南方医科大学学报 ›› 2026, Vol. 46 ›› Issue (1): 94-103.doi: 10.12122/j.issn.1673-4254.2026.01.10
赵锦燕1,2,3( ), 彭娇1, 林明和1, 朱晓勤1,2,3, 黄彬1,2,3, 林久茂1,2,3(
), 彭娇1, 林明和1, 朱晓勤1,2,3, 黄彬1,2,3, 林久茂1,2,3( )
)
收稿日期:2025-05-17
出版日期:2026-01-20
发布日期:2026-01-16
通讯作者:
林久茂
E-mail:zhaojinyan0928@163.com;linjiumao@fjtcm.edu.cn
作者简介:赵锦燕,博士,副研究员,E-mail: zhaojinyan0928@163.com
基金资助:
Jinyan ZHAO1,2,3( ), Jiao PENG1, Minghe LIN1, Xiaoqin ZHU1,2,3, Bin HUANG1,2,3, Jiumao LIN1,2,3(
), Jiao PENG1, Minghe LIN1, Xiaoqin ZHU1,2,3, Bin HUANG1,2,3, Jiumao LIN1,2,3( )
)
Received:2025-05-17
Online:2026-01-20
Published:2026-01-16
Contact:
Jiumao LIN
E-mail:zhaojinyan0928@163.com;linjiumao@fjtcm.edu.cn
Supported by:摘要:
目的 探讨清解扶正颗粒(QFG)缓解5-氟尿嘧啶(5-FU)导致骨骼肌萎缩的作用及其机制。 方法 采用CT26细胞制备小鼠皮下移植瘤模型,10只/组,待瘤体长至100 mm3,根据瘤体大小将小鼠分为对照(CT26)组、模型(5-FU,50 mg/kg)组、干预(5-FU,50 mg/kg+QFG,1 g/kg)组,同时设置正常组。模型组和干预组小鼠给予5-FU腹腔注射,1次/3 d;干预组同时灌胃 QFG,1次/d;正常组和对照组小鼠腹腔注射和灌胃等体积的生理盐水,连续干预21 d。在干预前1 d以及干预第20天进行抓力试验和悬挂试验;在第21天取材后称量瘤体质量和腓肠肌的质量;苏木素-伊红(HE)染色、透射电镜和原位末端转移酶标记(TUNEL)法观察腓肠肌组织形态改变;比色法检测腓肠肌三磷酸腺苷酶(ATP)含量;免疫组织化学(IHC)方法检测腓肠肌AMPK、PGC-1α、细胞色素(Cyto)C、凋亡诱导因子(AIF)、凋亡蛋白酶激活因子(Apaf)-1、第二个线粒体衍生半胱天冬氨酸蛋白酶激活剂(Smac)、B细胞淋巴瘤(Bcl)-2、Bcl-2相关X蛋白(Bax)和剪切的天冬氨酸特异性半胱氨酸蛋白酶(cleaved caspase-3)和cleaved caspase-9蛋白表达。 结果 与正常组比较,对照组腓肠肌质量、抓力和悬挂评分变小(P<0.05),腓肠肌纤维结构和超微结构损伤,线粒体减少,ATP含量降低(P<0.05),细胞凋亡率增加(P<0.05),腓肠肌AMPK、PGC-1α、Bcl-2表达降低(均P<0.05),Bax、Cyto C、AIF、Apaf-1、Smac、cleaved caspase-3 和cleaved caspase-9表达升高(均P<0.05)。与对照组比较,模型组腓肠肌质量、抓力和悬挂评分更小(P<0.05),腓肠肌纤维结构和超微结构损伤严重,ATP含量降低(P<0.05),细胞凋亡进一步增加(P<0.05),腓肠肌AMPK、PGC-1α、Bcl-2表达降低(均P<0.05),Bax、Cyto C、AIF、Apaf-1、Smac、cleaved caspase-3 和cleaved caspase-9 表达升高(均P<0.05)。与模型组比较,干预组腓肠肌质量、抓力和悬挂评分增加(均P<0.015),腓肠肌纤维结构和超微结构损伤减轻,细胞凋亡减少(P<0.05),腓肠肌AMPK、PGC-1α、Bcl-2表达升高(均P<0.05),Bax、Cyto C、AIF、Apaf-1、Smac、cleaved caspase-3 和cleaved caspase-9表达降低(均P<0.05)。 结论 QFG缓解5-FU所致荷瘤小鼠的骨骼肌疲劳,其机制与AMPK/PGC-1α通路活化,抑制腓肠肌线粒体依赖性细胞凋亡有关。
赵锦燕, 彭娇, 林明和, 朱晓勤, 黄彬, 林久茂. 清解扶正颗粒通过抑制线粒体依赖的凋亡、激活AMPK-PGC-1α通路缓解5-氟尿嘧啶引起的骨骼肌损伤[J]. 南方医科大学学报, 2026, 46(1): 94-103.
Jinyan ZHAO, Jiao PENG, Minghe LIN, Xiaoqin ZHU, Bin HUANG, Jiumao LIN. Qingjie Fuzheng Granules alleviates 5-fluorouracil-induced skeletal muscle injury in tumor-bearing mice by inhibiting mitochondria-dependent apoptosis and activating the AMPK-PGC-1α pathway[J]. Journal of Southern Medical University, 2026, 46(1): 94-103.
