南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (4): 751-759.doi: 10.12122/j.issn.1673-4254.2025.04.10
郭舒婷1( ), 曹福羊1,2(
), 曹福羊1,2( ), 郭永馨1, 李言响1,3, 郝新宇1, 张倬宁1, 周志康1, 仝黎1(
), 郭永馨1, 李言响1,3, 郝新宇1, 张倬宁1, 周志康1, 仝黎1( ), 曹江北1(
), 曹江北1( )
)
收稿日期:2024-12-27
出版日期:2025-04-20
发布日期:2025-04-28
通讯作者:
仝黎,曹江北
E-mail:gstanes@163.com;caofuyang840723@163.com;tongli301@aliyun.com;caojiangbei@301hospital.com.cn
作者简介:郭舒婷,在读硕士研究生,E-mail: gstanes@163.com基金资助:
Shuting GUO1( ), Fuyang CAO1,2(
), Fuyang CAO1,2( ), Yongxin GUO1, Yanxiang LI1,3, Xinyu HAO1, Zhuoning ZHANG1, Zhikang ZHOU1, Li TONG1(
), Yongxin GUO1, Yanxiang LI1,3, Xinyu HAO1, Zhuoning ZHANG1, Zhikang ZHOU1, Li TONG1( ), Jiangbei CAO1(
), Jiangbei CAO1( )
)
Received:2024-12-27
Online:2025-04-20
Published:2025-04-28
Contact:
Li TONG, Jiangbei CAO
E-mail:gstanes@163.com;caofuyang840723@163.com;tongli301@aliyun.com;caojiangbei@301hospital.com.cn
Supported by:摘要:
目的 探究下丘脑背内侧核(DMH)区星形胶质细胞在七氟烷麻醉觉醒中的调节作用。 方法 选用42只雄性C57小鼠,随机分为6组(n=7),研究星形胶质细胞在七氟烷麻醉中的活性变化时分为EGFP组和GCaMP6组,通过钙成像技术记录星形胶质细胞的活性;研究光遗传激活DMH区星形胶质细胞对麻醉觉醒的影响时,行为学实验分为EGFP组和ChR2组;光遗传脑电记录实验分为EGFP组和ChR2组。麻醉诱导与觉醒的评判标准以翻正反射的消失与恢复为准,在2.0%七氟烷浓度下记录脑电并分析爆发抑制率(BSR),在1.5%七氟烷浓度下分析脑电功率频谱。此外,采用免疫荧光染色观察GFAP阳性细胞(星形胶质细胞)与病毒蛋白信号的共定位。 结果 随着七氟烷浓度的增加,DMH区星形胶质细胞的活性逐渐降低。在2.0%七氟烷麻醉中,ChR2组的觉醒时间缩短(P<0.05),光遗传激活DMH区星形胶质细胞后BSR降低(P<0.001)。在1.5%七氟烷麻醉中,光激活后ChR2组小鼠的脑电γ波增加(P<0.001),δ波减少(P<0.01)。 结论 光遗传激活DMH区星形胶质细胞能够促进七氟烷麻醉后的觉醒,但对麻醉诱导过程无显著调节作用。这一发现为麻醉觉醒机制的研究提供了新的视角,可能为术后快速苏醒及麻醉并发症的干预提供潜在靶点。
郭舒婷, 曹福羊, 郭永馨, 李言响, 郝新宇, 张倬宁, 周志康, 仝黎, 曹江北. 激活下丘脑背内侧核区星形胶质细胞可加速七氟醚麻醉小鼠觉醒[J]. 南方医科大学学报, 2025, 45(4): 751-759.
Shuting GUO, Fuyang CAO, Yongxin GUO, Yanxiang LI, Xinyu HAO, Zhuoning ZHANG, Zhikang ZHOU, Li TONG, Jiangbei CAO. Activation of astrocytes in the dorsomedial hypothalamus accelerates sevoflurane anesthesia emergence in mice[J]. Journal of Southern Medical University, 2025, 45(4): 751-759.
