Journal of Southern Medical University ›› 2024, Vol. 44 ›› Issue (6): 1059-1069.doi: 10.12122/j.issn.1673-4254.2024.06.06
Jie ZHANG1( ), Junyan YAO3, Yinggui YANG4, Fei WANG6, Qingyou ZHENG4, Xin LI5, Changbai LIU2(
), Junyan YAO3, Yinggui YANG4, Fei WANG6, Qingyou ZHENG4, Xin LI5, Changbai LIU2( )
)
Received:2023-12-20
Online:2024-06-20
Published:2024-07-01
Contact:
Changbai LIU
E-mail:zhangjie5229@163.com;cbliu@ctgu.edu.cn
Supported by:Jie ZHANG, Junyan YAO, Yinggui YANG, Fei WANG, Qingyou ZHENG, Xin LI, Changbai LIU. Cell membrane-penetrating capacity of hPP10-Cu, Zn-SOD fusion protein and its antioxidant and anti-inflammatory activity[J]. Journal of Southern Medical University, 2024, 44(6): 1059-1069.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.j-smu.com/EN/10.12122/j.issn.1673-4254.2024.06.06
| Reagent | Control group | Assay group |
|---|---|---|
| Reagent I | 1.0 | 1.0 |
| Control supernatant | a* | |
| Sample group supernatant | a* | |
| Reagent II | 1.0 | 1.0 |
| Reagent III | 1.0 | 1.0 |
| Reagent IV | 1.0 | 1.0 |
| Mix thoroughly with a vortex mixer and place in a water bath at 37 ℃ for 40 minutes | ||
| Color developing agent | 2.0 | 2.0 |
Tab.1 SOD enzyme activity detection system (mL)
| Reagent | Control group | Assay group |
|---|---|---|
| Reagent I | 1.0 | 1.0 |
| Control supernatant | a* | |
| Sample group supernatant | a* | |
| Reagent II | 1.0 | 1.0 |
| Reagent III | 1.0 | 1.0 |
| Reagent IV | 1.0 | 1.0 |
| Mix thoroughly with a vortex mixer and place in a water bath at 37 ℃ for 40 minutes | ||
| Color developing agent | 2.0 | 2.0 |
| Gene | Primer sequences | |
|---|---|---|
| Bcl-2 | Forward (5'-3') | GGTGGGGTCATGTGTGTGG |
| Reverse (3'-5') | CGGTTCAGGTACTCAGTCATCC | |
| Deptor | Forward (5'-3') | TTAGCAGACCGGGGCATTATT |
| Reverse (3'-5') | GAAGGTGCCGTCATCCTTTCT | |
| NF-κB | Forward (5'-3') | AGTATTCCTGGCGAGAAAG |
| Reverse (3'-5') | CTGTTCCTGGTCCTGTGTAG | |
| IL-6 | Forward (5'-3') | GACTTCCATCCAGTTGCCTTCT |