| Group | Tumor weight | Gastrocnemius weight |
|---|---|---|
| Normal | - | 0.31±0.02 |
| Control | 10.93±1.92 | 0.25±0.01** |
| Model | 2.83±0.40## | 0.21±0.01## |
| Treatment | 2.74±0.57 | 0.25±0.01△ |
| F | 63.88 | 25.57 |
| P | <0.001 | <0.001 |
表1 各组小鼠瘤体质量和腓肠肌质量比较
Tab.1 Comparison of tumor weight and gastrocnemius muscle weight in each group (g, Mean±SD, n=4)
| Group | Tumor weight | Gastrocnemius weight |
|---|---|---|
| Normal | - | 0.31±0.02 |
| Control | 10.93±1.92 | 0.25±0.01** |
| Model | 2.83±0.40## | 0.21±0.01## |
| Treatment | 2.74±0.57 | 0.25±0.01△ |
| F | 63.88 | 25.57 |
| P | <0.001 | <0.001 |
| Group | Grip | Suspension score |
|---|---|---|
| Normal | 76.37±1.48 | 3 |
| Control | 63.45±1.66** | 2.33±0.47* |
| Model | 54.75±1.93## | 1.85±0.33# |
| Treatment | 69.12±2.96△△ | 2.67±0.27△△ |
| F | 76.63 | 9.74 |
| P | <0.001 | <0.01 |
表2 各组小鼠抓力和悬挂评分比较
Tab.2 Comparison of grip and suspension test scores in each group (Mean±SD, n=4)
| Group | Grip | Suspension score |
|---|---|---|
| Normal | 76.37±1.48 | 3 |
| Control | 63.45±1.66** | 2.33±0.47* |
| Model | 54.75±1.93## | 1.85±0.33# |
| Treatment | 69.12±2.96△△ | 2.67±0.27△△ |
| F | 76.63 | 9.74 |
| P | <0.001 | <0.01 |
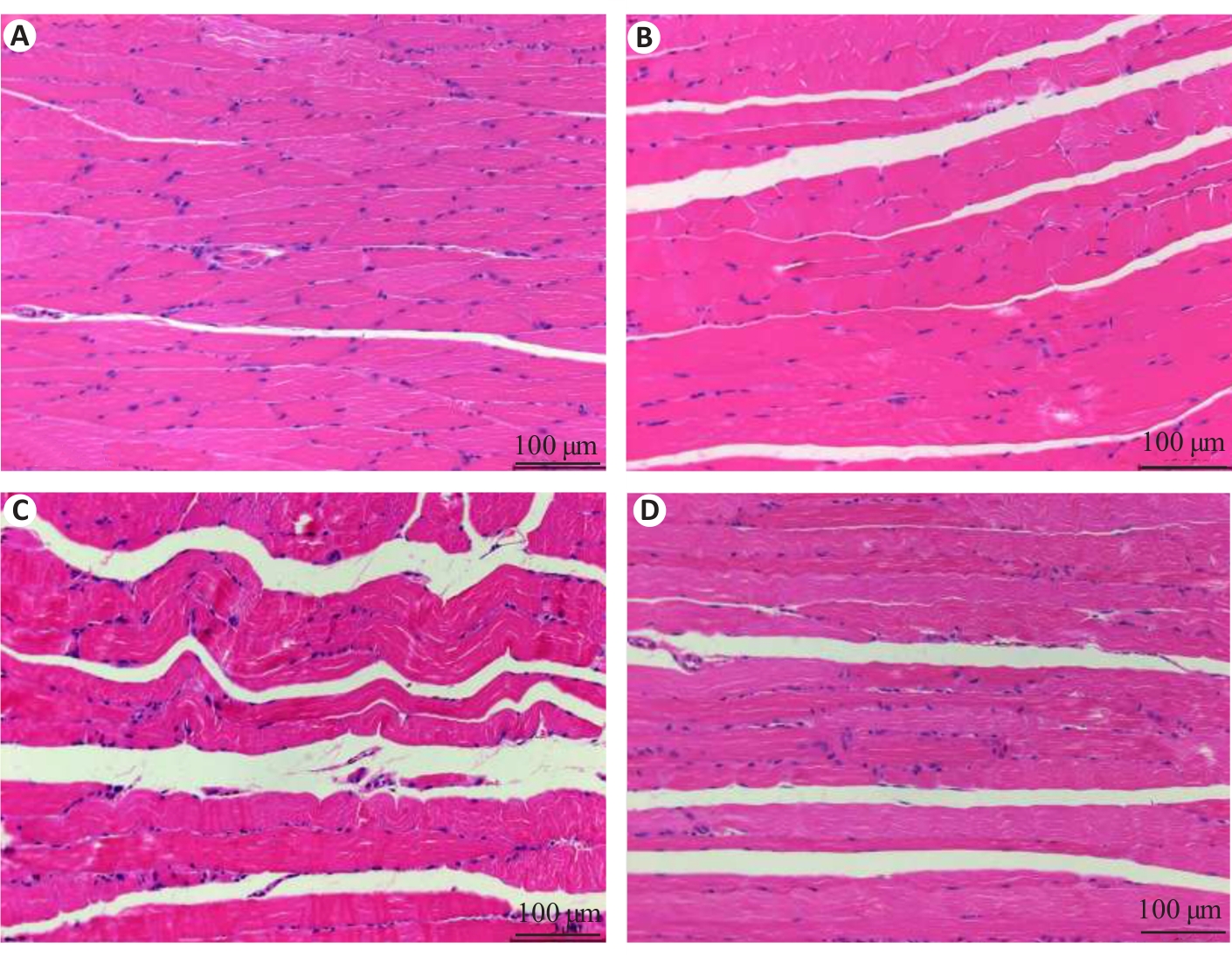
图1 各组小鼠腓肠肌HE染色结果
Fig.1 HE staining of the gastrocnemius muscle of the mice in each group. A: Normal group. B: Control group. C: Model group. D: Treatment group.
