南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (5): 801-809.doi: 10.12122/j.issn.1673-4254.2024.05.01
• 基础研究 • 下一篇
收稿日期:2023-11-03
接受日期:2024-01-05
出版日期:2024-05-20
发布日期:2024-06-06
通讯作者:
张俊伶,樊赛军
E-mail:yuantong1233@163.com;zhangjunling@irm-cams.ac.cn;fansaijun@irm-cams.ac.cn
作者简介:苑 通,在读博士研究生,E-mail: yuantong1233@163.com
基金资助:
Tong YUAN( ), Yuying GUO, Junling ZHANG(
), Yuying GUO, Junling ZHANG( ), Saijun FAN(
), Saijun FAN( )
)
Received:2023-11-03
Accepted:2024-01-05
Online:2024-05-20
Published:2024-06-06
Contact:
Junling ZHANG, Saijun FAN
E-mail:yuantong1233@163.com;zhangjunling@irm-cams.ac.cn;fansaijun@irm-cams.ac.cn
Supported by:摘要:
目的 探讨正常小鼠血清(NMS)对放射性肺炎的治疗作用及可能机制。 方法 建立胸腔照射诱导的放射性肺炎模型,将小鼠分为对照组、静脉注射血清组、照射组和照射后静脉注射血清组。注射血清组小鼠在照射后立即静脉注射正常小鼠血清100 μL,对照组小鼠注射100 μL 生理盐水,隔天注射1次,共注射8次。照射后15 d取材,HE染色检测肺组织形态学变化,ELISA检测小鼠肺组织和血清中炎症因子肿瘤坏死因子-α(TNF-α)、转化生长因子-β(TGF-β)、白细胞介素-1α(IL-1α)、白细胞介素-6(IL-6)水平;流式细胞术检测肺组织内淋巴细胞比例变化。外泌体miRNA高通量测序探索处理后小鼠的信号通路变化,qRT-PCR检测免疫相关基因的表达水平,使用Western blotting检测黏着斑通路talin-1、tensin 2、FAK、vinculin、α-actinin和paxillin蛋白的表达。 结果 与照射组相比,照射后注射血清组小鼠肺脏器系数、血清及肺组织上清液中炎症因子TNF-α、TGF-β、IL-1α、IL-6水平显著降低(P<0.05),CD45+、CD4+、Treg淋巴细胞在小鼠肺组织中的浸润程度显著下降(P<0.05);肺中Egfr和Pik3cd的mRNA和蛋白表达水平显著下调,talin-1、tensin 2、FAK、vinculin、α-actinin和paxillin蛋白的表达水平也显著降低(P<0.05)。 结论 正常小鼠血清通过抑制focal adhesion信号通路关键蛋白的表达减轻电离辐射诱发的小鼠放射性肺炎。
苑通, 郭玉莹, 张俊伶, 樊赛军. 正常小鼠血清通过抑制focal adhesion信号通路减轻小鼠放射性肺炎[J]. 南方医科大学学报, 2024, 44(5): 801-809.
Tong YUAN, Yuying GUO, Junling ZHANG, Saijun FAN. Normal mouse serum alleviates radiation pneumonitis in mice by inhibiting the focal adhesion signaling pathway[J]. Journal of Southern Medical University, 2024, 44(5): 801-809.
