南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (4): 725-735.doi: 10.12122/j.issn.1673-4254.2025.04.07
刘露玉1,2( ), 公茂伟1(
), 公茂伟1( ), 廖国松1,2, 赵维星1(
), 廖国松1,2, 赵维星1( ), 傅强1(
), 傅强1( )
)
收稿日期:2024-12-24
出版日期:2025-04-20
发布日期:2025-04-28
通讯作者:
赵维星,傅强
E-mail:luyuliu001@163.com;gongmwei2001@163.com;zwxsjrz@163.com;dr_fuqiang@hotmail.com
作者简介:刘露玉,在读硕士研究生,E-mail: luyuliu001@163.com基金资助:
Luyu LIU1,2( ), Maowei GONG1(
), Maowei GONG1( ), Guosong LIAO1,2, Weixing ZHAO1(
), Guosong LIAO1,2, Weixing ZHAO1( ), Qiang FU1(
), Qiang FU1( )
)
Received:2024-12-24
Online:2025-04-20
Published:2025-04-28
Contact:
Weixing ZHAO, Qiang FU
E-mail:luyuliu001@163.com;gongmwei2001@163.com;zwxsjrz@163.com;dr_fuqiang@hotmail.com
Supported by:摘要:
目的 探索高血压与术后认知功能障碍的关系及其可能的机制。 方法 采用七氟烷麻醉下颈动脉暴露手术构建术后学习记忆损伤动物模型,将12周龄自发性高血压大鼠(SHR)和正常血压对照Wistar-Kyoto 大鼠(WKY)根据是否手术分为4组,即 WKY+CON组、WKY+SUR组、SHR+CON组、SHR+SUR组,8只/组。行为学测试评估大鼠术后认知变化;生物学检测炎症反应和氧化应激水平。 结果 高血压大鼠术后发生了明显学习记忆损伤,表现为新物体识别指数明显降低(P<0.05);场景相关和声音相关恐惧测试僵直时间均缩短(P<0.05)。与WKY大鼠相比,SHR大鼠术后海马线粒体UCP2表达和膜电位明显降低,而ATP水平显著升高(P<0.05);血清活性氧、IL-6、IL-1β炎症因子升高(P<0.05);此外,SHR大鼠星形胶质细胞过度激活,神经元Nissl染色阳性细胞个数明显减少,差异均有统计学意义(P<0.05)。 结论 高血压可加重大鼠术后学习记忆损伤,这可能与UCP2介导的线粒体功能障碍和氧化应激损伤,进一步导致星型胶质细胞过度活化和神经元损伤有关。
刘露玉, 公茂伟, 廖国松, 赵维星, 傅强. 高血压通过UCP2下调介导的线粒体功能障碍加重大鼠术后学习记忆损伤[J]. 南方医科大学学报, 2025, 45(4): 725-735.
Luyu LIU, Maowei GONG, Guosong LIAO, Weixing ZHAO, Qiang FU. Hypertension exacerbates postoperative learning and memory impairment in rats possibly due to UCP2 downregulation-mediated mitochondrial dysfunction[J]. Journal of Southern Medical University, 2025, 45(4): 725-735.
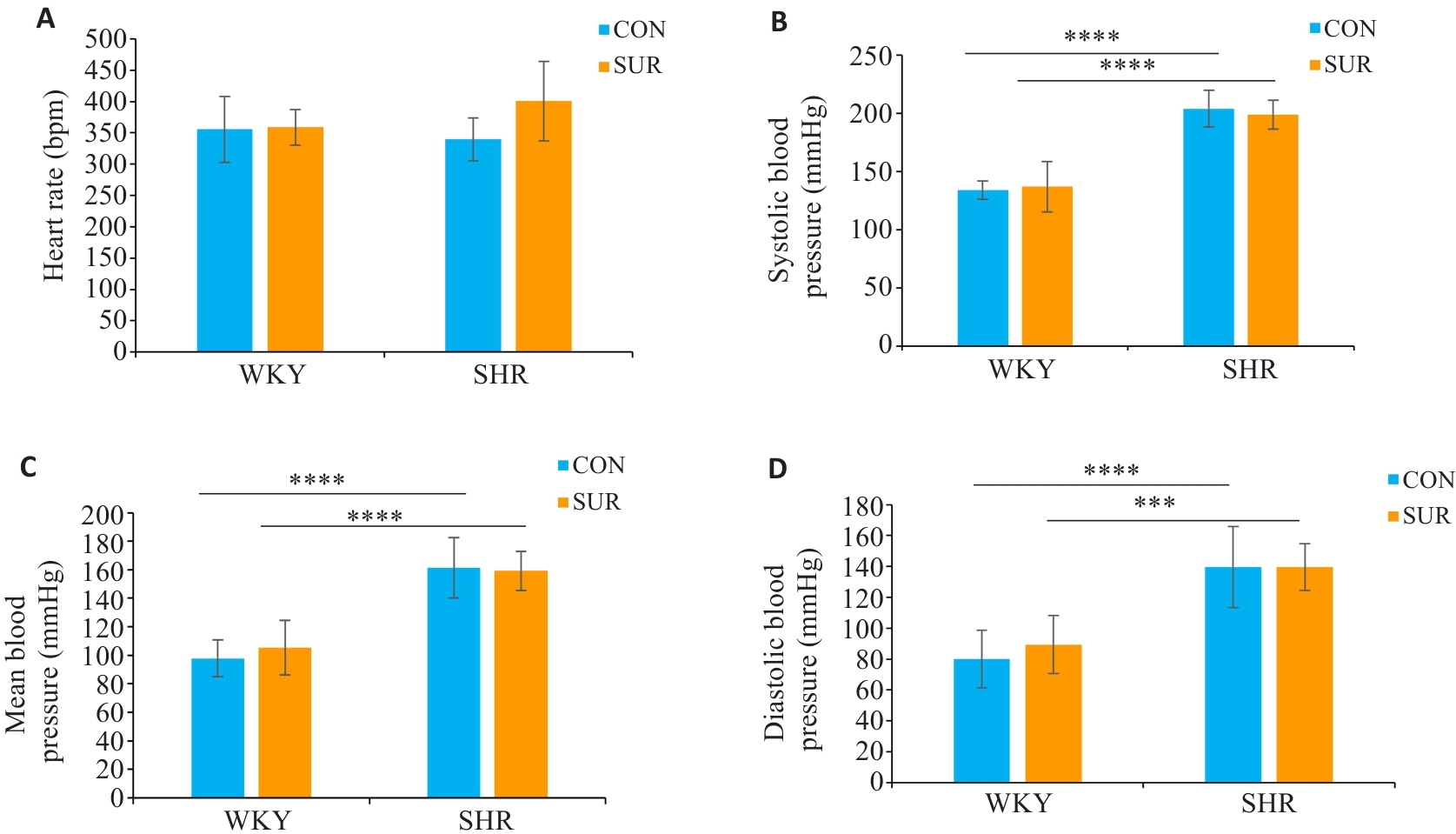
图1 SHR较WKY大鼠术前血压明显高
Fig. 1 Comparison of heart rate (A), systolic blood pressure (B), mean arterial pressure (C) and diastolic pressure (D) between SHRs and WKYs before surgery. ***P<0.001, ****P<0.0001.