| Reverse (3'-5') | AGACAGGTCTGTTGGGAGTGGTA | |
| IL-1β | Forward (5'-3') | GAAATGCCACCTTTTGACAGTG |
| Reverse (3'-5') | TGGATGCTCTCATCAGGACAG | |
| TNF-α | Forward (5'-3') | CAGGCGGTGCCTATGTCTC |
| Reverse (3'-5') | CGATCACCCCGAAGTTCAGTA | |
Tab.2 Primer sequences for quantitative real-time PCR analysis
| Gene | Primer sequences | |
|---|---|---|
| Bcl-2 | Forward (5'-3') | GGTGGGGTCATGTGTGTGG |
| Reverse (3'-5') | CGGTTCAGGTACTCAGTCATCC | |
| Deptor | Forward (5'-3') | TTAGCAGACCGGGGCATTATT |
| Reverse (3'-5') | GAAGGTGCCGTCATCCTTTCT | |
| NF-κB | Forward (5'-3') | AGTATTCCTGGCGAGAAAG |
| Reverse (3'-5') | CTGTTCCTGGTCCTGTGTAG | |
| IL-6 | Forward (5'-3') | GACTTCCATCCAGTTGCCTTCT |
| Reverse (3'-5') | AGACAGGTCTGTTGGGAGTGGTA | |
| IL-1β | Forward (5'-3') | GAAATGCCACCTTTTGACAGTG |
| Reverse (3'-5') | TGGATGCTCTCATCAGGACAG | |
| TNF-α | Forward (5'-3') | CAGGCGGTGCCTATGTCTC |
| Reverse (3'-5') | CGATCACCCCGAAGTTCAGTA | |
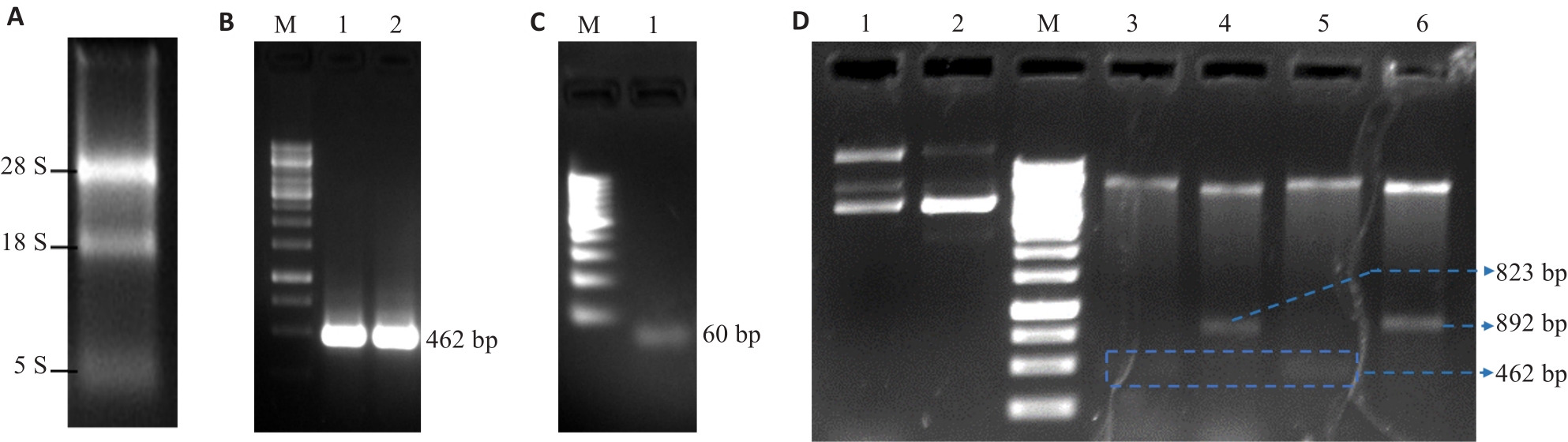
Fig.1 Construction and identification of the recombinant plasmids pET15b-Cu, Zn-SOD and pET15b-hPP10-Cu, Zn-SOD. A: Total RNA. B: Human Cu, Zn-SOD gene fragment (462 bp). C: hPP10 gene fragment (about 60 bp). D: Identification of the recombinant plasmids (Lane 1: pET15b-hPP10-SOD; Lane 2: pET15b-SOD; Lane 3: pET15b-Cu, Zn-SOD digested by Xho I and BamH I (462 bp); Lane 4: pET15b-Cu, Zn-SOD digested by Nco I and Hind III (823 bp); Lane 5: pET15b-hpp10-Cu, Zn-SOD digested by Xho I and BamH I (462 bp); Lane 6: pET15b-Cu, Zn-SOD digested by Nco I and Hind III (892 bp).
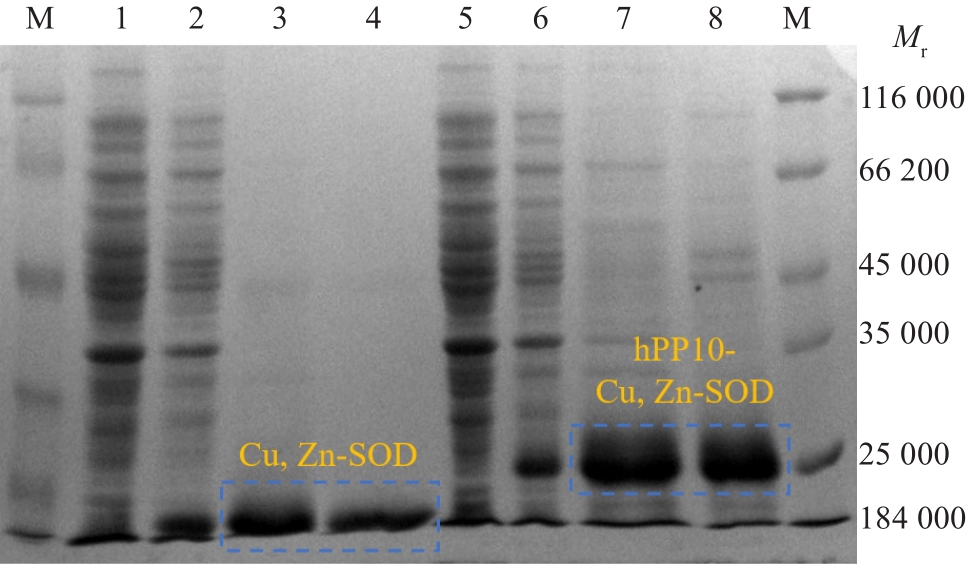
Fig.2 Induced expression and purification of Cu, Zn-SOD and the fusion protein hPP10-Cu, Zn-SOD. Lane 1: Uninduced group; Lane 2: Induced group; Lane 3: Cu, Zn-SOD protein purified by imidazole; Lane 4: Cu, Zn-SOD protein purified by urea; Lane 5: Uninduced group; Lane 6: Induced group; Lane 7: hPP10-Cu, Zn-SOD protein purified by imidazole; Lane 8: hPP10-Cu, Zn-SOD protein purified by urea.