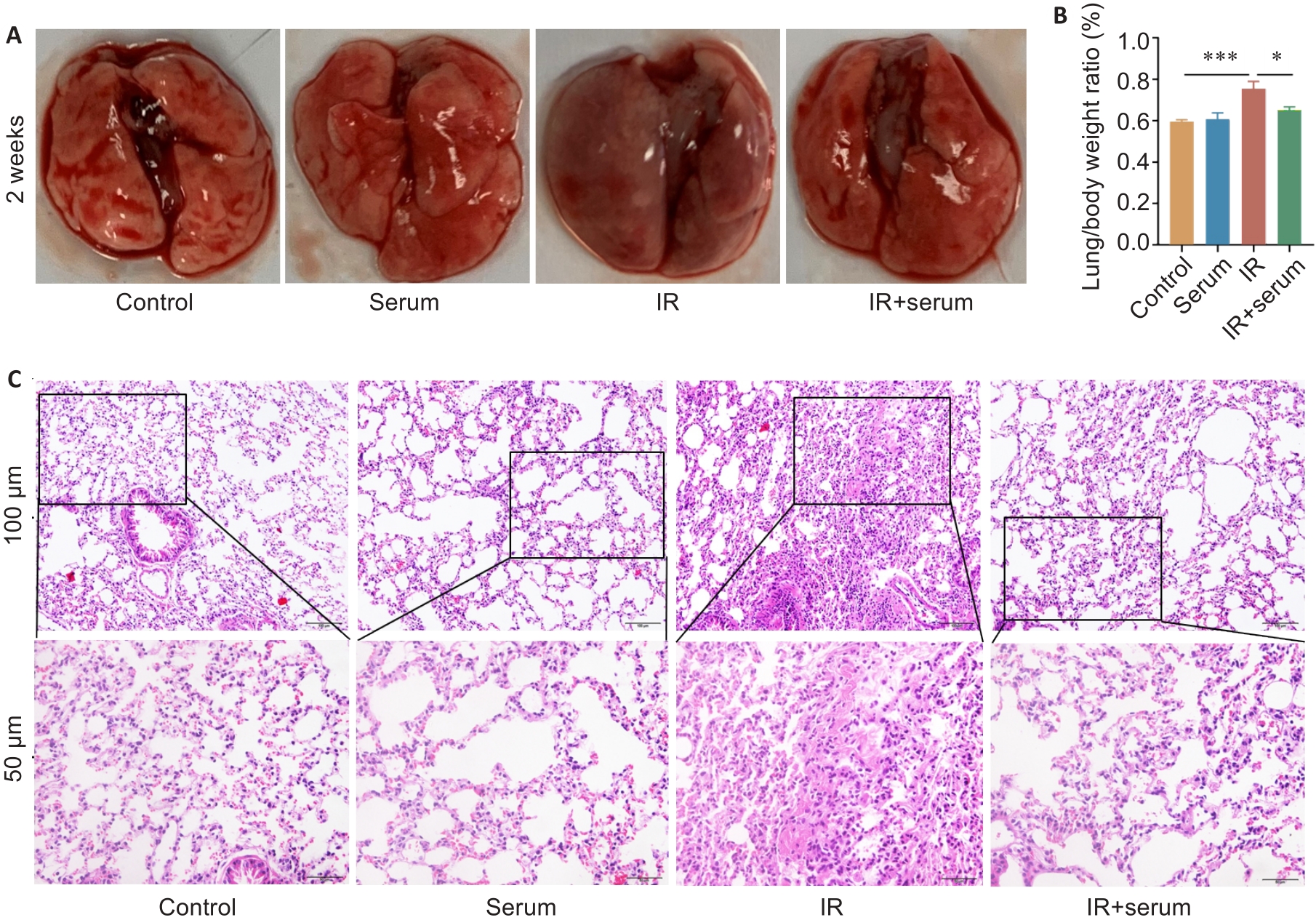
Fig.1 Normal mouse serum effectively protects normal histological structure of the lung tissue of irradiated mice. A: Gross observation of mouse lungs. B: Comparison of lung organ coefficients among the 4 groups. C: HE staining of the lung tissues in the 4 groups. Data are presented as Mean±SD (n=5). *P<0.05, ***P<0.001.

Fig.2 Intravenous injections of normal mouse serum down-regulate the levels of inflammatory factors in the lung tissue and serum of irradiated mice. A-D: Levels of TGF-β, TNF-α, IL-1α, and IL-6 in the lung tissue, respectively. E-H: Serum levels of TGF-β, TNF-α, IL-1α, and IL-6 in the serum, respectively. Data are presented as Mean±SD (n=8 in panel A and panel E, and n=5 in the rest panels). *P<0.05, **P<0.01, ***P<0.001.

Fig.3 NMS reduces lymphocyte infiltration in the lung tissue of irradiated mice A-D: Results of fluorescence-activated cell sorting showing gating for CD45+, CD4+ and Treg cells and their percentages in total cells. Data are presented as Mean±SD (n=5). *P<0.05, **P<0.01.