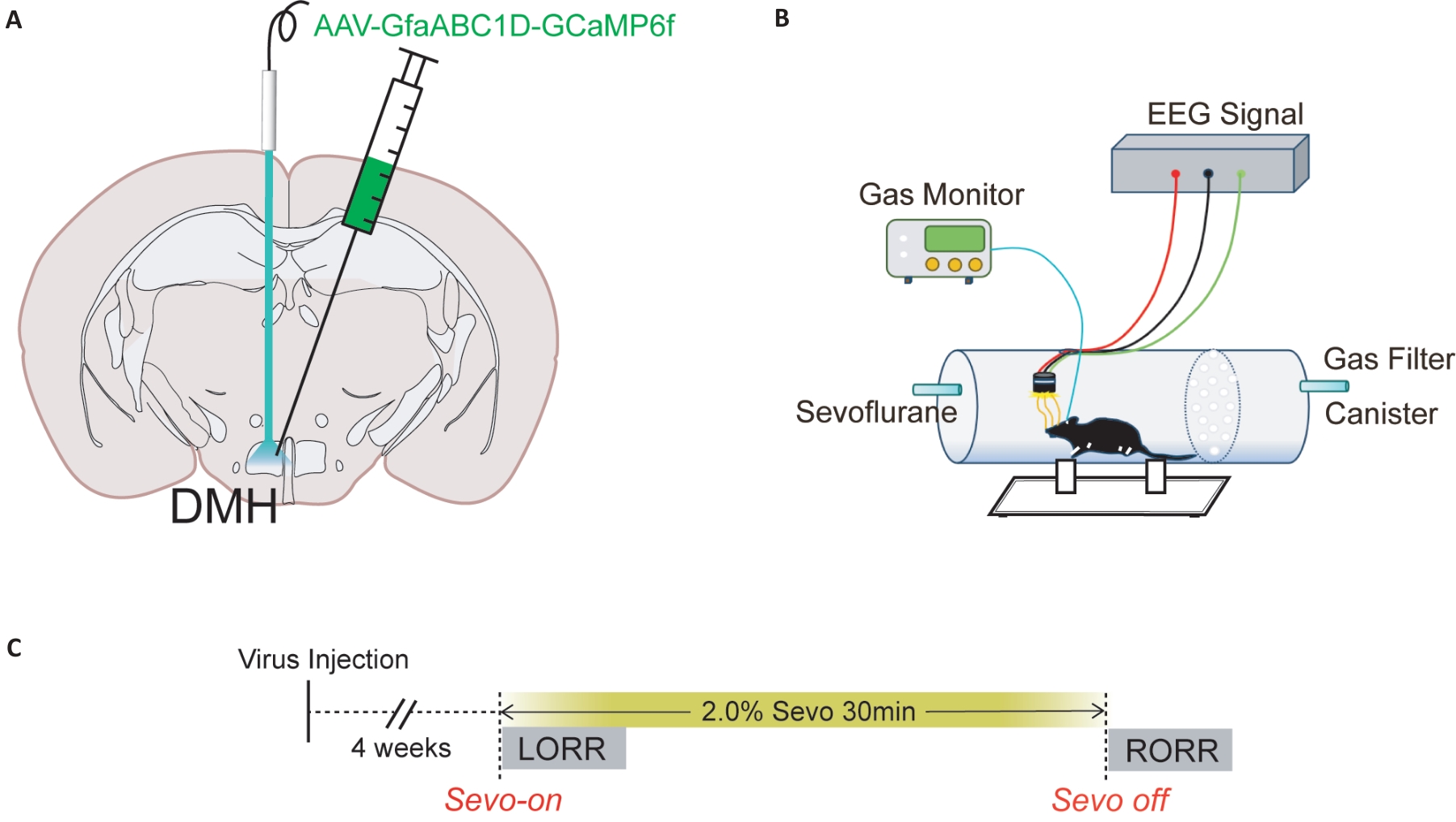
图1 病毒注射脑图谱及行为学实验示意图
Fig.1 Diagram of virus injection into the brain and schematic diagram of behavioral experiment. A: Schematic diagram of calcium imaging virus injection into the DMH of a mouse. B: Schematic diagram of the calcium signal recording system C: Detailed flow chart of calcium imaging experiment, including the time of sevoflurane on and off, LORR and RORR.
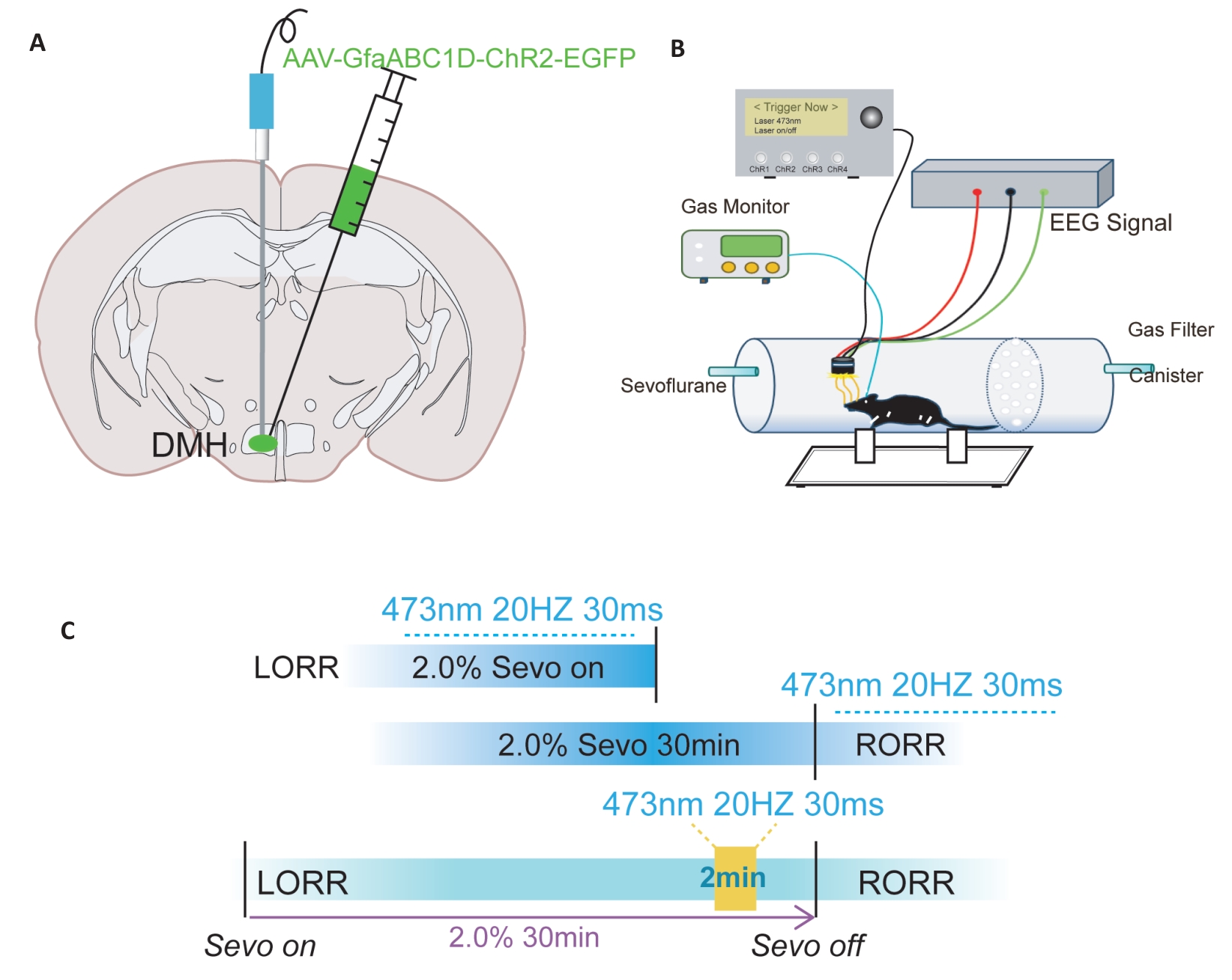
图2 光遗传病毒注射及行为学示意图