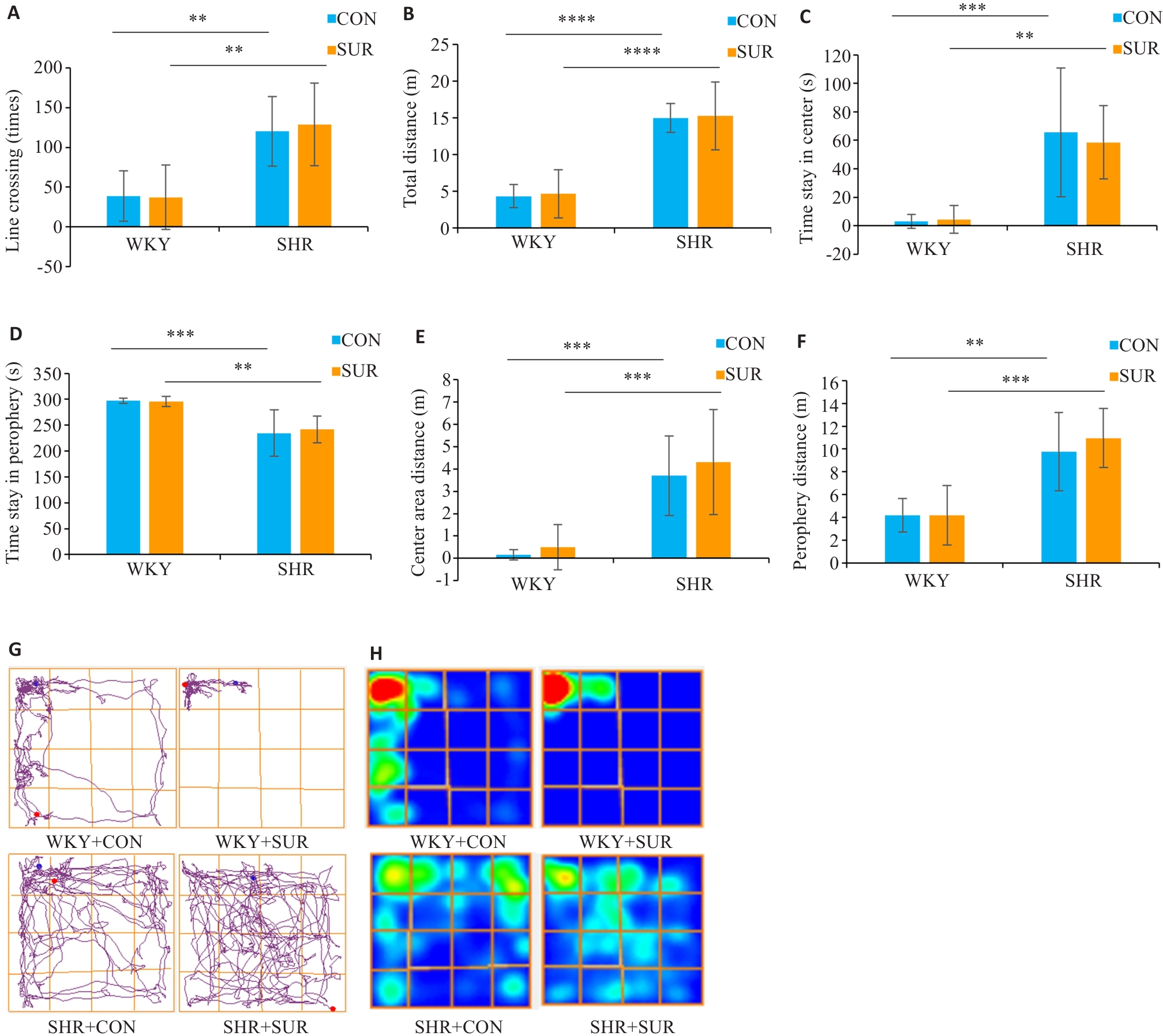
图2 高血压诱导大鼠旷场测试中的多动行为
Fig.2 Assessment of hypertension-induced hyperactive behaviors of rats in open field test. A: Performance of line crossing. B: Total moving distance in the test. C: Time spent in center zone. D: Time spent in the periphery zone. E: Center zone moving distance. F: Periphery zone moving distance. G: Representative motion trajectory diagram of the rats in each group. H: Representative motion trajectory heat map of each group. **P<0.01, ***P<0.001, ****P<0.0001.