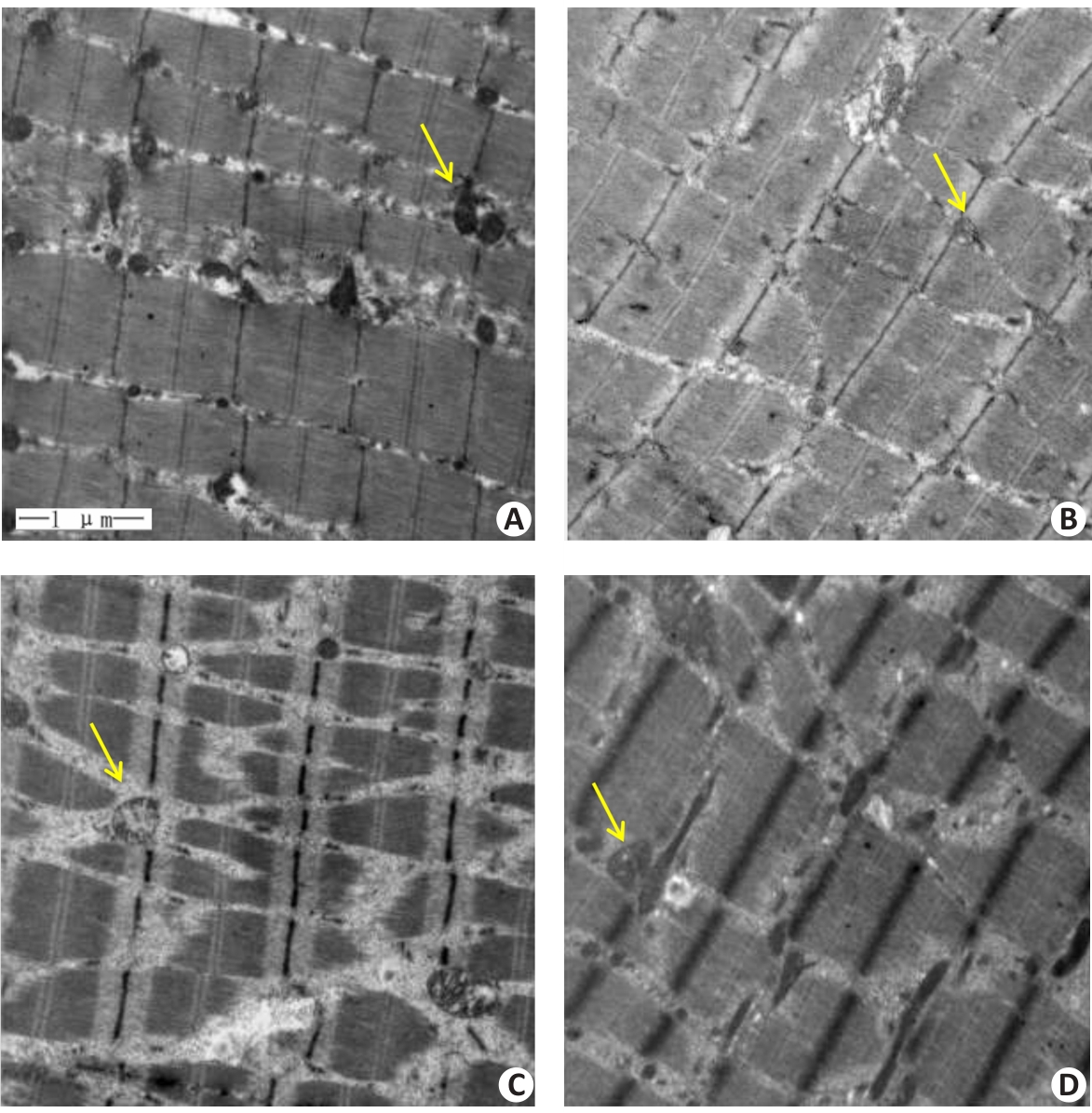
图2 各组小鼠腓肠肌透射电镜结果
Fig.2 Transmission electron microscopy of the gastrocnemius muscle in each group (Original magnification: ×20 000). A: Normal group. B: Control group. C: Model group. D: Treatment group. Yellow arrows indicate the mitochondria.
| Group | Content of ATP |
|---|---|
| Normal | 3.31±0.25 |
| Control | 2.06±0.11** |
| Model | 0.89±0.12## |
| Treatment | 1.44±0.17△△ |
| F | 113.85 |
| P | <0.001 |
表3 各组小鼠ATP含量比较
Tab.3 Comparison of ATP content in the gastrocnemius muscle in each group (μmol/g, Mean±SD, n=4)
| Group | Content of ATP |
|---|---|
| Normal | 3.31±0.25 |
| Control | 2.06±0.11** |
| Model | 0.89±0.12## |
| Treatment | 1.44±0.17△△ |
| F | 113.85 |
| P | <0.001 |
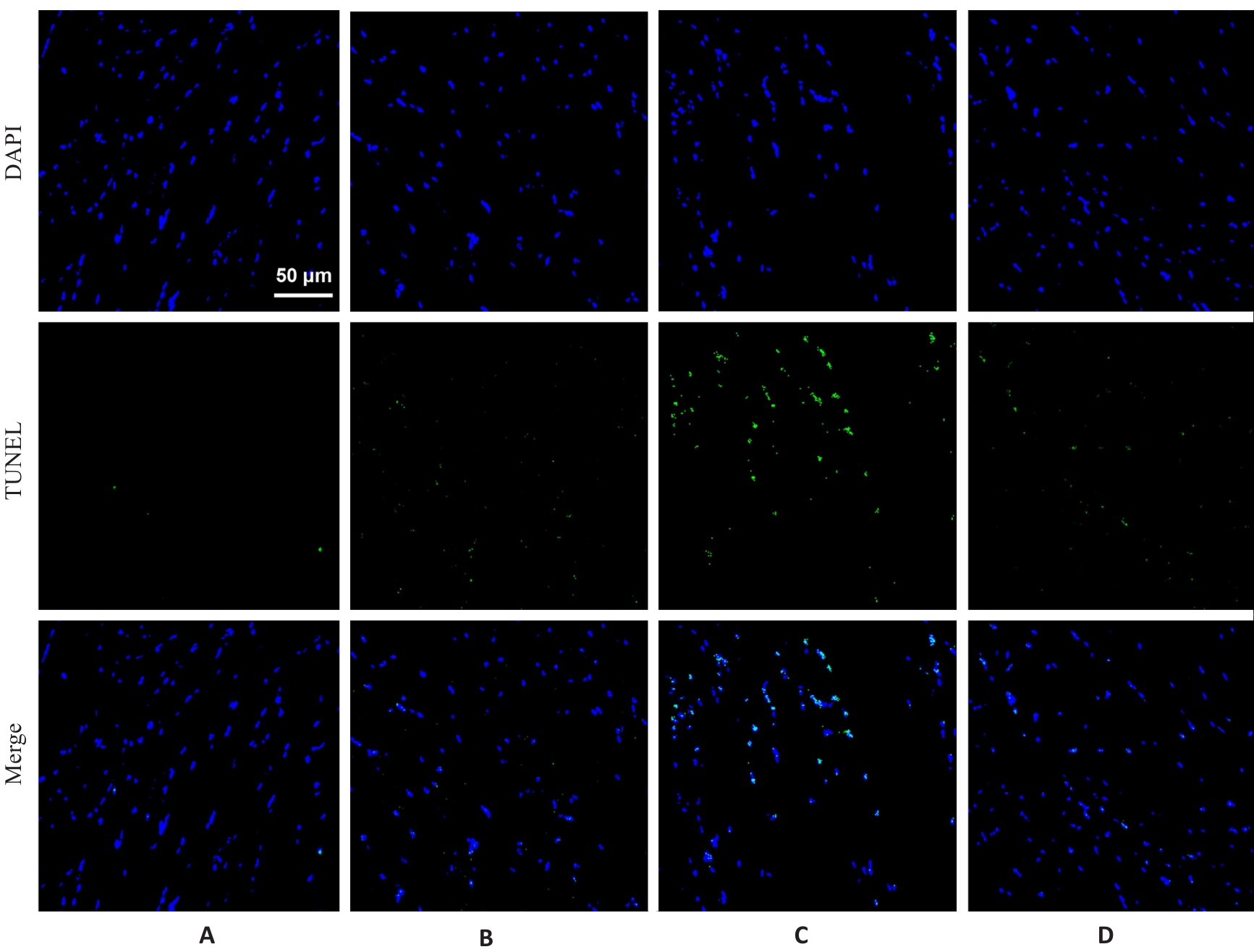
图3 各组小鼠腓肠肌TUNEL染色结果
Fig.3 TUNEL staining of the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | Apoptosis rate |
|---|---|
| Normal | 3.66±0.37 |
| Control | 14.13±0.60** |
| Model | 36.39±0.53## |
| Treatment | 13.61±1.06△△ |
| F | 1214.94 |
| P | <0.001 |
表4 各组小鼠凋亡率比较