Fig.2 Schematic diagram of optogenetic virus injection and behavioral experiments. A: Illustration of a coronal brain slice showing the locations of virus injection and optic fiber implantation. B: Schematic diagram of the equipment used for behavioral observation, optic fiber stimulation, and EEG recording during anesthesia. C: Flowchart of optogenetic modulation of behavioral testing under general anesthesia.
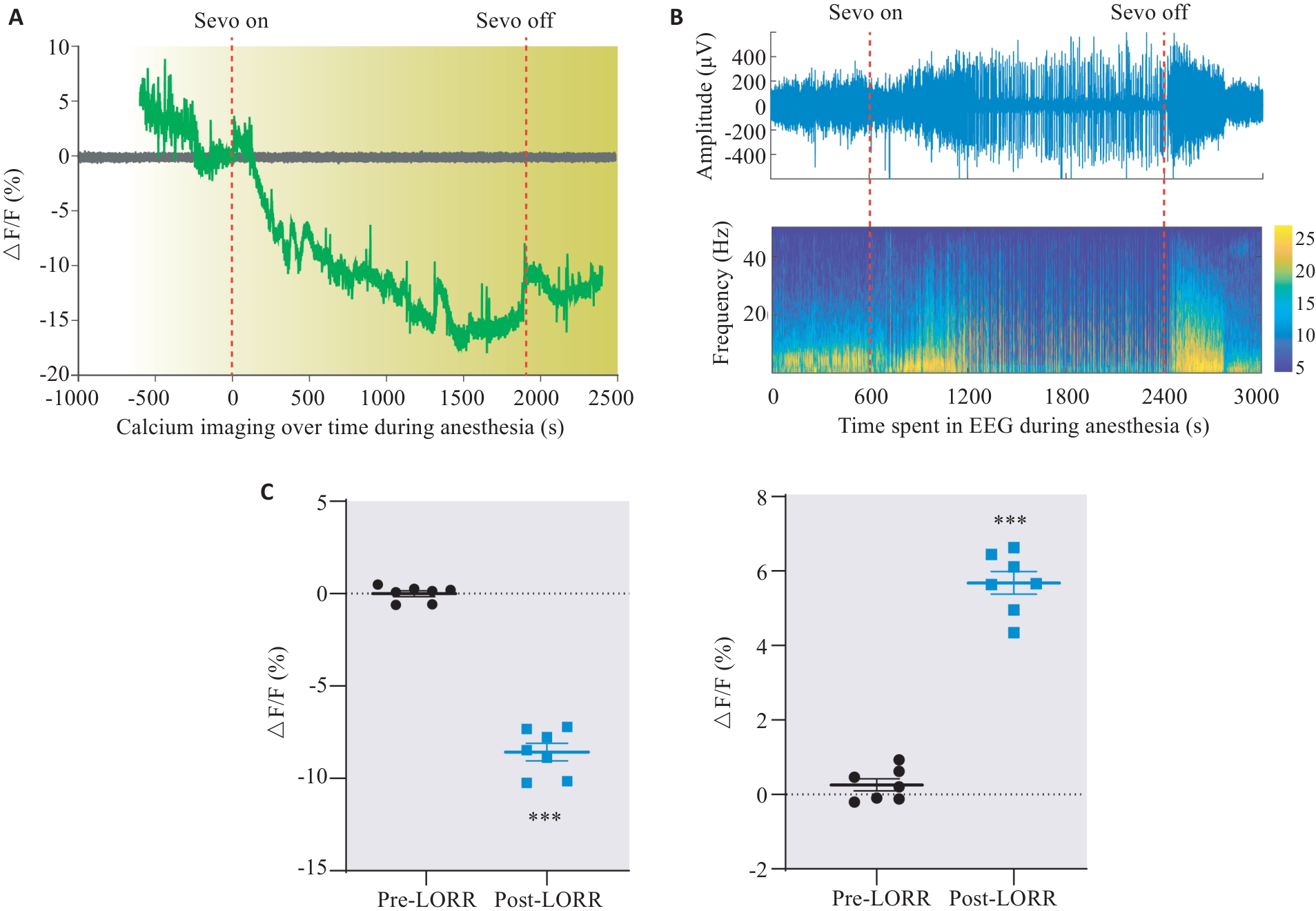
图4 DMH星形胶质细胞在七氟烷诱导觉醒过程中的钙活性变化
Fig.4 Changes of calcium activity in DMH astrocytes of rats during sevoflurane