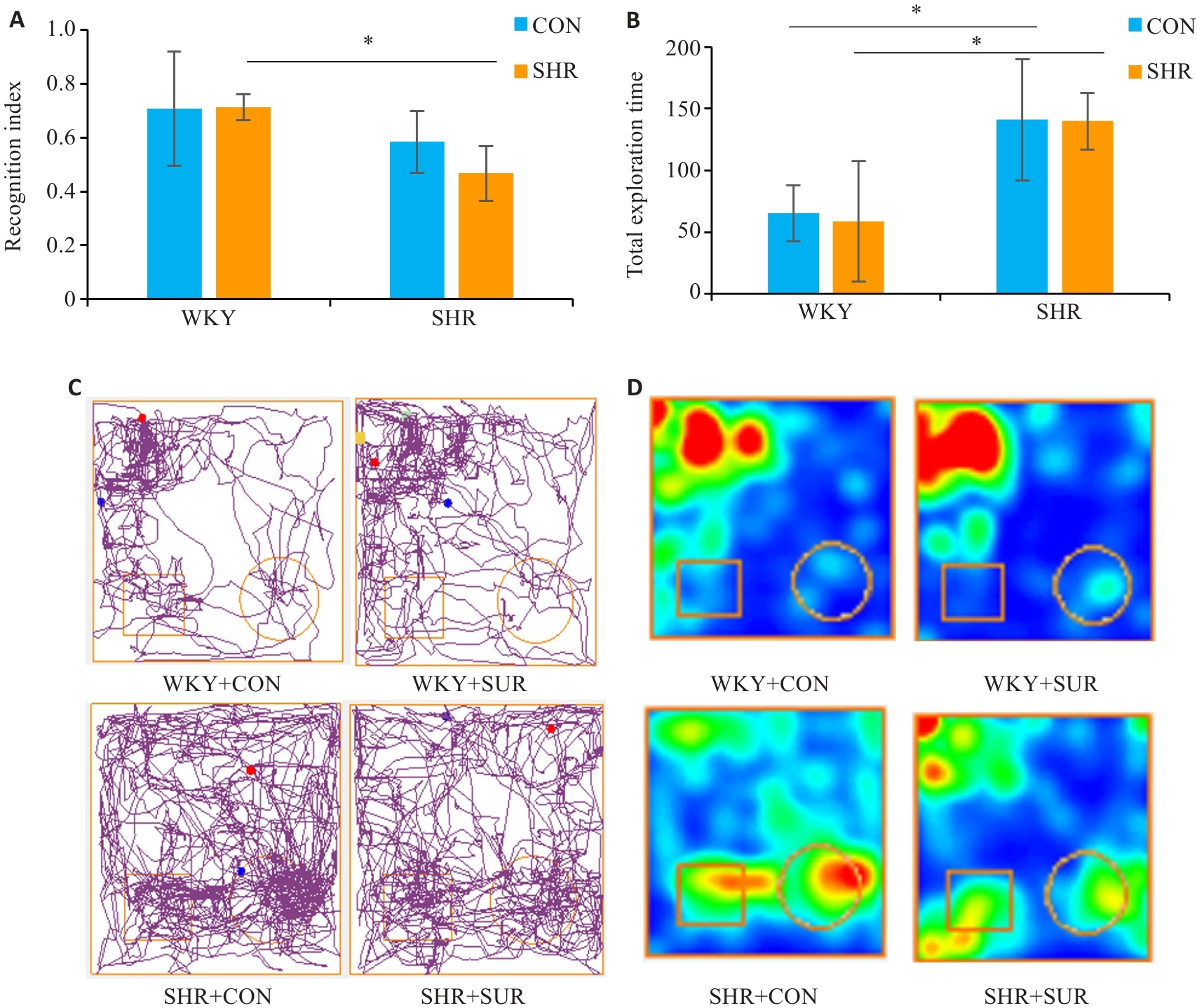
图3 高血压加重大鼠术后新物体识别测试中学习记忆功能损伤
Fig.3 Novel object recognition test for assessing the effect of hypertension on spatial memory impairment of the rats after surgery. A: Novel object recognition index. B: Total exploration times in the test. C: Representative motion trajectory diagrams, where the square represent an old object and the circle represent a novel object. D: Representative motion trajectory heat map. *P<0.05.
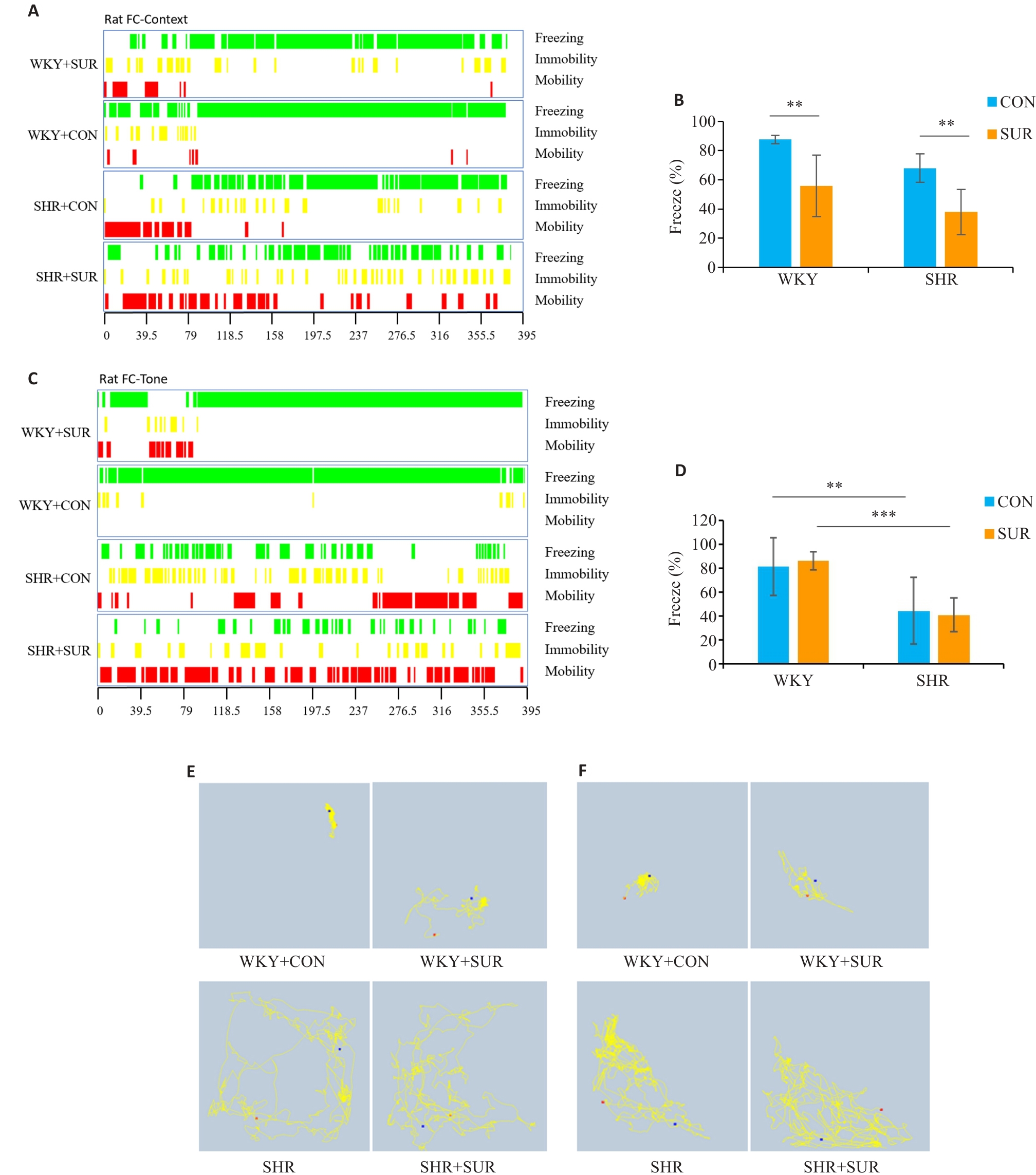
图4 高血压加重大鼠术后恐惧记忆损伤
Fig.4 Hypertension exacerbates fear memory impairment in SHRs after surgery. A: FC context-related freezing performance (green indicates freezing, yellow indicates immobility, and