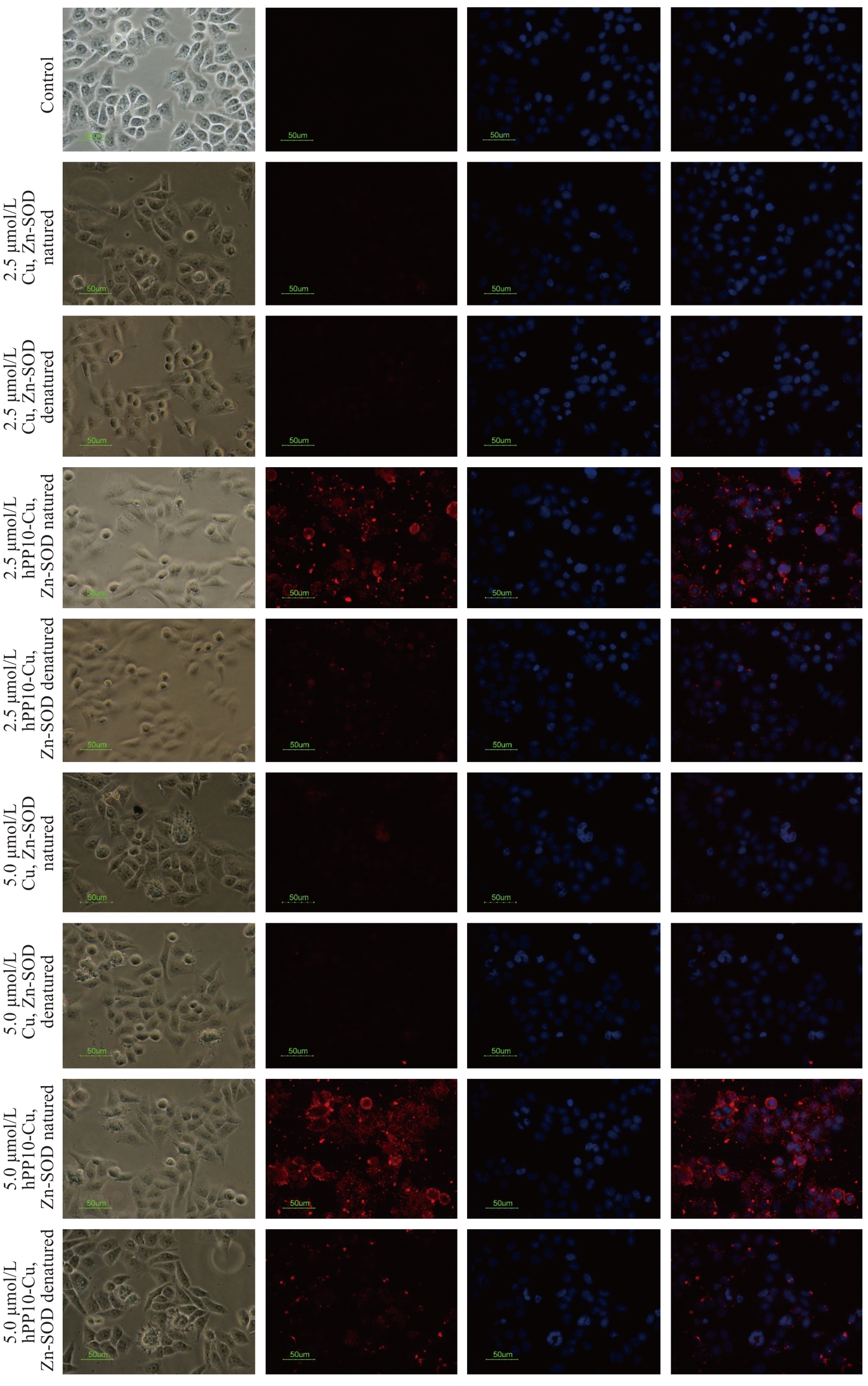
Fig.4 Assessment of cell membrane-penetrating capacity of Cu, Zn-SOD protein and hPP10-Cu, Zn-SOD fusion protein at different concentrations in HEK293 cells (scale bar=50 μm).
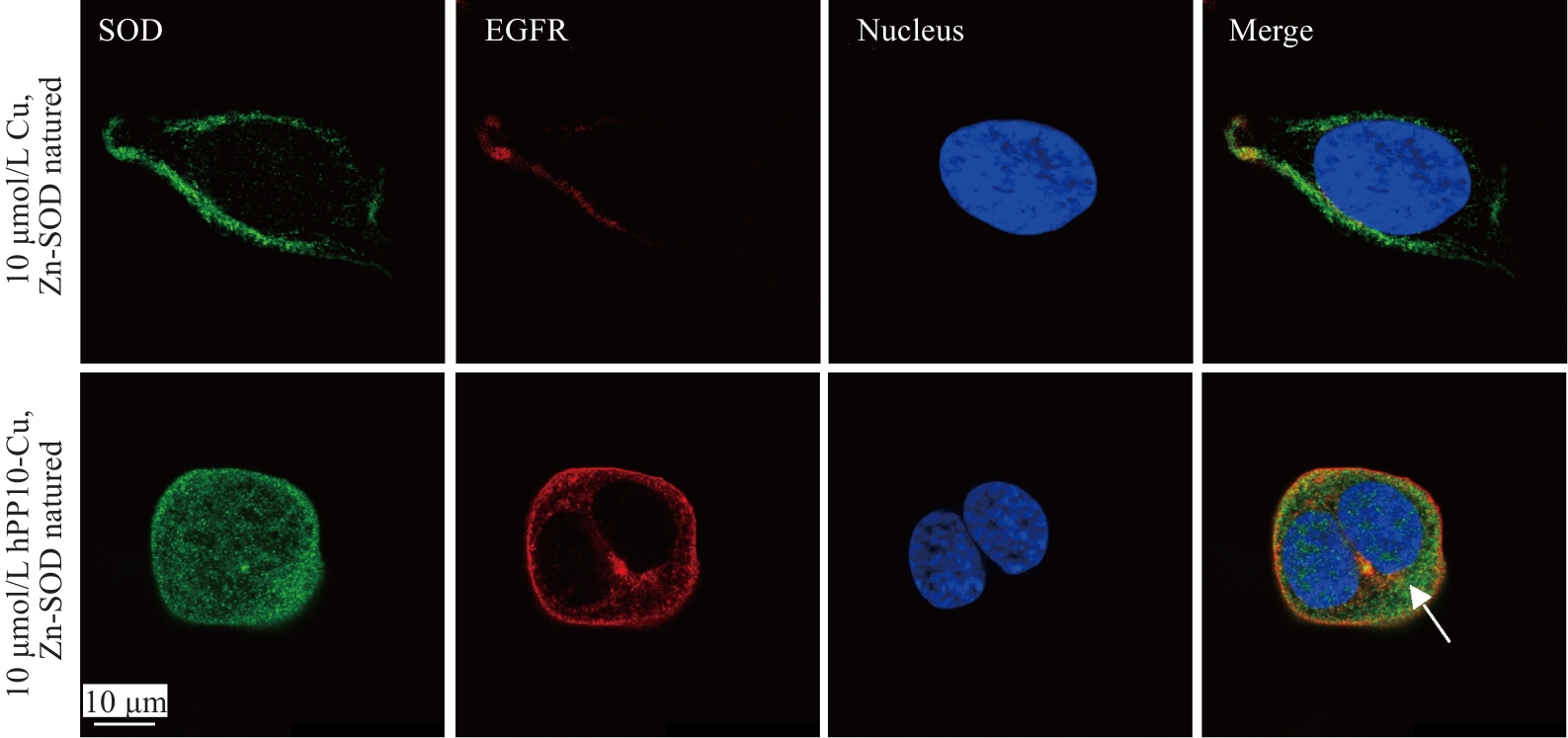
Fig.5 Intracellular localization of Cu, Zn-SOD protein and hPP10-Cu, Zn-SOD fusion protein at 10 μmol/L after cell membrane penetration (Arrow: hPP10-Cu, Zn-SOD).
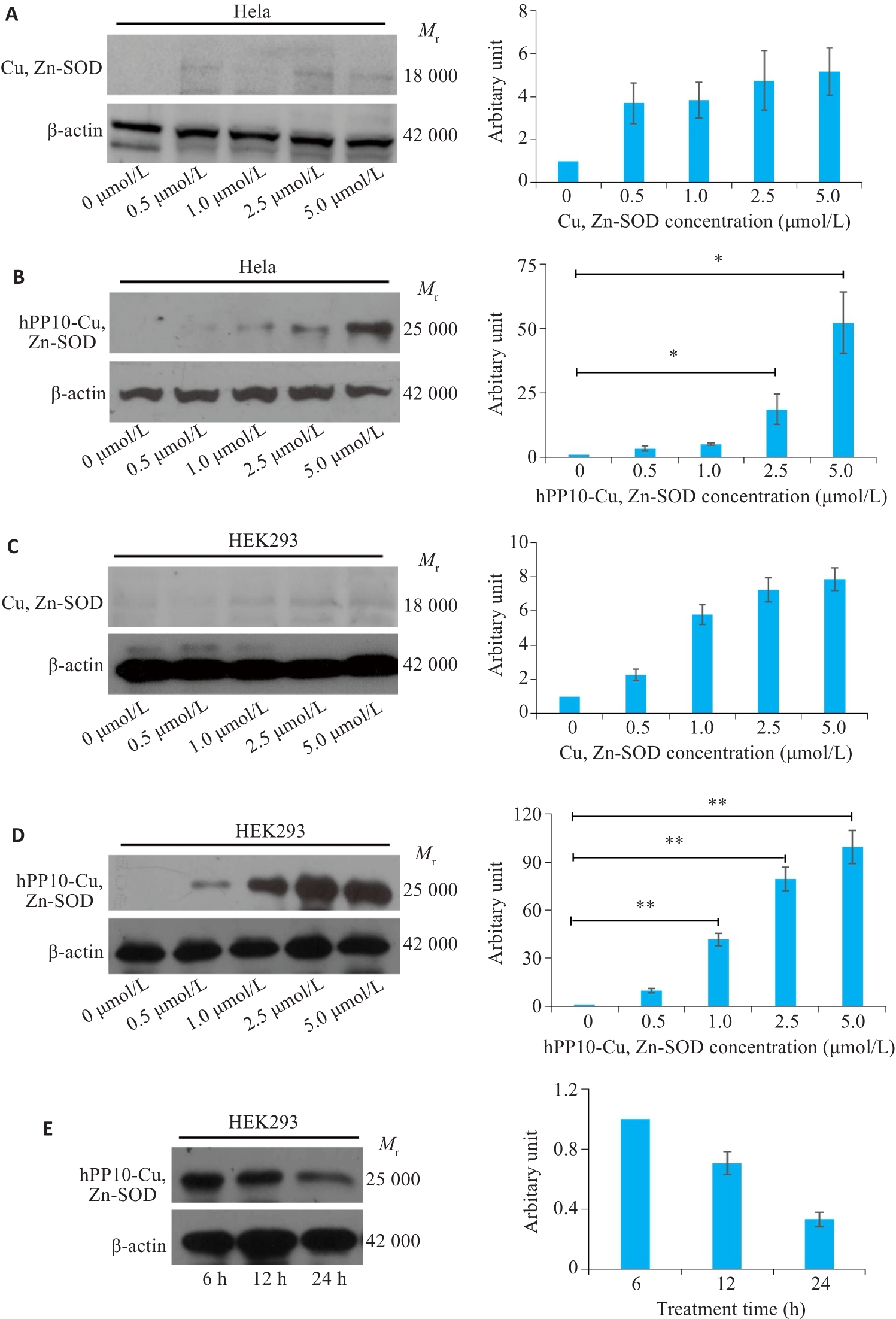