anesthesia emergence. A: Dynamic monitoring of changes in astrocyte activity during sevoflurane anesthesia. B: Changes in EEG activity during sevoflurane anesthesia corresponding to Fig.A. C: Changes of ΔF/F value of astrocytes in the DMH region during anesthesia induction and awakening. ***P<0.001 vs EGFP group.
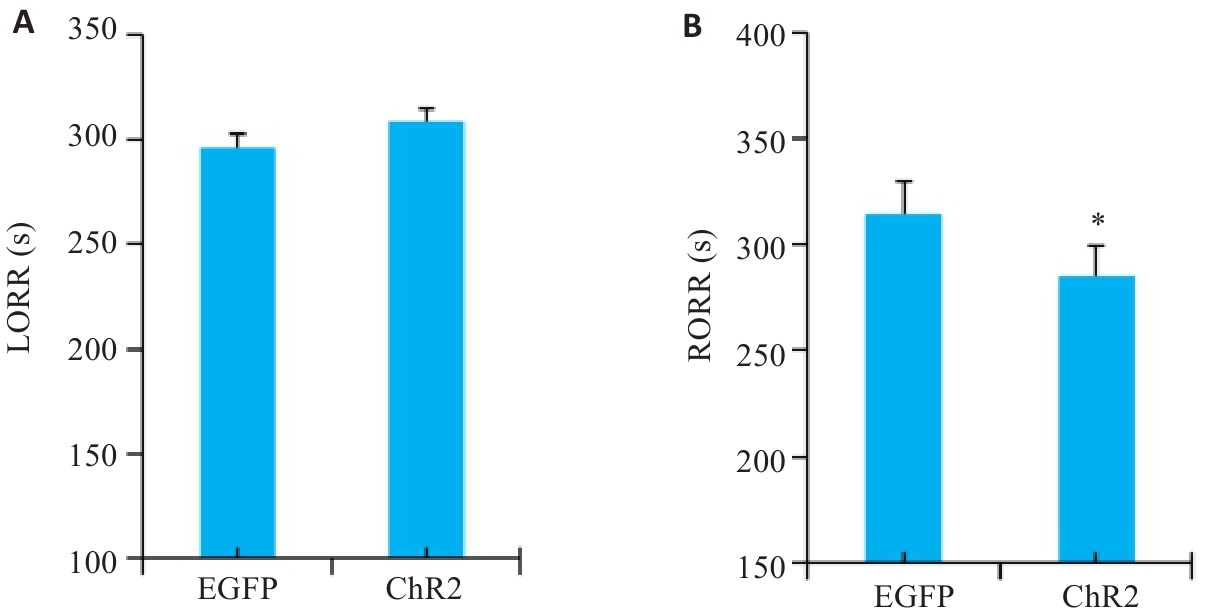
图6 光遗传激活DMH星形胶质细胞的LORR时间和RORR时间
Fig.6 Statistical analysis of LORR time (A) and RORR time (B) of optogenetically activated DMH astrocytes. *P<0.05 vs EGFP group.
