024, 44(1): 187-193. |
| [4] | GUO Jingjing, ZHANG Wenlong, LIANG Piao, ZHANG Longjun, PENG Lingyin, MIN Yuqi, PAN Xiaozhen, YANG Zhiying, DENG Huafei. Puerarin alleviates lipopolysaccharide-induced acute kidney injury in mice by modulating the SIRT1/NF-κB pathway [J]. Journal of Southern Medical University, 2023, 43(7): 1248-1253. |
| [5] | XIN Chen, WANG Xiaoying, LI Xiang, CHEN Yu, WANG Xue, NING Jiaxi, YANG Shi, WANG Zhongqiong. Silencing SIRT1 reduces 5-fluorouracil resistance of cholangiocarcinoma cells by inhibiting the FOXO1/Rab7 autophagy pathway [J]. Journal of Southern Medical University, 2023, 43(3): 454-459. |
| [6] | QIN Na, HUANG Lin, DONG Rui, LI Fen, TANG Xuheng, ZENG Zhenhua, WANG Xingmin, YANG Hong. Polydatin improves intestinal barrier injury after traumatic brain injury in rats by reducing oxidative stress and inflammatory response via activating SIRT1-mediated deacetylation of SOD2 and HMGB1 [J]. Journal of Southern Medical University, 2022, 42(1): 93-100. |
| [7] | . Angiotensin II inhibits AMPK/SIRT1 pathway by inducing oxidative stress in RAW264.7 macrophages [J]. Journal of Southern Medical University, 2021, 41(3): 384-390. |
| [8] | . Chaihu Guizhi decoction produces antidepressant-like effects via sirt1-p53 signaling pathway [J]. Journal of Southern Medical University, 2021, 41(3): 399-405. |
| [9] | . SIRT1 participates in epithelial-mesenchymal transition of EC-9706 and Eca-109 cells in vitro by regulating Snail expression [J]. Journal of Southern Medical University, 2018, 38(11): 1325-. |
| [10] | . Exendin-4 alleviates diabetic cardiomyopathy in mice by regulating Sirt1/PGC1α [J]. Journal of Southern Medical University, 2018, 38(05): 520-. |
| [11] | ZHANG Qi-wei1, YOU Shang-you2, SUN Ji-min2, WU Qi2, YU Chun-hua2, ZHANG Chu-yu1. Quantitative fluorogenic real-time PCR assay for respiratory syncytial virus detection [J]. Journal of Southern Medical University, 2005, 25(07): 847-852. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||