Tab.4 Comparison of cell apoptosis rate in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Apoptosis rate |
|---|---|
| Normal | 3.66±0.37 |
| Control | 14.13±0.60** |
| Model | 36.39±0.53## |
| Treatment | 13.61±1.06△△ |
| F | 1214.94 |
| P | <0.001 |
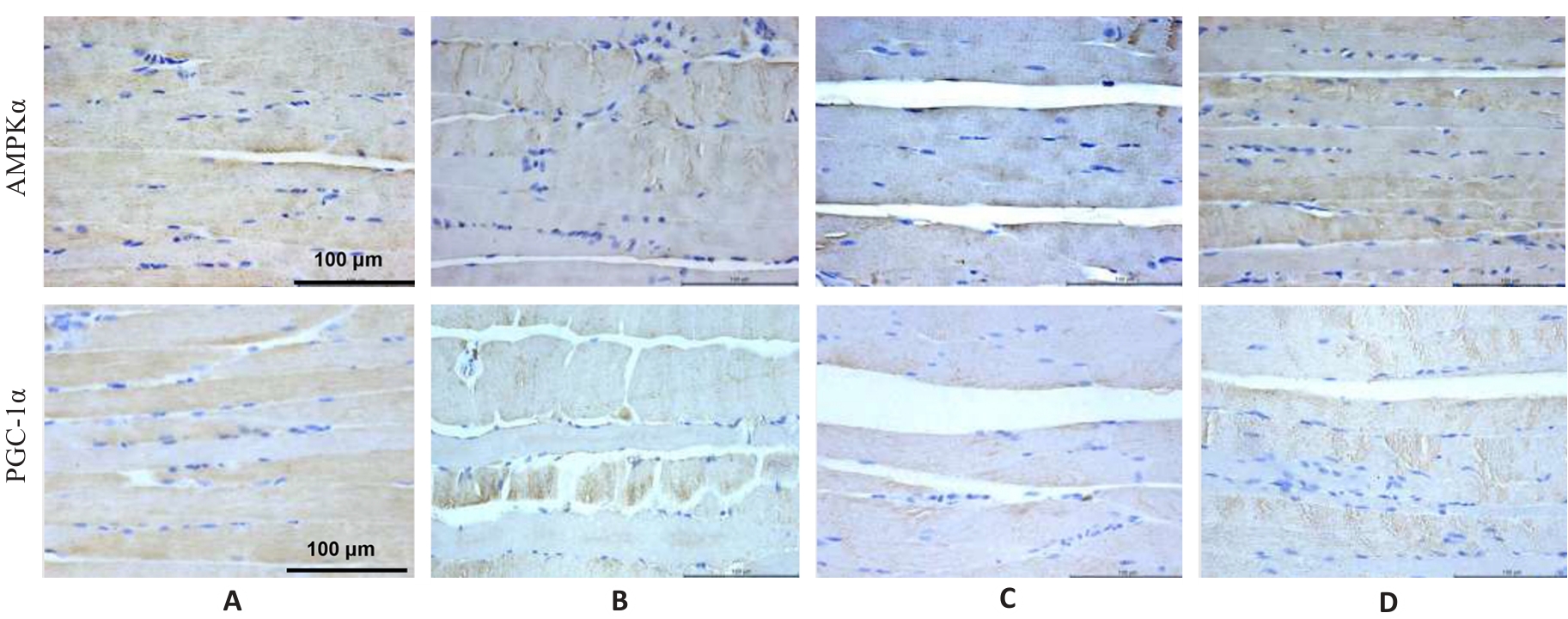
图4 各组小鼠腓肠肌AMPKα和PGC-1α蛋白表达
Fig.4 Immunohistochemistry for detecting AMPK and PGC-1α protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | AMPK | PGC-1α |
|---|---|---|
| Normal | 56.46±0.62 | 57.90±1.40 |
| Control | 42.48±1.88** | 25.67±1.01** |
| Model | 16.27±1.07## | 14.29±0.65## |
| Treatment | 21.8±1.17△△ | 20.67±2.00△△ |
| F | 648.10 | 406.17 |
| P | <0.001 | <0.01 |
表5 各组小鼠腓肠肌AMPK和PGC-1α蛋白表达
Tab.5 Protein expression levels of AMPK and PGC-1α in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | AMPK | PGC-1α |
|---|---|---|
| Normal | 56.46±0.62 | 57.90±1.40 |
| Control | 42.48±1.88** | 25.67±1.01** |
| Model | 16.27±1.07## | 14.29±0.65## |
| Treatment | 21.8±1.17△△ | 20.67±2.00△△ |
| F | 648.10 | 406.17 |
| P | <0.001 | <0.01 |
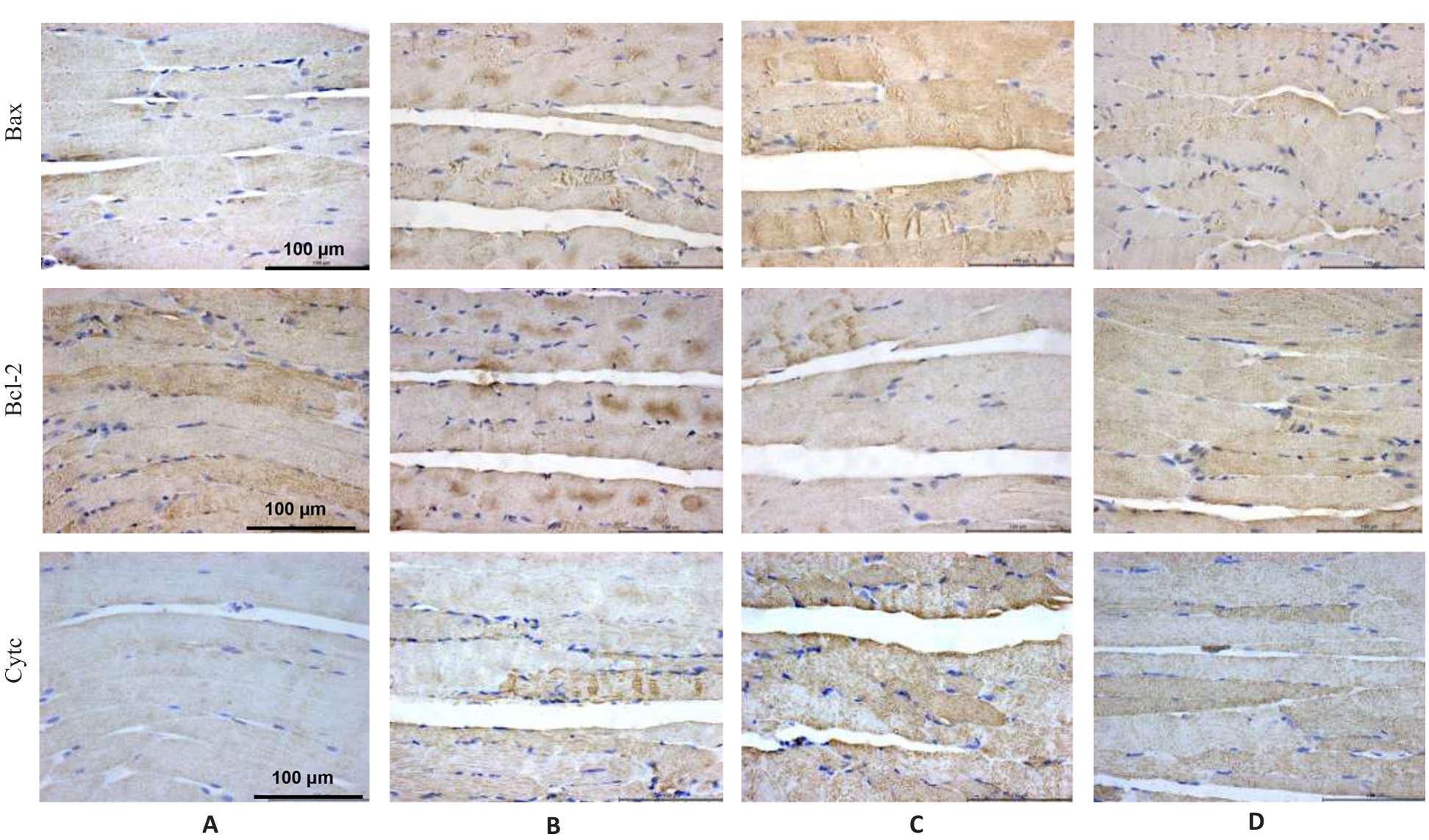
图5 各组小鼠腓肠肌Bax、Bcl-2和Cyt c蛋白表达