red indicates mobility). B: Statistical graph of context-related percent freezing time. C: Tone-related freezing performance. D: Statistical graph of tone-related percent freezing time. E: Representative motion trajectory corresponding to context-related FC test. F: Representative motion trajectory corresponding to tone-related freezing time. **P<0.01, ***P<0.001.
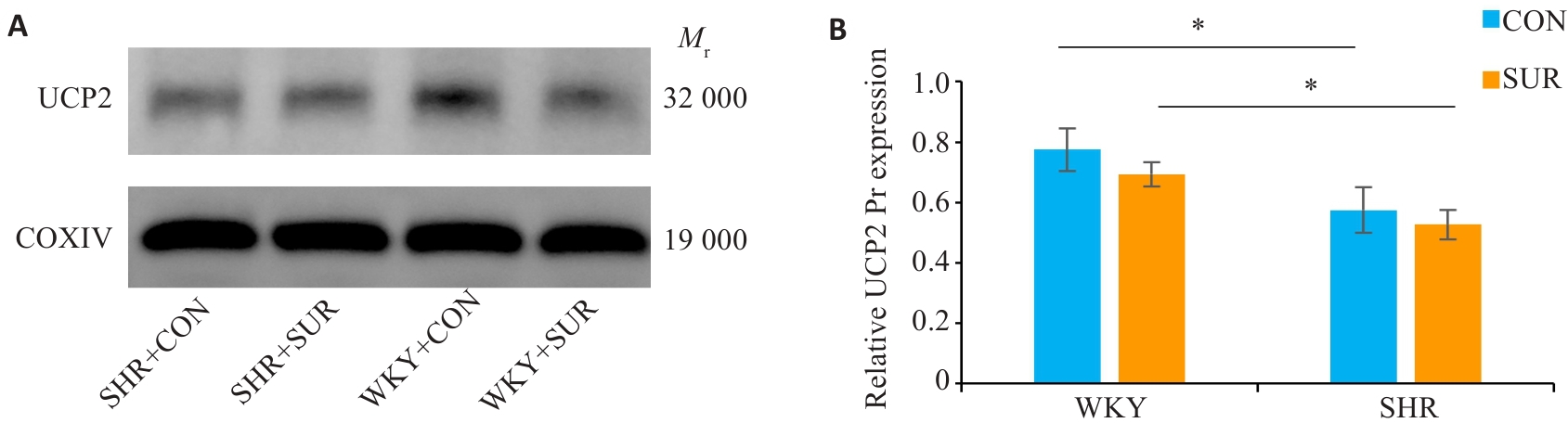
图5 术后SHR大鼠海马线粒体UCP2蛋白表达明显降低
Fig.5 Quantification of mitochondrial UCP2 protein expression in rat hippocampus. A: Western blotting bands of UCP2 protein and internal control COXIV in the mitochondria. B: Statistical graph of mitochondrial UCP2 protein expression in the hippocampus. *P<0.05.