Fig.5 Number of immune-related differential genes and their expression at mRNA and protein levels in irradiated and NMS-treated mice. A: Classification of KEGG pathways. B, C: Relative mRNA expressions of Gsk3b, Cul3, Tiam1, Prkci, Egfr and Pik3cd in the lung tissue. D, E: Western blots of EGFR and P110δ proteins and their relative expression levels. Data are presented as Mean±SD (n=5 in panel B and panel C, and n=3 in panel D and panel E). *P<0.05, **P<0.01, ***P<0.001.

Fig.6 Normal mouse serum significantly down-regulates the expression of Focal adhesion pathway-related proteins in irradiated mice. A: Enrichment bubble chart of KEGG pathway. B-D: Western blotting of the key proteins in the focal adhesion signaling pathway and their relative expression levels in the lung tissues. Data are presented as Mean±SD (n=3). *P<0.05, **P<0.01, ***P<0.001.
| 1 | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-49. DOI: 10.3322/caac.21660 |
| 2 | Arora A, Bhuria V, Singh S, et al. Amifostine analog, DRDE-30, alleviates radiation induced lung damage by attenuating inflammation and fibrosis[J]. Life Sci, 2022, 298: 120518. DOI: 10.1016/j.lfs.2022.120518 |
| 3 | Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-induced lung injury: assessment and management[J]. Chest, 2019, 156(1): 150-62. DOI: 10.1016/j.chest.2019.03.033 |
| 4 | Giridhar P, Mallick S, Rath GK, et al. Radiation induced lung injury: prediction, assessment and management[J]. Asian Pac J Cancer Prev, 2015, 16(7): 2613-7. DOI: 10.7314/apjcp.2015.16.7.2613 |
| 5 | Citrin DE, Shankavaram U, Horton JA, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis[J]. J Natl Cancer Inst, 2013, 105(19): 1474-84. DOI: 10.1093/jnci/djt212 |
| 6 | Abratt RP, Morgan GW, Silvestri G, et al. Pulmonary complications of radiation therapy[J]. Clin Chest Med, 2004, 25(1): 167-77. DOI: 10.1016/s0272-5231(03)00126-6 |
| 7 | Gao J, Peng S, Shan XN, et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis[J]. Cell Death Dis, 2019, 10(12): 957. DOI: 10.1038/s41419-019-2195-8 |
| 8 | Zhang JL, Han XD, Zhao Y, et al. Mouse serum protects against total body irradiation-induced hematopoietic system injury by improving the systemic environment after radiation[J]. Free Radic Biol Med, 2019, 131: 382-92. DOI: 10.1016/j.freeradbiomed.2018.12.021 |
| 9 | He CJ, Zheng S, Luo Y, et al. Exosome theranostics: biology and translational medicine[J]. Theranostics, 2018, 8(1): 237-55. DOI: 10.7150/thno.21945 |
| 10 | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. DOI: 10.1126/science.aau6977 |
| 11 | Gurunathan S, Kang MH, Jeyaraj M, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes[J]. Cells, 2019, 8(4): 307. DOI: 10.3390/cells8040307 |
| 12 | Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis[J]. Cancer Res, 2011, 71(11): 3792-801. DOI: 10.1158/0008-5472.can-10-4455 |