Fig.6 Cell membrane penetration by hPP10-Cu, Zn-SOD fusion protein in Hela and HEK293 cells. A: Concentration gradient effect of Cu, Zn-SOD protein transduction into Hela cells. B: Concentration gradient effect of hPP10-Cu, Zn-SOD fusion protein transduction into Hela cells (* P<0.05). C: Concentration gradient effect of Cu, Zn-SOD protein transduction into HEK293 cells. D: Concentration gradient effect of hPP10-Cu, Zn-SOD fusion protein transduction into HEK293 cells. ** P<0.01. E: Time gradient effect of hPP10-Cu, Zn-SOD fusion protein transduction into HEK293 cells.
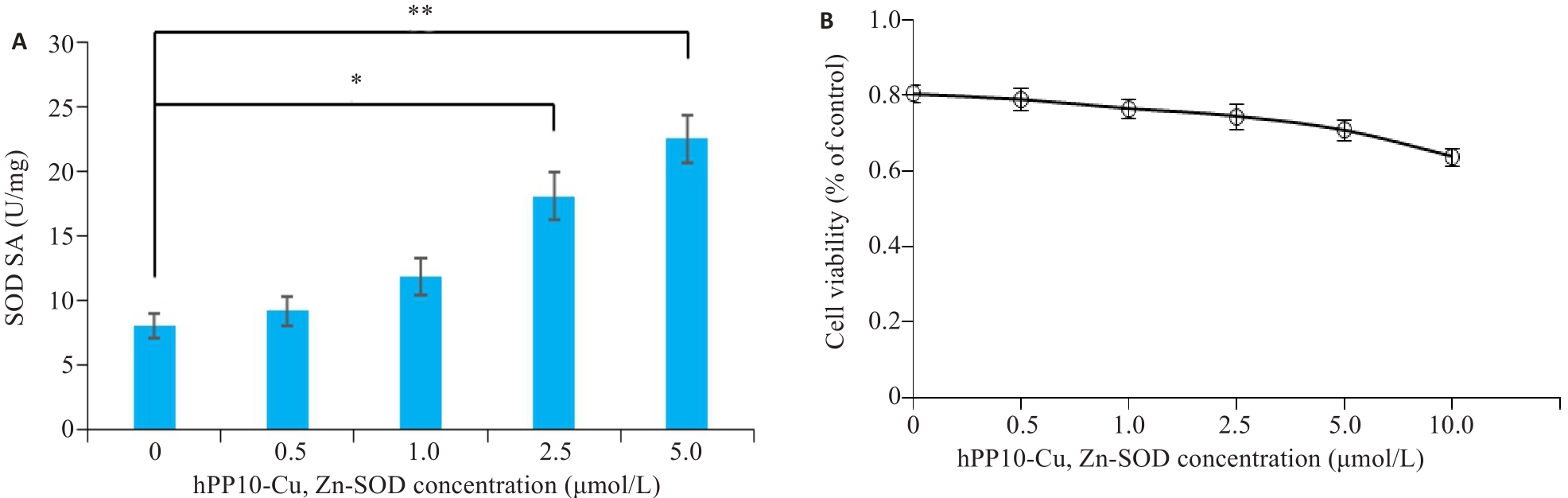
Fig.7 Analysis of enzyme activity and viability in cells treated with hPP10-Cu, Zn-SOD. A: Enzyme activities of hPP10-Cu, Zn-SOD treated cells detected by SOD kit. * P<0.05; ** P<0.01. B: The effect of hPP10-Cu, Zn-SOD treatment on cell viability detected by MTT assay.