Fig.5 Immunohistochemistry for detecting Bax, Bcl-2 and Cyt c protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | Bax | Bcl-2 | Cyt c |
|---|---|---|---|
| Normal | 29.42±0.79 | 71.08±1.53 | 20.41±0.53 |
| Control | 51.03±1.74** | 55.40±0.92** | 43.32±1.55** |
| Model | 80.67±1.36## | 37.53±1.41## | 69.45±1.13## |
| Treatment | 33.38±1.0△△ | 57.27±1.07△△ | 38.02±0.96△△ |
| F | 661.12 | 293.84 | 674.29 |
| P | <0.001 | <0.001 | <0.001 |
表6 各组小鼠腓肠肌Bax、Bcl-2和Cyt c蛋白表达
Tab.6 Protein expression levels of Bax, Bcl-2 and Cyt c in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Bax | Bcl-2 | Cyt c |
|---|---|---|---|
| Normal | 29.42±0.79 | 71.08±1.53 | 20.41±0.53 |
| Control | 51.03±1.74** | 55.40±0.92** | 43.32±1.55** |
| Model | 80.67±1.36## | 37.53±1.41## | 69.45±1.13## |
| Treatment | 33.38±1.0△△ | 57.27±1.07△△ | 38.02±0.96△△ |
| F | 661.12 | 293.84 | 674.29 |
| P | <0.001 | <0.001 | <0.001 |
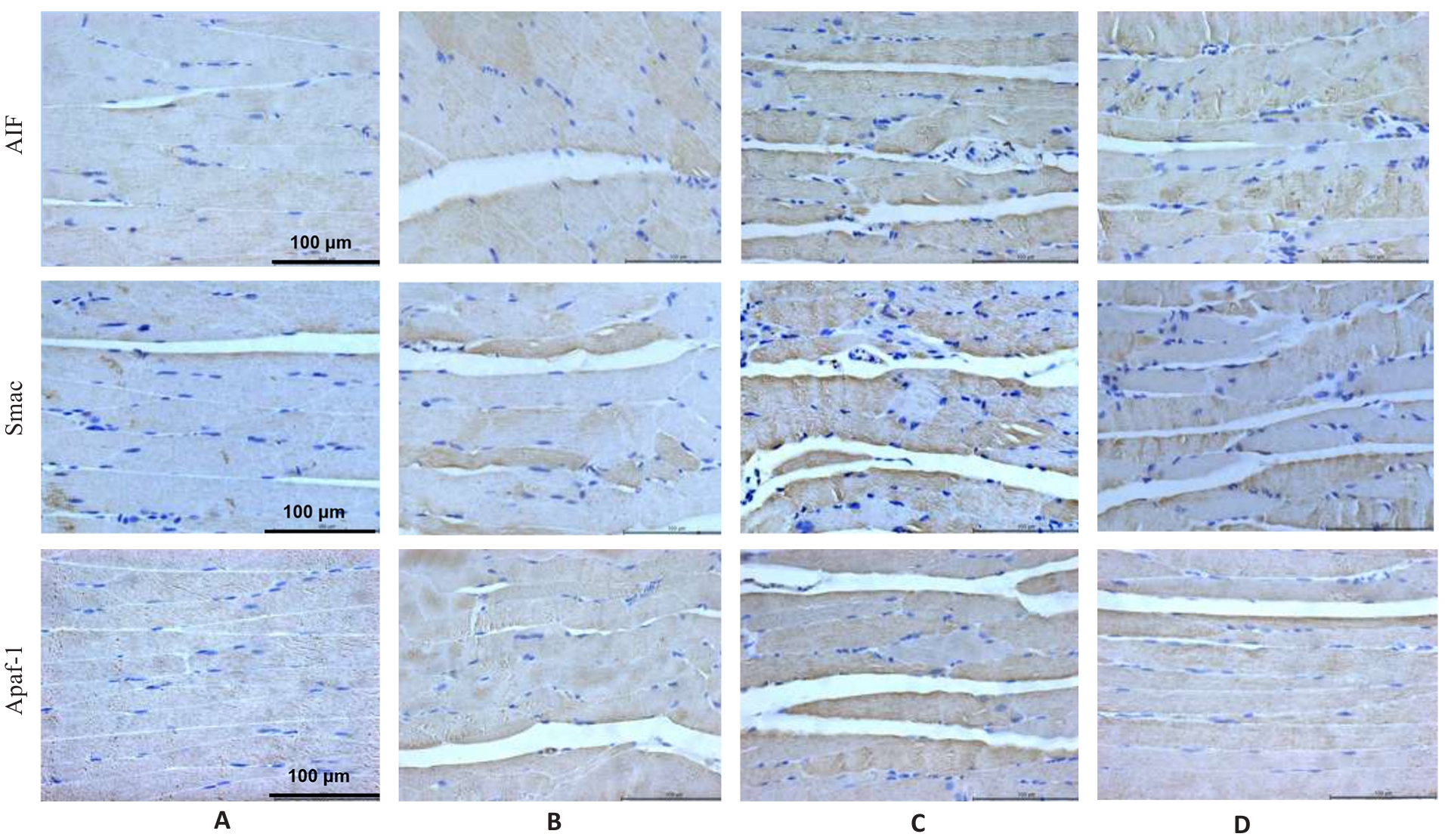
图6 各组小鼠腓肠肌AIF、Smac及Apaf1蛋白表达
Fig.6 Immunohistochemistry for detecting AIF, Smac and Apaf1 protein expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | AIF | Smac | Apaf1 |