| 13 | Zhang L, Yu DH. Exosomes in cancer development, metastasis, and immunity[J]. Biochim Biophys Acta Rev Cancer, 2019, 1871(2): 455-68. DOI: 10.1016/j.bbcan.2019.04.004 |
| 14 | Bobrie A, Colombo M, Raposo G, et al. Exosome secretion: molecular mechanisms and roles in immune responses[J]. Traffic, 2011, 12(12): 1659-68. DOI: 10.1111/j.1600-0854.2011.01225.x |
| 15 | Gregory CD, Pound JD. Microenvironmental influences of apoptosis in vivo and in vitro [J]. Apoptosis, 2010, 15(9): 1029-49. DOI: 10.1007/s10495-010-0485-9 |
| 16 | Howitt J, Hill AF. Exosomes in the pathology of neurodegenerative diseases[J]. J Biol Chem, 2016, 291(52): 26589-97. DOI: 10.1074/jbc.r116.757955 |
| 17 | Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future[J]. J Cell Biol, 2013, 200(4): 367-71. DOI: 10.1083/jcb.201212113 |
| 18 | Kang H, Kim SC, Oh Y. Fucoxanthin abrogates ionizing radiation-induced inflammatory responses by modulating sirtuin 1 in macrophages[J]. Mar Drugs, 2023, 21(12): 635. DOI: 10.3390/md21120635 |
| 19 | Téoule R. Radiation-induced DNA damage and its repair[J]. Int J Radiat Biol Relat Stud Phys Chem Med, 1987, 51(4): 573-89. DOI: 10.1080/09553008414552111 |
| 20 | Zheng Y, Li H, Bo XC, et al. Ionizing radiation damage and repair from 3D-genomic perspective[J]. Trends Genet, 2023, 39(1): 1-4. DOI: 10.1016/j.tig.2022.07.004 |
| 21 | Zhang JX, Wang XL, Vikash V, et al. ROS and ROS-mediated cellular signaling[J]. Oxid Med Cell Longev, 2016, 2016: 4350965. DOI: 10.1155/2016/4350965 |
| 22 | Xu TK, Zhang YY, Chang PY, et al. Mesenchymal stem cell-based therapy for radiation-induced lung injury[J]. Stem Cell Res Ther, 2018, 9(1): 18. DOI: 10.1186/s13287-018-0776-6 |
| 23 | Milane L, Singh A, Mattheolabakis G, et al. Exosome mediated communication within the tumor microenvironment[J]. J Control Release, 2015, 219: 278-94. DOI: 10.1016/j.jconrel.2015.06.029 |
| 24 | McCall CE, El Gazzar M, Liu TF, et al. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation[J]. J Leukoc Biol, 2011, 90(3): 439-46. DOI: 10.1189/jlb.0211075 |
| 25 | Wang CL, Xu MC, Fan QZ, et al. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases[J]. Asian J Pharm Sci, 2023, 18(1): 100772. DOI: 10.1016/j.ajps.2022.100772 |
| 26 | Zheng X, Zhao D, Liu Y, et al. Regeneration and anti-inflammatory effects of stem cells and their extracellular vesicles in gynecological diseases[J]. Biomed Pharmacother, 2023, 168: 115739. DOI: 10.1016/j.biopha.2023.115739 |
| 27 | Liu C, Xiao K, Xie LX. Advances in the use of exosomes for the treatment of ALI/ARDS[J]. Front Immunol, 2022, 13: 971189. DOI: 10.3389/fimmu.2022.971189 |
| 28 | Xu CJ, Zhao J, Li QY, et al. Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model[J]. Stem Cell Res Ther, 2020, 11(1): 503. DOI: 10.1186/s13287-020-02023-9 |