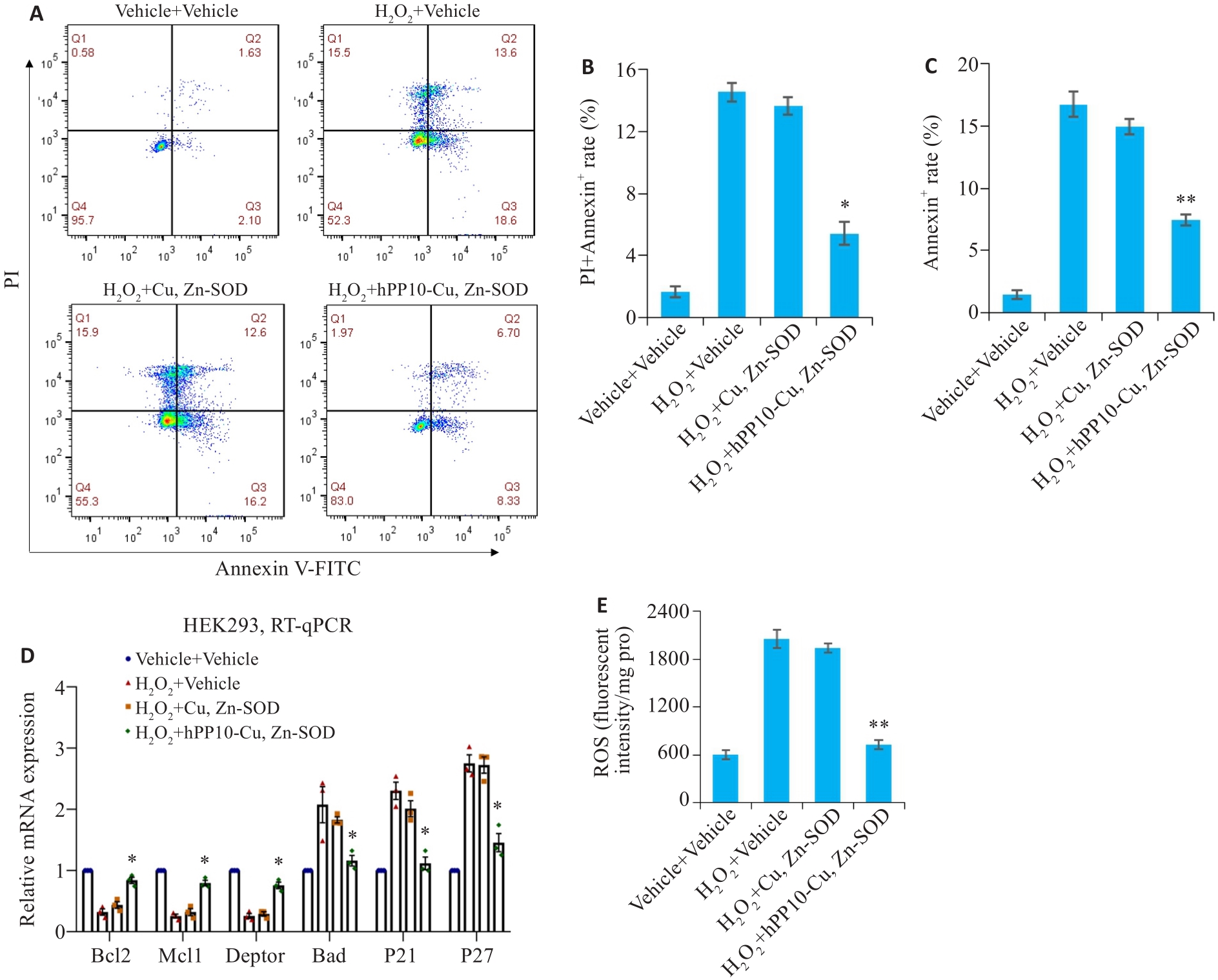
Fig.8 Effect of hPP10-Cu, Zn-SOD on apoptosis and antioxidant activity in cells treated with H2O2. A: hPP10-Cu,Zn-SOD inhibits cell apoptosis induced by H2O2. B: hPP10-Cu, Zn-SOD inhibits early apoptosis induced by H2O2 (* P<0.05 vs H2O2+Cu, Zn-SOD). C: hPP10-Cu, Zn-SOD inhibited the early and late apoptosis induced by H2O2 (** P<0.01 vs H2O2+Cu, Zn-SOD). D: hPP10-Cu, Zn-SOD fusion protein promotes expression of anti-apoptotic factors and inhibits the transcription of pro-apoptotic factors (* P<0.05 vs H2O2+Cu, Zn-SOD). E: SOD fusion protein significantly reduces oxygen free radicals in the cells (** P<0.01 vs H2O2+Cu, Zn-SOD).