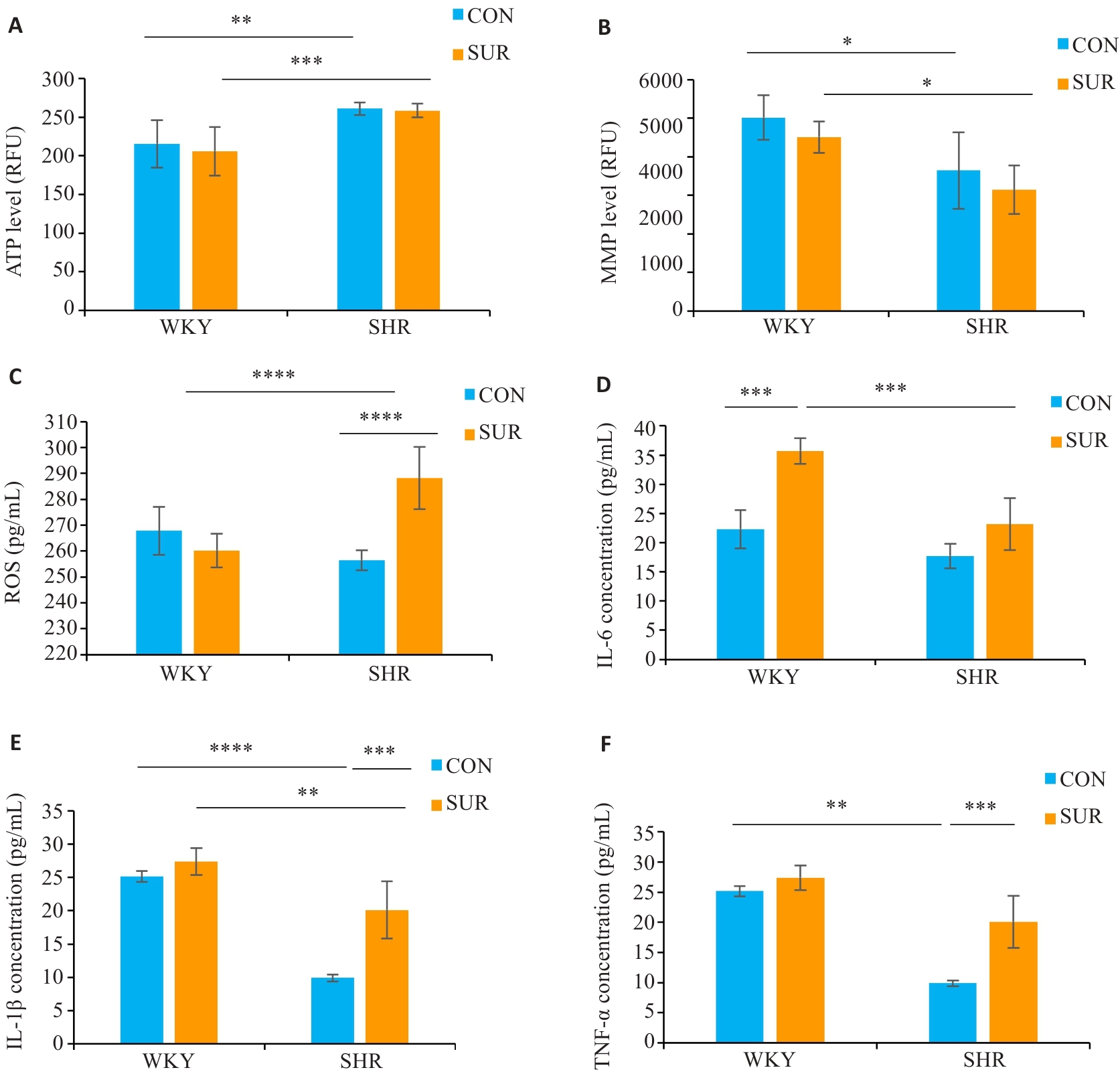
图6 高血压加重大鼠术后海马线粒体功能障碍、氧化应激和炎症反应
Fig.6 Hypertension aggravates hippocampal mitochondrial dysfunction, oxidative stress, and inflammatory response after surgery. A: Hippocampal ATP level. B: Hippocampal mitochondria membrane potential (MMP). C: Reactive oxygen species level in serum. D: Serum IL-6 level. E: Serum IL-1β level. F: Serum TNF-α level. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
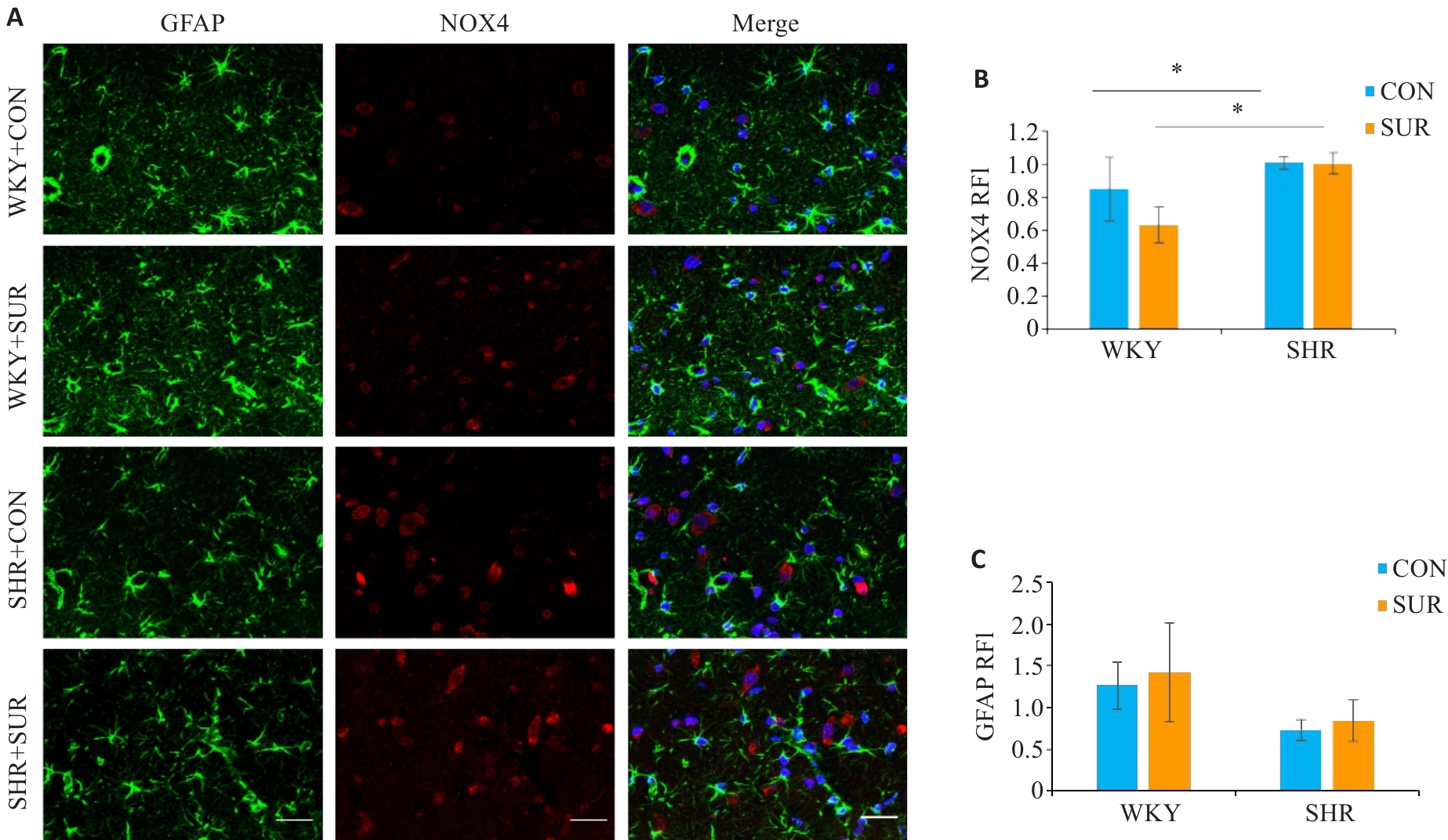
图7 高血压加重大鼠术后海马星型胶质细胞的过度激活
Fig. 7 Immunofluorescence staining of the hippocampus of SHR and WKY rats for detecting expressions of astrocyte markers in the CA1 region. A: Combo graph of immunofluorescence (Scale bar=20 μm; green represents GFAP, and red represents NOX4). B, C: Statistical graphs of NOX4 and GFAP. *P<0.05.