| 29 | Noonin C, Thongboonkerd V. Exosome-inflammasome crosstalk and their roles in inflammatory responses[J]. Theranostics, 2021, 11(9): 4436-51. DOI: 10.7150/thno.54004 |
| 30 | Sun ZQ, Yang SX, Zhou QB, et al. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment[J]. Mol Cancer, 2018, 17(1): 82. DOI: 10.1186/s12943-018-0831-z |
| 31 | Li H, Zhao SY, Jiang M, et al. Biomodified extracellular vesicles remodel the intestinal microenvironment to overcome radiation enteritis[J]. ACS Nano, 2023, 17(14): 14079-98. DOI: 10.1021/acsnano.3c04578 |
| 32 | Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling[J]. Nat Med, 2011, 18(1): 148-52. DOI: 10.1038/nm.2574 |
| 33 | Chen WC, Yu WK, Su VYF, et al. NLRP3 inflammasome activates endothelial-to-mesenchymal transition via focal adhesion kinase pathway in bleomycin-induced pulmonary fibrosis[J]. Int J Mol Sci, 2023, 24(21): 15813. DOI: 10.3390/ijms242115813 |
| [1] | 涂舒谕, 陈祥宇, 李程辉, 黄丹萍, 张莉. 补阳还五汤通过调控外泌体miR-590-5p介导的巨噬细胞极化延缓大鼠血管衰老[J]. 南方医科大学学报, 2025, 45(6): 1251-1259. |
| [2] | 陈志亮, 杨永刚, 黄霞, 成彦, 瞿媛, 衡琪琪, 符羽佳, 李可薇, 顾宁. 外泌体miRNA差异表达可作为诊断慢性心力衰竭合并高尿酸血症患者新型分子标志物及靶基因功能分析[J]. 南方医科大学学报, 2025, 45(1): 43-51. |
| [3] | 何思齐, 文楠, 陈勋, 王跃, 张艇, 牟雁东. 枸杞糖肽可减轻放射治疗后人牙龈成纤维细胞来源的外泌体导致的成骨抑制[J]. 南方医科大学学报, 2024, 44(9): 1752-1759. |
| [4] | 戴荣, 曹泽平, 刘传娇, 葛永, 程梦, 王伟丽, 陈义珍, 张磊, 王亿平. 清肾颗粒通过调控外泌体、miR-330-3p以及CREBBP表达抑制小鼠肾纤维化[J]. 南方医科大学学报, 2024, 44(8): 1431-1440. |
| [5] | 陈光亚, 向星亮, 曾兆祥, 黄荣增, 金姝娜, 肖明中, 宋成武. 地五养肝方对非酒精性脂肪肝小鼠循环外泌体溶血甘油磷脂的调控作用[J]. 南方医科大学学报, 2024, 44(7): 1382-1388. |
| [6] | 李粉霞, 林浩生, 黎一琳, 朱雯倩, 孙元洁, 黄源, 裘毓雯, 秦霞, 常清贤. 孤立性侧脑室扩张胎儿孕妇羊水外泌体miRNA差异表达谱[J]. 南方医科大学学报, 2024, 44(11): 2256-2264. |
| [7] | 孙晓鹏, 史 航, 张 磊, 刘 中, 李克威, 钱玲玲, 朱星宇, 杨康佳, 付 强, 丁 华. 外胚层间充质干细胞来源的外泌体通过控制炎症和氧化损伤减少M1型小胶质细胞并促进H2O2处理后PC12细胞的存活[J]. 南方医科大学学报, 2024, 44(1): 119-128. |
| [8] | 何艳娟, 李卓颖, 申 琳, 石丁华, 李申堂. 心脏祖细胞来源的外泌体可减轻心肌梗死小鼠的心肌损伤:基于mTOR途径诱导Treg细胞分化[J]. 南方医科大学学报, 2023, 43(9): 1644-1650. |
| [9] | 徐梦歧, 石宇彤, 刘俊平, 吴敏敏, 张凤梅, 何志强, 唐 敏. JAG1影响单核-巨噬细胞重塑三阴性乳腺癌转移前微环境:基于外泌体中的LncRNA MALAT1[J]. 南方医科大学学报, 2023, 43(9): 1525-1535. |
| [10] | 林嘉宜, 娄安妮, 李 旭. 脂多糖刺激巨噬细胞分泌含miR-155-5p的外泌体促进肝星状细胞的活化及迁移[J]. 南方医科大学学报, 2023, 43(6): 994-1001. |
| [11] | 王 丽, 严志锐, 夏耀雄. 抑制RAB27a能够抑制三阴乳腺癌细胞的增殖、侵袭和粘附[J]. 南方医科大学学报, 2023, 43(4): 560-567. |
| [12] | 刘 屿, 曾 莲, 王卫红, 杨艳玲, 王 洲, 刘建启, 李 卫, 孙婧宇, 余晓宏. 人骨髓间充质干细胞外泌体来源的miR-335-5p促进人牙周膜干细胞的成骨分化:基于下调DKK1表达[J]. 南方医科大学学报, 2023, 43(3): 420-427. |
| [13] | 张梦莹, 李 志, 裴纬亚, 李雪琴, 杨 辉, 朱小龙, 吕 坤. M2型巨噬细胞来源的外泌体lncRNA NR_028113.1通过激活JAK2/STAT3通路促进巨噬细胞的极化[J]. 南方医科大学学报, 2023, 43(3): 393-399. |
| [14] | 黄永祺, 喻 伟, 游月华. 槟榔碱通过促进巨噬细胞分泌含miR-155-5p外泌体诱导人口腔成纤维细胞的活化[J]. 南方医科大学学报, 2023, 43(1): 60-67. |
| [15] | 吴小凤, 詹日明, 程大钊, 陈 黎, 王天雨, 唐旭东. 非小细胞肺癌细胞外泌体源性FZD10促进体外血管生成[J]. 南方医科大学学报, 2022, 42(9): 1351-1358. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||