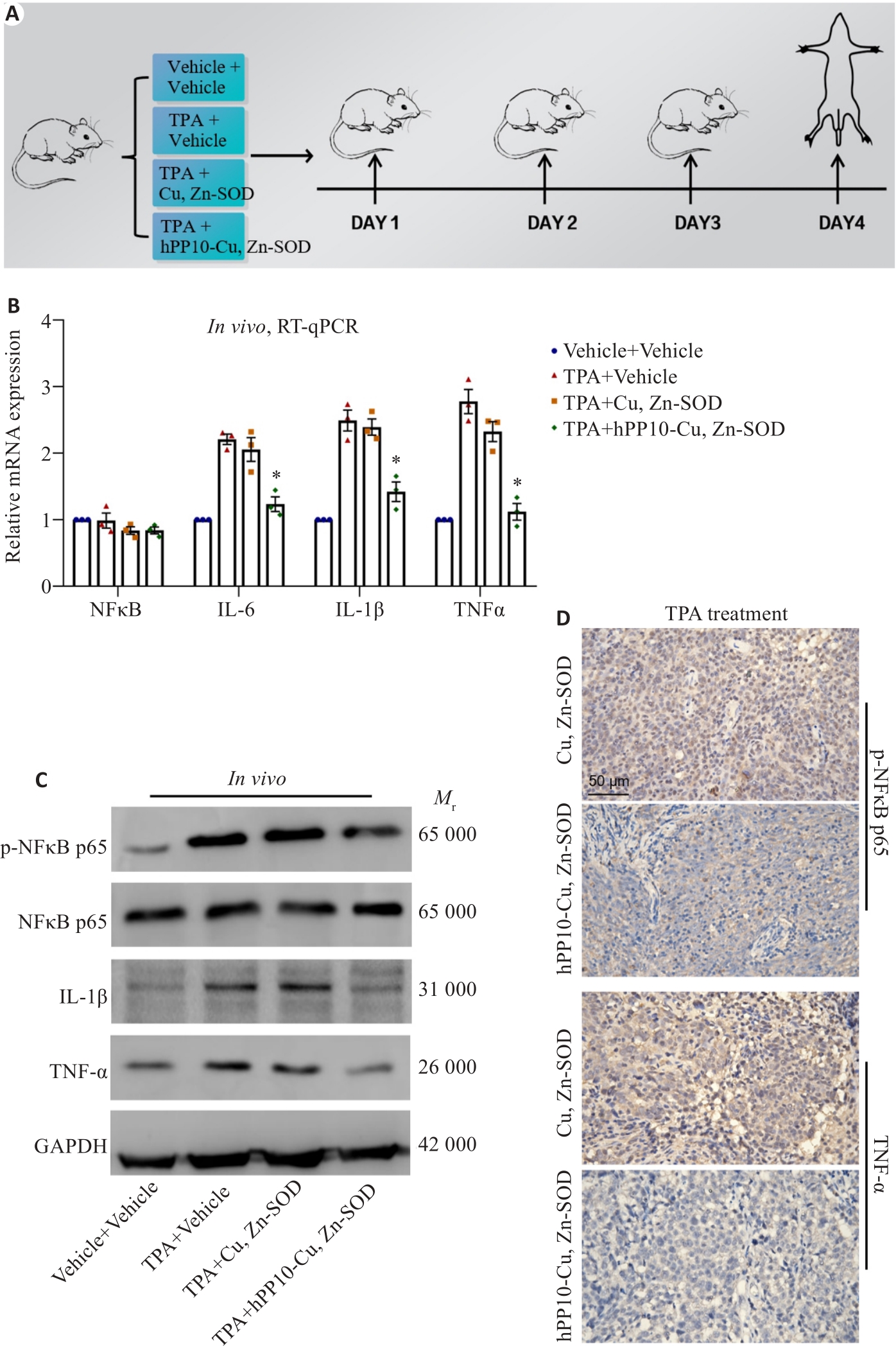
Fig.9 hPP10-Cu, Zn-SOD reduces the inflammatory response induced by TPA. A: Modeling of ear inflammation by TPA in mice. B: Inhibitory effect of hPP10-Cu, Zn-SOD on TPA-induced ear inflammation detected by RT-PCR (* P<0.05 vs TPA+Cu, Zn-SOD). C: Inhibitory effect of hPP10-Cu, Zn-SOD on TPA-induced ear inflammation detected by Western blotting. D: Inhibitory effects of hPP10-Cu, Zn-SOD on TPA-induced ear inflammation analyzed by immunohistochemistry.
| 1 | Kwon MJ, Jeon YJ, Lee KY, et al. Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice[J]. Antioxid Redox Signal, 2012, 17( 10): 1376- 92. |
| 2 | Teoh ML, Fitzgerald MP, Oberley LW, et al. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion[J]. Cancer Res, 2009, 69( 15): 6355- 63. |
| 3 | Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: a natural cargo[J]. Int J Biol Macromol, 2019, 123: 979- 90. |
| 4 | Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal[J]. Biochim Biophys Acta, 2006, 1763( 7): 747- 58. |
| 5 | Liu TP, Chen YP, Chou CM, et al. Therapeutic evaluation of HIV transduction basic domain-conjugated superoxide dismutase solution on suppressive effects of the formation of peroxynitrite and expression of COX-2 in murine skin[J]. J Biomed Sci, 2016, 23: 11. |
| 6 | Eleutherio ECA, Silva Magalhães RS, de Araújo Brasil A, et al. SOD1, more than just an antioxidant[J]. Arch Biochem Biophys, 2021, 697: 108701. |
| 7 | 杨方瑶, 简甜甜, 蒋立翔, 等. 人源SOD1及其突变体的表达纯化及酶学性质[J]. 生物学杂志, 2023, 40( 2): 20- 6. DOI: 10.3969/j.issn.2095-1736.2023.02.020 |
| 8 | Perry JJ, Shin DS, Getzoff ED, et al. The structural biochemistry of the superoxide dismutases[J]. Biochim Biophys Acta, 2010, 1804( 2): 245- 62. |
| 9 | Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein[J]. Cell, 1988, 55( 6): 1179- 88. |
| 10 | Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus[J]. Cell, 1988, 55( 6): 1189- 93. |
| 11 | Pescina S, Ostacolo C, Gomez-Monterrey IM, et al. Cell penetrating peptides in ocular drug delivery: state of the art[J]. J Control Release, 2018, 284: 84- 102. |
| 12 | Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics[J]. Trends Pharmacol Sci, 2017, 38( 4): 406- 24. |
| 13 | Yu M, Li XL, Huang XF, et al. New cell-penetrating peptide (KRP) with multiple physicochemical properties endows doxorubicin with tumor targeting and improves its therapeutic index[J]. ACS Appl Mater Interfaces, 2019, 11( 2): 2448- 58. |
| 14 | Wang LK, Chen HY, Wang FS, et al. The development of peptide-drug conjugates (PDCs) strategies for paclitaxel[J]. Expert Opin Drug Deliv, 2022, 19( 2): 147- 61. |
| 15 | Sajid MI, Moazzam M, Stueber R, et al. Applications of amphipathic and cationic cyclic cell-penetrating peptides: significant therapeutic delivery tool[J]. Peptides, 2021, 141: 170542. |
| 16 | Rádis-Baptista G, Campelo IS, Morlighem JRL, et al. Cell-penetrating peptides (CPPs): from delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis[J]. J Biotechnol, 2017, 252: 15- 26. |