|---|---|---|---|
| Normal | 17.09±0.58 | 18.21±0.66 | 19.54±0.51 |
| Control | 36.43±0.50** | 36.41±0.69** | 39.32±0.69** |
| Model | 51.70±0.55## | 51.23±1.72## | 49.32±1.27## |
| Treatment | 27.24±0.32△△ | 23.59±1.59△△ | 29.63±1.68△△ |
| F | 1719.99 | 270.47 | 252.82 |
| P | <0.001 | <0.001 | <0.001 |
表7 各组小鼠腓肠肌AIF、Smac和Apaf1蛋白表达
Tab.7 Protein expression levels of AIF, Smac and Apaf1 in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | AIF | Smac | Apaf1 |
|---|---|---|---|
| Normal | 17.09±0.58 | 18.21±0.66 | 19.54±0.51 |
| Control | 36.43±0.50** | 36.41±0.69** | 39.32±0.69** |
| Model | 51.70±0.55## | 51.23±1.72## | 49.32±1.27## |
| Treatment | 27.24±0.32△△ | 23.59±1.59△△ | 29.63±1.68△△ |
| F | 1719.99 | 270.47 | 252.82 |
| P | <0.001 | <0.001 | <0.001 |
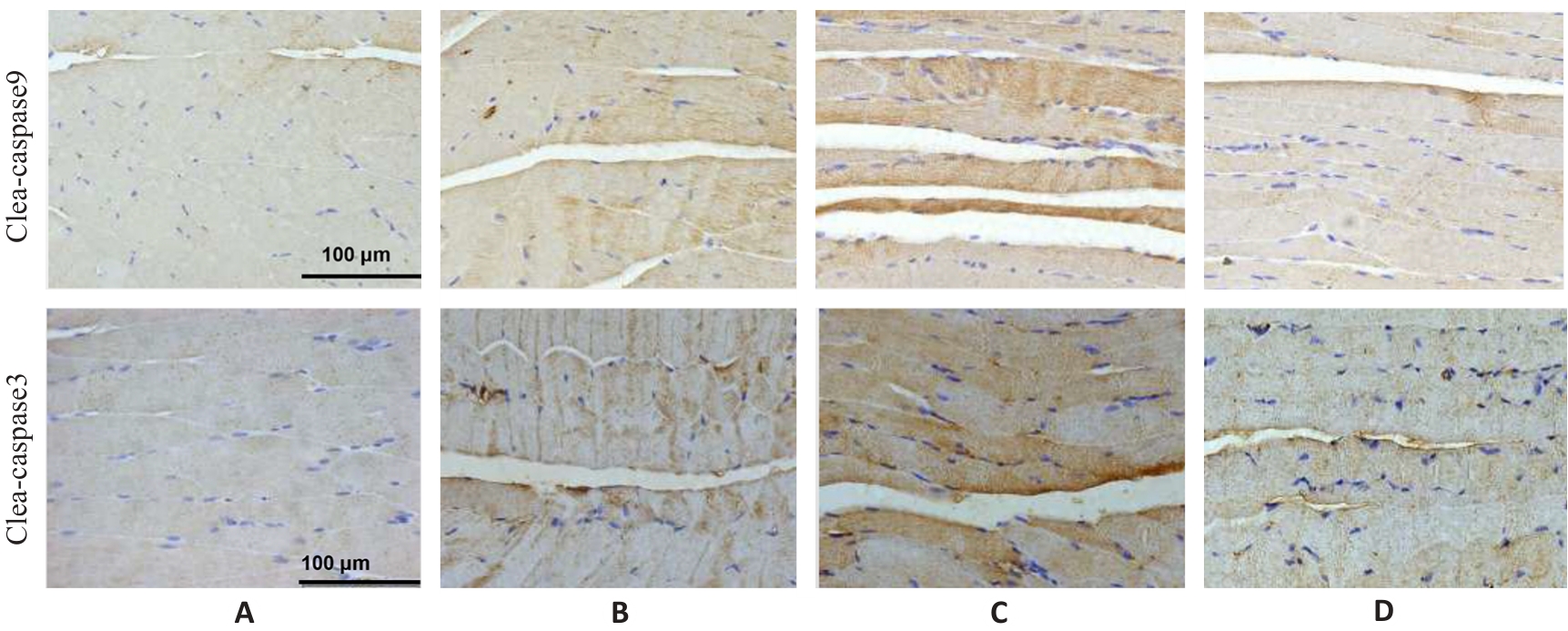
图7 各组小鼠腓肠肌cleaved caspase-9和cleaved caspase-3蛋白表达
Fig.7 Immunohistochemistry for detecting cleaved caspase-9 and cleaved caspase-3 expressions in the gastrocnemius muscle in each group (×400). A: Normal group. B: Control group. C: Model group. D: Treatment group.