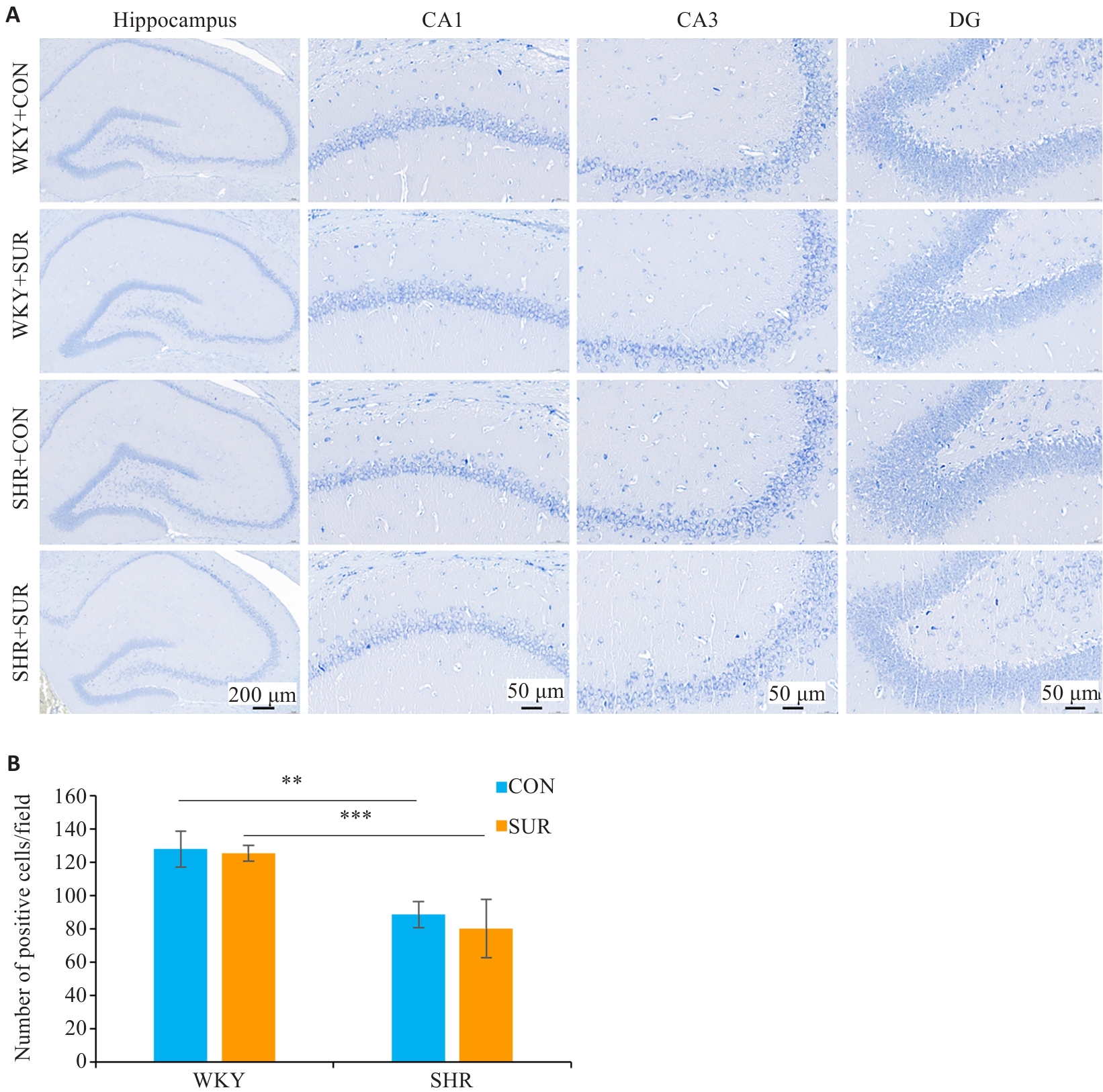
图8 高血压加重大鼠术后神经元损伤
Fig.8 Nissl staining images of different regions in rat hippocampus (A) and comparison of positive cell numbers in the CA1 region among the groups (B). **P<0.01, ***P<0.001
| 1 | Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018[J]. Br J Anaesth, 2018, 121(5): 1005-12. |
| 2 | Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1-2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction[J]. Acta Anaesthesiol Scand, 2000, 44(10): 1246-51. |
| 3 | Anand N, Gupta R, Mishra SP, et al. Postoperative cognitive dysfunction: a review[J]. Asian J Anesthesiol, 2024, 62(1): 1-11. |
| 4 | Yang X, Huang X, Li M, et al. Identification of individuals at risk for postoperative cognitive dysfunction (POCD)[J]. Ther Adv Neurol Disord, 2022, 15: 17562864221114356. |
| 5 | Gonzales MM, Garbarino VR, ollet E, et al. Biological aging processes underlying cognitive decline and neurodegenerative disease [J]. J Clin Invest, 20, 132(10):e158453 |
| 6 | Lövdén M, Fratiglioni L, Maria Glymour M, et al. Education and cognitive functioning across the life span[J]. Psychol Sci Public Interest, 2020, 21(1): 6-41. |
| 7 | Feinkohl I, Winterer G, Spies CD, et al. Cognitive reserve and the risk of postoperative cognitive dysfunction[J]. Dtsch Arztebl Int, 2017, 114(7): 110-7. |
| 8 | Butz M, Gerriets T, Sammer G, et al. Effects of postoperative cognitive training on neurocognitive decline after heart surgery: a randomized clinical trial [J]. Eur J Cardiothorac Surg, 20, 62(5):ezac251. |
| 9 | Rappold T, Laflam A, Hori D, et al. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery[J]. Br J Anaesth, 2016, 116(1): 83-9. |
| 10 | Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health[J]. Nat Rev Nephrol, 2021, 17(10): 639-54. |
| 11 | Qiu CX, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia[J]. Lancet Neurol, 2005, 4(8): 487-99. |
| 12 | Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis[J]. JAMA, 2020, 323(19): 1934-44. |
| 13 | Siedlinski M, Carnevale L, Xu X, et al. Genetic analyses identify brain structures related to cognitive impairment associated with elevated blood pressure[J]. Eur Heart J, 2023, 44(23): 2114-25. |
| 14 | Sure VN, Sakamuri SSVP, Sperling JA, et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice[J]. Geroscience, 2018, 40(4): 365-75. |
| 15 | Zong Y, Li H, Liao P, et al. Mitochondrial dysfunction: mechanisms and advances in therapy[J]. Signal Transduct Target Ther, 2024, 9(1): 124. |
| 16 | Wallace DC. Mitochondrial diseases in man and mouse[J]. Science, 1999, 283(5407): 1482-8. |
| 17 | Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival[J]. Nat Rev Neurosci, 2005, 6(11): 829-40. |
| 18 | Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3[J]. Cell Metab, 2005, 2(2): 85-93. |
| 19 | Chan SHH, Wu CA, Wu KLH, et al. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension[J]. Circ Res, 2009, 105(9): 886-96. |