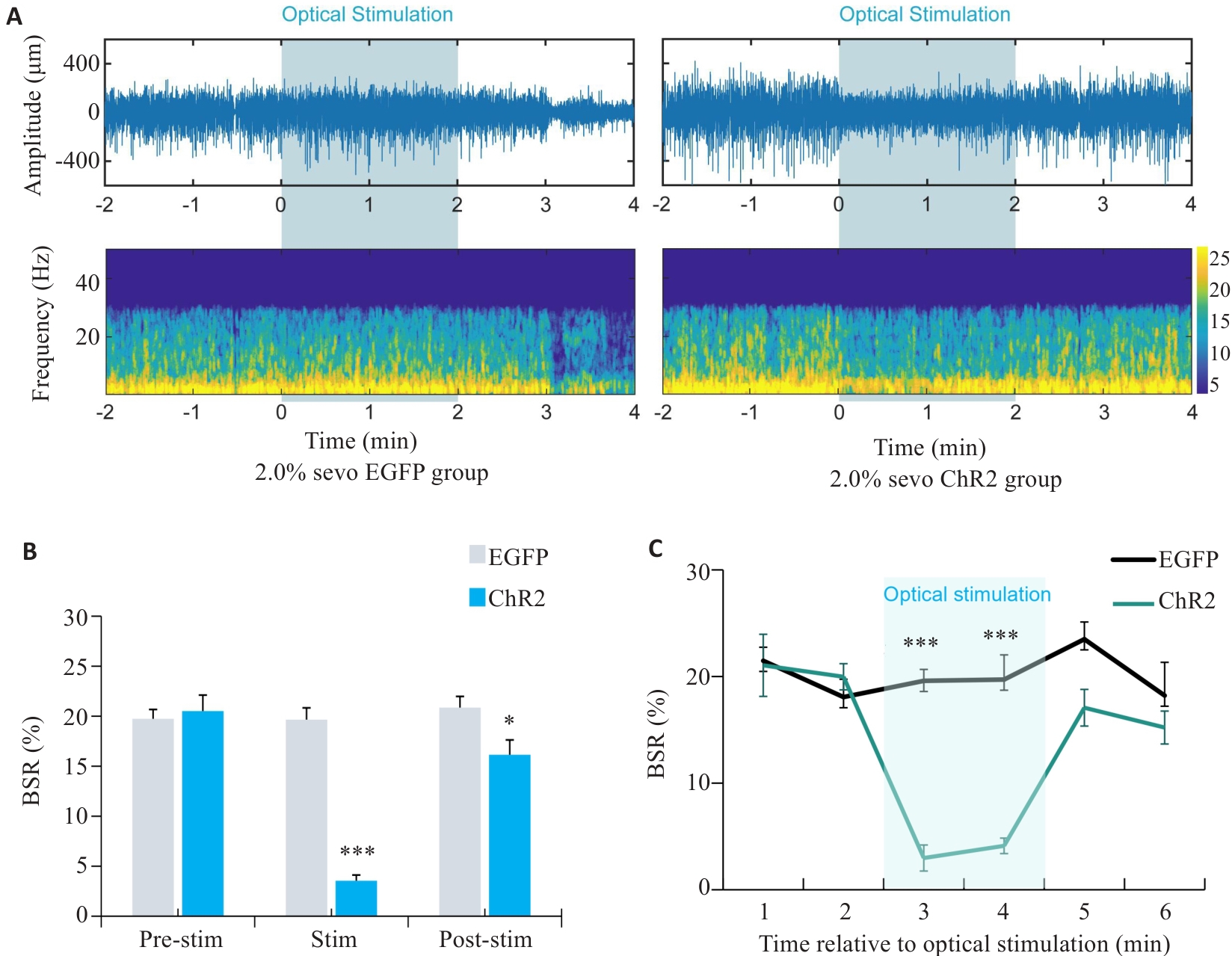
图7 在2.0%七氟醚麻醉下光遗传学激活DMH星形胶质细胞的脑电变化
Fig.7 EEG of rats with optogenetically activated DMH astrocytes under 2.0% sevoflurane anesthesia. A: Comparison of representative EEG spectra between EGFP group and ChR2 group under 2.0% sevoflurane anesthesia. B: Comparison of EEG burst suppression rate between EGFP group and ChR2 group after 2 min of light stimulation at 25th min of sevoflurane anesthesia. C: Changes in the brain spontaneous rhythm (BSR%) curve per minute before and after light stimulation. *P<0.05, ***P<0.001 vs EGFP group.