| Group | Cleaved-caspase9 | Cleaved-caspase3 |
|---|---|---|
| Normal | 16.35±0.34 | 19.61±0.50 |
| Control | 44.50±0.84** | 29.94±1.60** |
| Model | 90.65±0.80## | 58.86±1.33## |
| Treatment | 20.08±0.43△△ | 20.67±2.00△△ |
| F | 2244.19 | 569.43 |
| P | <0.001 | <0.01 |
表8 各组小鼠腓肠肌cleaved caspase-9和cleaved caspase-3蛋白表达
Tab.8 Protein expression levels of cleaved caspase-9 and cleaved caspase-3 in the gastrocnemius muscle in each group (%, Mean±SD, n=3)
| Group | Cleaved-caspase9 | Cleaved-caspase3 |
|---|---|---|
| Normal | 16.35±0.34 | 19.61±0.50 |
| Control | 44.50±0.84** | 29.94±1.60** |
| Model | 90.65±0.80## | 58.86±1.33## |
| Treatment | 20.08±0.43△△ | 20.67±2.00△△ |
| F | 2244.19 | 569.43 |
| P | <0.001 | <0.01 |
| [1] | Cao WY, Li JH, Yang KP, et al. An overview of autophagy: Mechanism, regulation and research progress[J]. Bull Cancer, 2021, 108(3): 304-22. doi:10.1016/j.bulcan.2020.11.004 |
| [2] | Beesley VL, Ross TL, King MT, et al. Evaluating patient-reported symptoms and late adverse effects following completion of first-line chemotherapy for ovarian cancer using the MOST (Measure of Ovarian Symptoms and Treatment concerns)[J]. Gynecol Oncol, 2022, 164(2): 437-45. doi:10.1016/j.ygyno.2021.12.006 |
| [3] | Fox RS, Ancoli-Israel S, Roesch SC, et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy[J]. Support Care Cancer, 2020, 28(2): 845-55. doi:10.1007/s00520-019-04834-w |
| [4] | Liu WM, Liu J, Ma L, et al. Effect of mindfulness Yoga on anxiety and depression in early breast cancer patients received adjuvant chemotherapy: a randomized clinical trial[J]. J Cancer Res Clin Oncol, 2022, 148(9): 2549-60. doi:10.1007/s00432-022-04167-y |
| [5] | Zhang H, Meng YT, Jiang RX, et al. Effect of multimodal exercise on cancer-related fatigue in patients undergoing simultaneous radiotherapy and chemotherapy: a randomized trial in patients with breast cancer[J]. Altern Ther Health Med, 2023, 29(5): 233-7. |
| [6] | Mallard J, Hucteau E, Charles AL, et al. Chemotherapy impairs skeletal muscle mitochondrial homeostasis in early breast cancer patients[J]. J Cachexia Sarcopenia Muscle, 2022, 13(3): 1896-907. doi:10.1002/jcsm.12991 |
| [7] | Mallard J, Hucteau E, Bender L, et al. A single chemotherapy administration induces muscle atrophy, mitochondrial alterations and apoptosis in breast cancer patients[J]. J Cachexia Sarcopenia Muscle, 2024, 15(1): 292-305. doi:10.1002/jcsm.13414 |
| [8] | Jahnke VE, Peterson JM, Van Der Meulen JH, et al. Mitochondrial dysfunction and consequences in calpain-3-deficient muscle[J]. Skelet Muscle, 2020, 10(1): 37. doi:10.1186/s13395-020-00254-1 |
| [9] | 黄燕峰, 朱达坚, 鲁 路. 黄芪多糖对慢性疲劳小鼠骨骼肌线粒体功能的影响及作用机制[J]. 广东医学, 2017, 38(12): 1789-94. |
| [10] | 张 璐, 丁焕章, 许浩燃, 等. 参芪补中方通过激活AMPK/SIRT1/PGC-1α改善COPD肺脾气虚证大鼠线粒体功能障碍[J].南方医科大学学报,2025, 45(5): 969-976. |
| [11] | 关海燕, 张洪亮. 恶性肿瘤患者化疗引起癌因性疲乏的中西医研究进展[J]. 新疆中医药, 2018, 36(5): 119-21. |
| [12] | 华杭菊, 林久茂, 任丽萍, 等. 清解扶正方联合mFOLFOX4方案治疗晚期大肠癌的疗效观察[J]. 福建中医药, 2019, 50(1): 20-1, 24. |
| [13] | 王泽坤, 陈晓琦, 陈召起, 等. 癌因性疲乏的中西医研究进展[J]. 中华中医药杂志, 2023, 38(3): 1185-9. |
| [14] | 周 婷, 吴泳蓉, 熊家青, 等. 癌因性疲乏的中医病因探析[J]. 中华中医药杂志, 2022, 37(2): 982-5. |
| [15] | Wei XT, Xin JY, Chen W, et al. Astragalus polysaccharide ameliorated complex factor-induced chronic fatigue syndrome by modulating the gut microbiota and metabolites in mice[J]. Biomed Pharmacother, 2023, 163: 114862. doi:10.1016/j.biopha.2023.114862 |
| [16] | Dong J, Wang S, Gui YR, et al. Astragalus membranaceus (Huang Qi) for cancer-related fatigue: a protocol for systematic review and meta-analysis[J]. Medicine (Baltimore), 2022, 101(3): e28633. doi:10.1097/md.0000000000028633 |
| [17] | 余 意, 胡明华, 张丹丹, 等. 黄芪多糖对气虚大鼠的补气作用及其机制探讨[J]. 中药新药与临床药理, 2021, 32(4): 505-10. |
| [18] | 徐振秋, 李雪峰, 张海弢, 等. 麦芽油软胶囊缓解体力疲劳作用的研究[J]. 食品与发酵科技, 2015, 51(5): 17-9, 26. |
| [19] | 郜浩帆, 姚佳霖, 王宝亮, 等. 黄芪及其有效成分治疗重症肌无力的作用机制研究进展[J]. 中华中医药学刊, 2025, 43(10): 171-5. |
| [201] | 柴梦音, 寇卜心, 豆双双, 等. 黄芪超微粉抗急性肝损伤、抗疲劳作用及黄芪甲苷含量的变化[J]. 现代中医药, 2022, 42(5): 26-32. |
| [21] | 吴 娇, 仝芳超. 黄芪的化学成分、药理作用及临床应用[J]. 滨州医学院学报, 2024, 47(1): 68-75. |
| [22] | Wu ZH, Yin B, You FM. Molecular mechanism of anti-colorectal cancer effect of Hedyotis diffusa willd and its extracts[J]. Front Pharmacol, 2022, 13: 820474. doi:10.3389/fphar.2022.820474 |