| 20 | Xin J, Shan WR, Li J, et al. Activation of the lateral habenula-ventral tegmental area neural circuit contributes to postoperative cognitive dysfunction in mice[J]. Adv Sci, 2022, 9(22): e2202228. |
| 21 | Zheng B, Lai RC, Li J, et al. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery[J]. Brain Behav Immun, 2017, 61: 365-74. |
| 22 | Yuan HJ, Na WN, Li BZ, et al. Optogenetic cortical spreading depression originating from the primary visual cortex induces migraine-like pain and anxiety behaviors in freely moving C57BL/6J mice[J]. J Headache Pain, 2025, 26(1): 44. |
| 23 | Preveza NJ, Setenet G, Gwin P, et al. Decreases in K63 polyubiquitination in the hippocampus promote the formation of contextual fear memories in both males and females[J]. Hippocampus, 2025, 35(1): e23650. |
| 24 | Yamagishi A, Yonemochi N, Kimura A, et al. AMP-activated protein kinase in the amygdala and hippocampus contributes to enhanced fear memory in diabetic mice[J]. Br J Pharmacol, 2024, 181(24): 5028-40. |
| 25 | Collaborators G 2 DF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019[J]. Lancet Public Health, 2022, 7(2): e105-25. |
| 26 | Chen JXY, Vipin A, Sandhu GK, et al. Blood-brain barrier integrity disruption is associated with both chronic vascular risk factors and white matter hyperintensities[J]. J Prev Alzheimers Dis, 2025, 12(2): 100029. |
| 27 | Feng XM, Valdearcos M, Uchida Y, et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice[J]. JCI Insight, 2017, 2(7): e91229. |
| 28 | Klemmensen MM, Borrowman SH, Pearce C, et al. Mitochondrial dysfunction in neurodegenerative disorders[J]. Neurotherapeutics, 2024, 21(1): e00292. |
| 29 | McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia[J]. Neurology, 2017, 89(24): 2447-54. |
| 30 | Li CL, Zhu YD, Ma YJ, et al. Association of cumulative blood pressure with cognitive decline, dementia, and mortality[J]. J Am Coll Cardiol, 2022, 79(14): 1321-35. |
| 31 | Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American heart association[J]. Hypertension, 2016, 68(6): e67-94. |
| 32 | Jamwal S, Blackburn JK, Elsworth JD. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders[J]. Pharmacol Ther, 2021, 219: 107705. |
| 33 | Ashleigh T, Swerdlow RH, Beal MF. The role of mitochondrial dysfunction in Alzheimer's disease pathogenesis [J]. Alzheimers Dement, 20, 19(1): 333-4. |
| 34 | Yu HY, Yang CW, Chen S, et al. Comparison of the glycopattern alterations of mitochondrial proteins in cerebral cortex between rat Alzheimer's disease and the cerebral ischemia model[J]. Sci Rep, 2017, 7: 39948. |
| 35 | Liu YJ, Sulc J, Auwerx J. Mitochondrial genetics, signalling and stress responses[J]. Nat Cell Biol, 2025 Mar 10.doi: 10.1038/s41556-025-01625-w . |
| 36 | Franco C, Sciatti E, Favero G, et al. Essential Hypertension and Oxidative Stress: Novel Future Perspectives [J]. Int J Mol Sci, 20, 23(22):14489. |
| 37 | Skvarc DR, Berk M, Byrne LK, et al. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies[J]. Neurosci Biobehav Rev, 2018, 84: 116-33. |