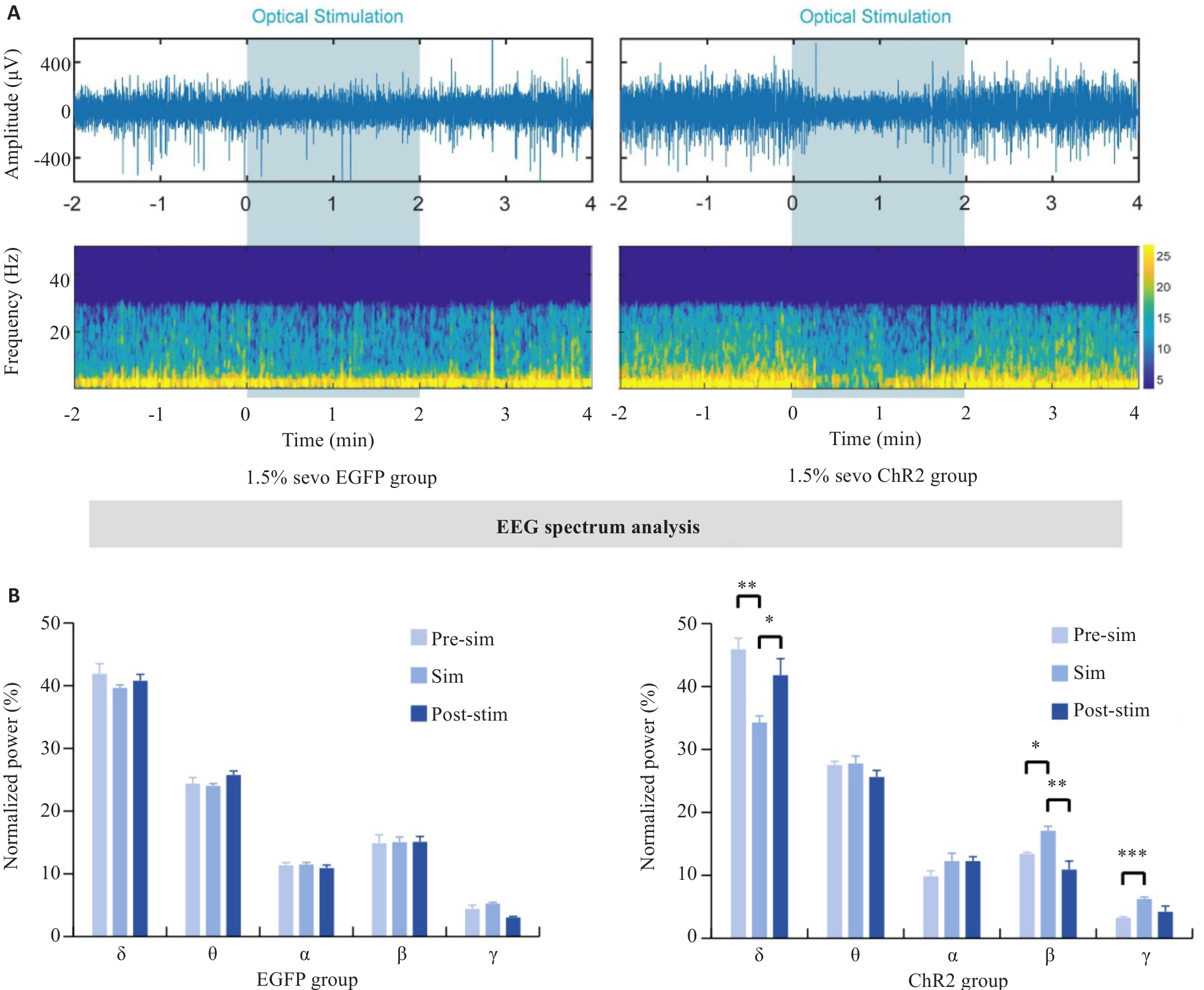
图8 在1.5%七氟醚麻醉下光遗传学激活DMH星形胶质细胞的脑电
Fig.8 EEG of rats with optogenetically activated DMH astrocytes under 1.5% sevoflurane anesthesia. A: Comparison of representative EEG spectra between EGFP group and ChR2 group under 1.5% sevoflurane anesthesia. B: Quantification of EEG power percentage during optogenetic activation in EGFP and ChR2 mice. *P<0.05, **P<0.01, ***P<0.001.