| 17 | Gu Q, Feng TN, Cao H, et al. HIV-TAT mediated protein transduction of Cu/Zn-superoxide dismutase-1 (SOD1) protects skin cells from ionizing radiation[J]. Radiat Oncol, 2013, 8: 253. |
| 18 | Yoo DY, Kim DW, Chung JY, et al. Cu, Zn-superoxide dismutase increases the therapeutic potential of adipose-derived mesenchymal stem cells by maintaining antioxidant enzyme levels[J]. Neurochem Res, 2016, 41( 12): 3300- 7. |
| 19 | Kim W, Kim DW, Yoo DY, et al. Neuroprotective effects of PEP-1-Cu, Zn-SOD against ischemic neuronal damage in the rabbit spinal cord[J]. Neurochem Res, 2012, 37( 2): 307- 13. |
| 20 | 王 伟, 彭 昊. 穿透肽介导的铜-锌超氧化物歧化酶1融合蛋白的抗氧化作用及对脂肪干细胞移植修复脊髓损伤的促进作用[J]. 中华实验外科杂志, 2017, 34( 10): 1721- 4. DOI: 10.3760/cma.j.issn.1001-9030.2017.10.031 |
| 21 | Jia J, Chen F, Wu Y. Recombinant PEP-1-SOD1 improves functional recovery after neural stem cell transplantation in rats with traumatic brain injury[J]. Exp Ther Med, 2018, 15( 3): 2929- 35. |
| 22 | 孟丽华, 薛荣亮, 雷晓鸣, 等. 免疫荧光法检测融合蛋白PTD4-Cu, Zn-SOD在人星形胶质细胞中穿膜能力及保护作用[J]. 西安交通大学学报: 医学版, 2023, 44( 2): 309- 14. |
| 23 | 万婷婷, 魏兰璎, 程 艳, 等. SOD融合蛋白外用对皮肤自然老化的影响[J]. 中国皮肤性病学杂志, 2022, 36( 4): 381- 90. |
| 24 | Ding Y, Zhao XL, Geng JP, et al. Intracellular delivery of nucleic acid by cell-permeable hPP10 peptide[J]. J Cell Physiol, 2019, 234( 7): 11670- 8. |
| 25 | Wang H, Ma JL, Yang YG, et al. Efficient therapeutic delivery by a novel cell-permeant peptide derived from KDM4A protein for antitumor and antifibrosis[J]. Oncotarget, 2016, 7( 31): 49075- 90. |
| 26 | 赵雪丽, 宋 阳, 张 明, 等. 穿膜肽凋亡素融合蛋白诱导黑色素瘤细胞凋亡的实验研究[J]. 中国药学杂志, 2017, 52( 2): 115- 8. DOI: 10.11669/cpj.2017.02.007 |
| 27 | Guo JK, Xu JS, Chen TB, et al. Effects of TAT-SOD at acupoints on essential hypertension by monitoring meridians electrical potential[J]. Chin J Integr Med, 2020, 26( 9): 694- 700. |
| 28 | Reigado GR, Adriani PP, dos Santos JF, et al. Delivery of superoxide dismutase by TAT and abalone peptides for the protection of skin cells against oxidative stress[J]. Biotechnol Appl Biochem, 2022, 69( 6): 2673- 85. |
| 29 | Paul A, Belton A, Nag S, et al. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging[J]. Mech Ageing Dev, 2007, 128( 11/12): 706- 16. |
| 30 | Levin ED. Extracellular superoxide dismutase (EC-SOD) quenches free radicals and attenuates age-related cognitive decline: opportunities for novel drug development in aging[J]. Curr Alzheimer Res, 2005, 2( 2): 191- 6. |
| 31 | Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system[J]. Nat Immunol, 2002, 3( 1): 20- 6. |
| 32 | Sabio G, Davis RJ. TNF and MAP kinase signalling pathways[J]. Semin Immunol, 2014, 26( 3): 237- 45. |
| 33 | Lee YS, Cheon IS, Kim BH, et al. Loss of extracellular superoxide dismutase induces severe IL-23-mediated skin inflammation in mice[J]. J Invest Dermatol, 2013, 133( 3): 732- 41. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||