| 38 | Liu Y, Yang W, Xue JQ, et al. Neuroinflammation: The central enabler of postoperative cognitive dysfunction[J]. Biomed Pharmacother, 2023, 167: 115582. |
| 39 | Tchekalarova J, Krushovlieva D, Ivanova P, et al. Spontaneously hypertensive rats vs. Wistar Kyoto and Wistar rats: an assessment of anxiety, motor activity, memory performance, and seizure susceptibility[J]. Physiol Behav, 2023, 269: 114268. |
| 40 | Tchekalarova J, Ivanova P, Krushovlieva D. Age-related effects of AT1 receptor antagonist losartan on cognitive decline in spontaneously hypertensive rats[J]. Int J Mol Sci, 2024, 25(13): 7340. |
| 41 | JöHREN O, Golsch C, Ddendorfer A, et al. Differential expression of AT1 receptors in the pituitary and adrenal gland of SHR and WKY [J]. Hypertension, 200, 41(4): 984-90. |
| 42 | Bak J, Pyeon HI, Seok JI, et al. Effect of rotation preference on spontaneous alternation behavior on Y maze and introduction of a new analytical method, entropy of spontaneous alternation[J]. Behav Brain Res, 2017, 320: 219-24. |
| [1] | 张璐, 丁焕章, 许浩燃, 陈珂, 许博文, 杨勤军, 吴迪, 童佳兵, 李泽庚. 参芪补中方通过激活AMPK/SIRT1/PGC-1α改善COPD肺脾气虚证大鼠线粒体功能障碍[J]. 南方医科大学学报, 2025, 45(5): 969-976. |
| [2] | 廖茗, 钟文华, 张冉, 梁娟, 徐文陶睿, 万文珺, 吴超, 李曙. 源自蛇毒的蛋白C激活剂通过调控HIF-1α抑制BNIP3活性氧生成保护人脐静脉内皮细胞免受缺氧-复氧损伤[J]. 南方医科大学学报, 2025, 45(3): 614-621. |
| [3] | 蔡巧燕, 许瑶瑶, 林雨星, 林浩伟, 郑俊鹏, 张伟祥, 赵春雨, 林育鹏, 张铃. 清达颗粒通过调控miR-124/STAT3信号轴减轻自发性高血压大鼠脑损伤[J]. 南方医科大学学报, 2025, 45(1): 18-26. |
| [4] | 郭克磊, 李颖利, 宣晨光, 侯紫君, 叶松山, 李林运, 陈丽平, 韩立, 卞华. 益气养阴化浊通络方通过调控miR-21a-5p/FoxO1/PINK1介导的线粒体自噬减轻糖尿病肾病小鼠的足细胞损伤[J]. 南方医科大学学报, 2025, 45(1): 27-34. |
| [5] | 展俊平, 黄硕, 孟庆良, 范围, 谷慧敏, 崔家康, 王慧莲. 缺氧微环境下补阳还五汤通过抑制BNIP3-PI3K/Akt通路抑制类风湿关节炎滑膜成纤维细胞的线粒体自噬[J]. 南方医科大学学报, 2025, 45(1): 35-42. |
| [6] | 左志威, 孟庆良, 崔家康, 郭克磊, 卞华. 基于硬皮病线粒体相关基因的人工神经网络模型的构建[J]. 南方医科大学学报, 2024, 44(5): 920-929. |
| [7] | 王梓凝, 杨 明, 李双磊, 迟海涛, 王军惠, 肖苍松. 心肌梗死后心肌纤维化小鼠心肌线粒体功能和能量代谢重塑相关性的转录组学分析[J]. 南方医科大学学报, 2024, 44(4): 666-674. |
| [8] | 姜诚诚, 李洋洋, 段可欣, 詹婷婷, 陈子龙, 王永雪, 赵蕊, 马彩云, 郭俣, 刘长青. Parkin通过介导PINK 1/Parkin线粒体自噬信号通路加速小鼠帕金森病发展及加剧神经炎症发生[J]. 南方医科大学学报, 2024, 44(12): 2359-2366. |
| [9] | 胡嘉伟, 杜芳, 丁璐, 王路翔, 赵巍峰. 合并高血压病的乙型肝炎肝硬化患者发生肝细胞癌的风险评估:一项基于倾向性匹配评分的回顾性队列研究[J]. 南方医科大学学报, 2024, 44(11): 2243-2249. |
| [10] | 王磊, 卞芬兰, 马飞扬, 方舒, 凌梓涵, 刘梦然, 孙红燕, 付程文, 倪诗垚, 赵晓阳, 冯心茹, 孙正宇, 卢国庆, 康品方, 吴士礼. 激活ALDH2通过上调SIRT1/PGC-1α信号通路减轻小鼠缺氧性肺动脉高压[J]. 南方医科大学学报, 2024, 44(10): 1955-1964. |
| [11] | 叶红伟, 张钰明, 云 琦, 杜若丽, 李 璐, 李玉萍, 高 琴. 白藜芦醇可减轻高糖诱导的心肌细胞肥大:基于促进SIRT1表达维持线粒体稳态[J]. 南方医科大学学报, 2024, 44(1): 45-51. |
| [12] | 于佳池, 李芮冰, 夏 天, 王佳楠, 金家丞, 袁漫秋, 李绵洋. 沉默PDCD4表达可减轻脓毒症血管内皮细胞损伤:基于改善线粒体动力学[J]. 南方医科大学学报, 2024, 44(1): 25-35. |
| [13] | 王 炼, 夏勇生, 张 震, 刘馨悦, 施金冉, 王月月, 李 静, 张小凤, 耿志军, 宋 雪, 左芦根. 高表达MRPL13促进胃癌细胞增殖并影响患者预后:基于抑制p53信号[J]. 南方医科大学学报, 2023, 43(9): 1558-1566. |
| [14] | 黄 奕, 林丽珊, 黄浩华, 董航明. VDAC1通过诱导气道上皮细胞铁死亡参与屋尘螨诱导的哮喘小鼠气道炎症[J]. 南方医科大学学报, 2023, 43(8): 1333-1338. |
| [15] | 王丽娅, 田美惠, 李 蓉, 吴 越, 王莎莎, 吕 恒, 刘忠义, 于 影. 乙醛脱氢酶2改善急性肺损伤小鼠的肺内皮屏障及维持线粒体动力学平衡[J]. 南方医科大学学报, 2023, 43(8): 1388-1395. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||