| 1 | Palanca BA, Avidan MS, Mashour GA. Human neural correlates of sevoflurane-induced unconsciousness[J]. Br J Anaesth, 2017, 119(4): 573-82. |
| 2 | Xu W, Wang L, Yuan XS, et al. Sevoflurane depresses neurons in the medial parabrachial nucleus by potentiating postsynaptic GABAA receptors and background potassium channels[J]. Neuropharm-acology, 2020, 181: 108249. |
| 3 | Yi TT, Wang N, Huang J, et al. A sleep-specific midbrain target for sevoflurane anesthesia[J]. Adv Sci, 2023, 10(15): 2300189. |
| 4 | Alhadidi QM, Bahader GA, Arvola O, et al. Astrocytes in functional recovery following central nervous system injuries[J]. J Physiol, 2024, 602(13): 3069-96. |
| 5 | He QQ, Ji LW, Wang YY, et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption[J]. Cell Metab, 2024, 36(9): 1998-2014.e15. |
| 6 | Cai P, Huang SN, Lin ZH, et al. Regulation of wakefulness by astrocytes in the lateral hypothalamus[J]. Neuropharmacology, 2022, 221: 109275. |
| 7 | Wang YF, Song YP, Tong L, et al. GABAergic neurons in the dorsomedial hypothalamus regulate states of consciousness in sevoflurane anesthesia[J]. iScience, 2023, 26(1): 105913. |
| 8 | Zhong HX, Tong L, Gu N, et al. Endocannabinoid signaling in hypothalamic circuits regulates arousal from general anesthesia in mice[J]. J Clin Invest, 2017, 127(6): 2295-309. |
| 9 | Yoshimoto A, Morikawa S, Kato E, et al. Top-down brain circuits for operant bradycardia[J]. Science, 2024, 384(6702): 1361-8. |
| 10 | Wang D, Bao C, Wu HM, et al. A hypothalamus-lateral periaqueductal gray GABAergic neural projection facilitates arousal following sevoflurane anesthesia in mice[J]. CNS Neurosci Ther, 2024, 30(9): e70047. |
| 11 | Gompf HS, Aston-Jones G. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity[J]. Brain Res, 2008, 1224: 43-52. |
| 12 | Brazhe A, Verisokin A, Verveyko D, et al. Astrocytes: new evidence, new models, new roles[J]. Biophys Rev, 2023, 15(5): 1303-33. |
| 13 | Zhu YW, Ma JL, Li YL, et al. Adenosine-dependent arousal induced by astrocytes in a brainstem circuit[J]. Adv Sci, 2024, 11(48): 2407706. |
| 14 | Kumar VJ, Scheffler K, Grodd W. The structural connectivity mapping of the intralaminar thalamic nuclei[J]. Sci Rep, 2023, 13(1): 11938. |
| 15 | Zhang Z, Huang YC, Chen XY, et al. State-specific regulation of electrical stimulation in the intralaminar thalamus of macaque monkeys: network and transcriptional insights into arousal[J]. Adv Sci, 2024, 11(33): e2402718. |
| 16 | Liu Q, Bell BJ, Kim DW, et al. A clock-dependent brake for rhythmic arousal in the dorsomedial hypothalamus[J]. Nat Commun, 2023, 14(1): 6381. |
| 17 | Tsuji S, Brace CS, Yao RQ, et al. Sleep-wake patterns are altered with age, Prdm13 signaling in the DMH, and diet restriction in mice[J]. Life Sci Alliance, 2023, 6(6): e202301992. |
| 18 | Li L, Zhang MQ, Sun X, et al. Role of dorsomedial hypothalamus GABAergic neurons in sleep-wake states in response to changes in ambient temperature in mice[J]. Int J Mol Sci, 2022, 23(3): 1270. |
| 19 | Fuente-Martín E, García-Cáceres C, Argente-Arizón P, et al. Ghrelin regulates glucose and glutamate transporters in hypothalamic astrocytes[J]. Sci Rep, 2016, 6: 23673. |
| 20 | García-Marín V, García-López P, Freire M. Cajal's contributions to Glia research[J]. Trends Neurosci, 2007, 30(9): 479-87. |
| 21 | Ingiosi AM, Hayworth CR, Frank MG. Activation of basal forebrain astrocytes induces wakefulness without compensatory changes in sleep drive[J]. J Neurosci, 2023, 43(32): 5792-809. |