| [23] | Zhu DY, Yuan SM, Chen C. Hedyotis diffusa-Sculellaria barbata (HD-SB) suppresses the progression of colorectal cancer cells via the hsa_circ_0039933/hsa-miR-204-5p/wnt11 axis[J]. Sci Rep, 2023, 13(1): 13331. doi:10.1038/s41598-023-40393-1 |
| [24] | Yang ZP, Lu S, Tang HZ, et al. Molecular targets and mechanisms of Hedyotis diffusa- Scutellaria barbata herb pair for the treatment of colorectal cancer based on network pharmacology and molecular docking[J]. Evid Based Complement Alternat Med, 2022, 2022: 6186662. doi:10.1155/2022/6186662 |
| [25] | Yang SW, Chu SF, Gao Y, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis[J]. Cells, 2019, 8(7): 738. doi:10.3390/cells8070738 |
| [26] | Deng XH, Zhang SW, Wu JZ, et al. Promotion of mitochondrial biogenesis via activation of AMPK-PGC1ɑ signaling pathway by ginger (Zingiber officinale Roscoe) extract, and its major active component 6-gingerol[J]. J Food Sci, 2019, 84(8): 2101-11. doi:10.1111/1750-3841.14723 |
| [27] | Sun SN, Yang SR, Cheng Y, et al. Jinlida granules alleviate podocyte apoptosis and mitochondrial dysfunction via the AMPK/PGC-1α pathway in diabetic nephropathy[J]. Int J Mol Med, 2025, 55(2): 26. doi:10.3892/ijmm.2024.5467 |
| [28] | Fontana F, Macchi C, Anselmi M, et al. PGC1-α-driven mitochondrial biogenesis contributes to a cancer stem cell phenotype in melanoma[J]. Biochim Biophys Acta Mol Basis Dis, 2024, 1870(1): 166897. doi:10.1016/j.bbadis.2023.166897 |
| [29] | 苏东东, 靳庆瑞, 赵 梦, 等. 参兰颗粒通过AMPK/PGC-1α/NRF1介导的线粒体保护改善阿霉素性心脏毒性[J].中药药理与临床, 2025, 41(9): 2-9. |
| [30] | Yang XF, Xue PP, Chen HR, et al. Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis[J]. Theranostics, 2020, 10(3): 1415-32. doi:10.7150/thno.40857 |
| [31] | Piao LM, Huang Z, Inoue A, et al. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice[J]. Stem Cell Res Ther, 2022, 13(1): 226. doi:10.1186/s13287-022-02895-z |
| [32] | Cao YY, Wang Z, Yu T, et al. Sepsis induces muscle atrophy by inhibiting proliferation and promoting apoptosis via PLK1-AKT signalling[J]. J Cell Mol Med, 2021, 25(20): 9724-39. doi:10.1111/jcmm.16921 |
| [33] | Nguyen TT, Wei SB, Nguyen TH, et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease[J]. Exp Mol Med, 2023, 55(8): 1595-619. doi:10.1038/s12276-023-01046-5 |
| [34] | Gu J, Rauniyar S, Wang Y, et al. Chrysophanol induced glioma cells apoptosis via activation of mitochondrial apoptosis pathway[J]. Bioengineered, 2021, 12(1): 6855-68. doi:10.1080/21655979.2021.1972079 |
| [35] | Cosentino K, Hertlein V, Jenner A, et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation[J]. Mol Cell, 2022, 82(5): 933-49.e9. doi:10.1016/j.molcel.2022.01.008 |
| [36] | Zhang S, Rao SJ, Yang MW, et al. Role of mitochondrial pathways in cell apoptosis during He-patic ischemia/reperfusion injury[J]. Int J Mol Sci, 2022, 23(4): 2357. doi:10.3390/ijms23042357 |
| [37] | Wang HJ, Zhang CW, Li MN, et al. Antimicrobial peptides mediate apoptosis by changing mitochondrial membrane permeability[J]. Int J Mol Sci, 2022, 23(21): 12732. doi:10.3390/ijms232112732 |
| [38] | Flores-Romero H, Dadsena S, García-Sáez AJ. Mitochondrial pores at the crossroad between cell death and inflammatory signaling[J]. Mol Cell, 2023, 83(6): 843-56. doi:10.1016/j.molcel.2023.02.021 |
| [39] | Cetraro P, Plaza-Diaz J, MacKenzie A, et al. A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer[J]. Cancers (Basel), 2022, 14(7): 1671. doi:10.3390/cancers14071671 |
| [40] | Picca A, Calvani R, Coelho-Junior HJ, et al. Cell death and inflammation: the role of mitochondria in health and disease[J]. Cells, 2021, 10(3): 537. doi:10.3390/cells10030537 |
| [41] | Barroso T, Melo-Alvim C, Ribeiro LA, et al. Targeting inhibitor of apoptosis proteins to overcome chemotherapy resistance-a marriage between targeted therapy and cytotoxic chemotherapy[J]. Int J Mol Sci, 2023, 24(17): 13385. doi:10.3390/ijms241713385 |
| [1] | 马金苗, 顼志兵, 朱 杰, 刘迎庆, 王青青, 奚希相, 张莉芬, 黄 静, 孝玲玲. 附萸汤改善心力衰竭大鼠的心室重构:基于抑制AMPK/mTOR通路介导的细胞自噬[J]. 南方医科大学学报, 2023, 43(3): 466-473. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||