| 22 | Srinivasan R. Calcium signals in astrocytes of the fly brain promote sleep[J]. Cell Calcium, 2021, 94: 102341. |
| 23 | Garofalo S, Picard K, Limatola C, et al. Role of Glia in the regulation of sleep in health and disease[J]. Compr Physiol, 2020, 10(2): 687-712. |
| 24 | Chao DHM, Kirchner MK, Pham C, et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance[J]. Cell Metab, 2022, 34(10): 1532-47.e6. |
| 25 | Chen CR, Zhong YH, Jiang S, et al. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice[J]. eLife, 2021, 10: e69909. |
| 26 | Peng WL, Wu ZF, Song K, et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons[J]. Science, 2020, 369(6508): eabb0556. |
| 27 | Liu PC, Yao W, Chen XY, et al. Parabrachial nucleus astrocytes regulate wakefulness and isoflurane anesthesia in mice[J]. Front Pharmacol, 2023, 13: 991238. |
| 28 | Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information[J]. Trends Neurosci, 2009, 32(8): 421-31. |
| 29 | Péter M, Héja L. High-frequency imaging reveals synchronised delta- and Theta-band Ca2+ oscillations in the astrocytic soma in vivo [J]. Int J Mol Sci, 2024, 25(16): 8911. |
| 30 | Kuga N, Sasaki T, Takahara Y, et al. Large-scale calcium waves traveling through astrocytic NetworksIn vivo[J]. J Neurosci, 2011, 31(7): 2607-14. |
| 31 | Dong R, Han YQ, Jiang LH, et al. Connexin 43 gap junction-mediated astrocytic network reconstruction attenuates isoflurane-induced cognitive dysfunction in mice[J]. J Neuroinflammation, 2022, 19(1): 64. |
| 32 | Nuriya M, Yasui D, Yamada T, et al. Direct posttranslational modification of astrocytic connexin 43 proteins by the general anesthetic propofol in the cerebral cortex[J]. Biochem Biophys Res Commun, 2018, 497(2): 734-41. |
| 33 | Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits[J]. Neuron, 2016, 91(2): 260-92. |
| [1] | 唐东宁, 康赟赟, 何文杰, 夏青. 针康结合促进C57/BL6J小鼠脑缺血后星形胶质细胞转分化为神经元[J]. 南方医科大学学报, 2025, 45(7): 1434-1441. |
| [2] | 黄鹏伟, 陈洁, 邹金虎, 高雪锋, 曹虹. 槲皮素促进应激颗粒G3BP1解聚改善HIV-1 gp120诱导的星形胶质细胞神经毒性[J]. 南方医科大学学报, 2025, 45(2): 304-312. |
| [3] | 陈洁, 刘晨旭, 王春, 李丽, 陶伟婷, 徐婧茹, 唐红辉, 黄丽. 外源性瘦素通过上调星形胶质细胞GLT-1和GLAST的表达减轻小鼠脑缺血再灌注引起的谷氨酸兴奋毒性损伤[J]. 南方医科大学学报, 2024, 44(6): 1079-1087. |
| [4] | 满 豪, 王建伟, 吴 毛, 邵 阳, 杨俊锋, 李绍烁, 吕锦业, 周 悦. 脊髓康通过激活星形胶质细胞的YAP/PKM2信号轴促进脊髓损伤大鼠神经功能的恢复[J]. 南方医科大学学报, 2024, 44(4): 636-643. |
| [5] | 谷昱琛,童旭辉,于丽,焦浩,余彬彬,董淑英. Src 激酶抑制剂PP2对星形胶质细胞缺氧/复氧损伤的保护作用[J]. 南方医科大学学报, 2015, 35(02): 239-. |
| [6] | 夏海坚,刘丹,钟东,夏永智,晏怡,唐文渊,孙晓川. 聚甲基丙烯酸甲酯静电纺丝纳米纤维对大鼠原代星形胶质细胞生长的影响[J]. 南方医科大学学报, 2014, 34(11): 1569-. |
| [7] | 张砡,李俣,李伟光,张成岗,叶铁虎. 丙泊酚对新生大鼠大脑皮层星形胶质细胞合成凝血酶敏感蛋白1的影响[J]. 南方医科大学学报, 2013, 33(09): 1316-. |
| [8] | 董淑英,童旭辉,蒋国君,谷昱琛,焦浩,李俊. 星形胶质细胞中缝隙连接蛋白connexin 43的表达及其功能调控[J]. 南方医科大学学报, 2012, 32(10): 1423-. |
| [9] | 周金桥,刘秋红,王景涛,郭新宾,宋来君. 过氧化物酶1,6 和GFAP在人脑星形胶质细胞瘤中的表达及临床意义[J]. 南方医科大学学报, 2012, 32(09): 1255-. |
| [10] | 唐兆华,廖正步,谢延风,石全红,何朝晖,詹彦. P38信号通路在星形胶质细胞氧糖剥夺/复氧后水通道蛋白4表达及细胞水肿形成中的作用[J]. 南方医科大学学报, 2012, 32(02): 141-. |
| [11] | 刘犇,陈清,郭江,周浩,李劲,俞守义. 白藜芦醇对脂多糖诱导的星型胶质细胞炎症损伤的保护作用[J]. 南方医科大学学报, 2011, 31(12): 2052-. |
| [12] | 颜晓慧, 陈雪梅, 邹飞. 缺氧对体外培养的大鼠脑星形胶质细胞存活的影响[J]. 南方医科大学学报, 2005, 25(04): 399-402. |
| [13] | 邹西峰1, 张海燕2, 赵春礼2, 李铁林1, 徐群渊2. 大鼠骨髓源干细胞在损伤脑组织中的分化趋势[J]. 南方医科大学学报, 2004, 24(10): 1102-1106. |
| [14] | 莫永炎1, 邢飞跃2, 张宝3, 陈瑗3, 姜勇1. 星形胶质细胞条件培养液对tbOOH致PC12细胞凋亡的抑制作用[J]. 南方医科大学学报, 2004, 24(09): 998-1000. |
| [15] | 杨志军1, 饶志仁2, 徐如祥1, 魏玲3, 王晓斌2, 姜晓丹1. 大鼠海马CA3区神经元和星形胶质细胞三维结构重塑[J]. 南方医科大学学报, 2004, 24(04): 426-429. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||