南方医科大学学报 ›› 2025, Vol. 45 ›› Issue (3): 577-586.doi: 10.12122/j.issn.1673-4254.2025.03.15
收稿日期:2024-09-10
出版日期:2025-03-20
发布日期:2025-03-28
通讯作者:
张学武
E-mail:13691216793@163.com;zhangxuewu@ybu.edu.cn
作者简介:李泽涵,在读本科生,E-mail: 13691216793@163.com
基金资助:
Zehan LI1( ), Meng LIANG2, Gencheng HAN2, Xuewu ZHANG1(
), Meng LIANG2, Gencheng HAN2, Xuewu ZHANG1( )
)
Received:2024-09-10
Online:2025-03-20
Published:2025-03-28
Contact:
Xuewu ZHANG
E-mail:13691216793@163.com;zhangxuewu@ybu.edu.cn
Supported by:摘要:
目的 探究菊淀粉型巴戟天寡糖(IOMO)对小鼠肺炎链球菌脑膜炎(SPM)的治疗作用及可能的作用机制。 方法 将120只雄性C57BL/6J小鼠随机分为假手术组、SPM+生理盐水(Saline)组、SPM+IOMO 25 mg/kg剂量组、SPM+IOMO 50 mg/kg剂量组,n=30。从造模当天起(0 d),SPM+Saline组和SPM+IOMO组小鼠分别灌胃生理盐水或IOMO 25 mg/kg、50 mg/kg进行干预,持续7 d,记录症状评分和死亡情况;采用脑组织HE染色和尼氏染色评价病理状态和神经元损伤情况,qRT-PCR检测皮质中炎症相关分子mRNA水平,干湿重法测脑含水量和伊文思蓝染色评价脑水肿程度和血脑屏障通透性,Western blotting检测皮质中BBB相关蛋白水平,流式细胞分析检测浸润淋巴细胞IFN-γ水平;SPM造模后21 d采用旷场实验和新物体识别实验评价小鼠学习记忆功能。 结果 灌胃给予IOMO 50 mg/kg(1次/d,连续7 d)可降低SPM小鼠的症状评分和死亡率(P<0.05),缓解脑组织病理损伤,降低皮质炎症相关分子(IL-6、TNF-α、IL-1β、IL-18、IFN-γ、iNOS、NLRP3、ASC、Caspase-1、GSDMD)mRNA水平(P<0.05); IOMO可以降低SPM小鼠脑含水量和伊文思蓝渗透量(P<0.05);IOMO可以上调脑皮质血管内皮钙黏蛋白VE-Cadherin和闭合蛋白Occludin水平、下调水通道蛋白AQP4和BBB损伤的关键调控因子iNOS、IFN-γ水平(P<0.05);建模21d后,与SPM + Saline组小鼠相比,IOMO可以改善SPM小鼠学习记忆能力(P<0.05)。 结论 首次发现IOMO可降低SPM小鼠的症状评分和死亡率,并改善其学习记忆能力,其作用机制可能与IOMO抑制过度炎症反应并保护血脑屏障有关。
李泽涵, 梁萌, 韩根成, 张学武. 菊淀粉型巴戟天寡糖降低肺炎链球菌脑膜炎小鼠的症状评分和死亡率[J]. 南方医科大学学报, 2025, 45(3): 577-586.
Zehan LI, Meng LIANG, Gencheng HAN, Xuewu ZHANG. Therapeutic effects of inulin-type oligosaccharides of Morinda officinalis on Streptococcus pneumoniae meningitis in mice[J]. Journal of Southern Medical University, 2025, 45(3): 577-586.
| Gene | Primer sequence (5'-3') |
|---|---|
| 18S | F:TTGACGGAAGGGCACCACCAG |
| R:GCACCACCACCACGGAATCG | |
| TNF-α | F:CCCTCACACTCAGATCATCTTCT |
| R:GCTACGACGTGGGCTACAG | |
| IL-6 | F:GATGGATGCTACCAAACTGGA |
| R:TCTGAAGGACTCTGGCTTTG | |
| IL-1β | F:TGAAGCAGCTATGGCAACTG |
| R:AGGTCAAAGGTTTGGAAGCA | |
| IL-18 | F:CCAAATCACTTCCTCTTGGC |
| R:GGCCAAAGTTGTCTGATTCC | |
| IFN-γ | F:AAGCGTCATTGAATCACACCTG |
| R:TGACCTCAAACTTGGCAATACTC | |
| iNOS | F:GTTCTCAGCCCAACAATACAAGA |
| R:GTGGACGGGTCGATGTCAC | |
| NLRP3 | F:ATTACCCGCCCGAGAAAGG |
| R:TCGCAGCAAAGATCCACACAG | |
| ASC | F:CTTGTCAGGGGATGAACTCAAAA |
| R:GCCATACGACTCCAGATAGTAGC | |
| Caspase-1 | F:ACAAGGCACGGGACCTATG |
| R:TCCCAGTCAGTCCTGGAAATG | |
| GSDMD | F:CCATCGGCCTTTGAGAAAGTG |
| R:ACACATGAATAACGGGGTTTCC |
表1 qRT-PCR引物序列
Tab.1 Primer sequence used in qRT-PCR
| Gene | Primer sequence (5'-3') |
|---|---|
| 18S | F:TTGACGGAAGGGCACCACCAG |
| R:GCACCACCACCACGGAATCG | |
| TNF-α | F:CCCTCACACTCAGATCATCTTCT |
| R:GCTACGACGTGGGCTACAG | |
| IL-6 | F:GATGGATGCTACCAAACTGGA |
| R:TCTGAAGGACTCTGGCTTTG | |
| IL-1β | F:TGAAGCAGCTATGGCAACTG |
| R:AGGTCAAAGGTTTGGAAGCA | |
| IL-18 | F:CCAAATCACTTCCTCTTGGC |
| R:GGCCAAAGTTGTCTGATTCC | |
| IFN-γ | F:AAGCGTCATTGAATCACACCTG |
| R:TGACCTCAAACTTGGCAATACTC | |
| iNOS | F:GTTCTCAGCCCAACAATACAAGA |
| R:GTGGACGGGTCGATGTCAC | |
| NLRP3 | F:ATTACCCGCCCGAGAAAGG |
| R:TCGCAGCAAAGATCCACACAG | |
| ASC | F:CTTGTCAGGGGATGAACTCAAAA |
| R:GCCATACGACTCCAGATAGTAGC | |
| Caspase-1 | F:ACAAGGCACGGGACCTATG |
| R:TCCCAGTCAGTCCTGGAAATG | |
| GSDMD | F:CCATCGGCCTTTGAGAAAGTG |
| R:ACACATGAATAACGGGGTTTCC |
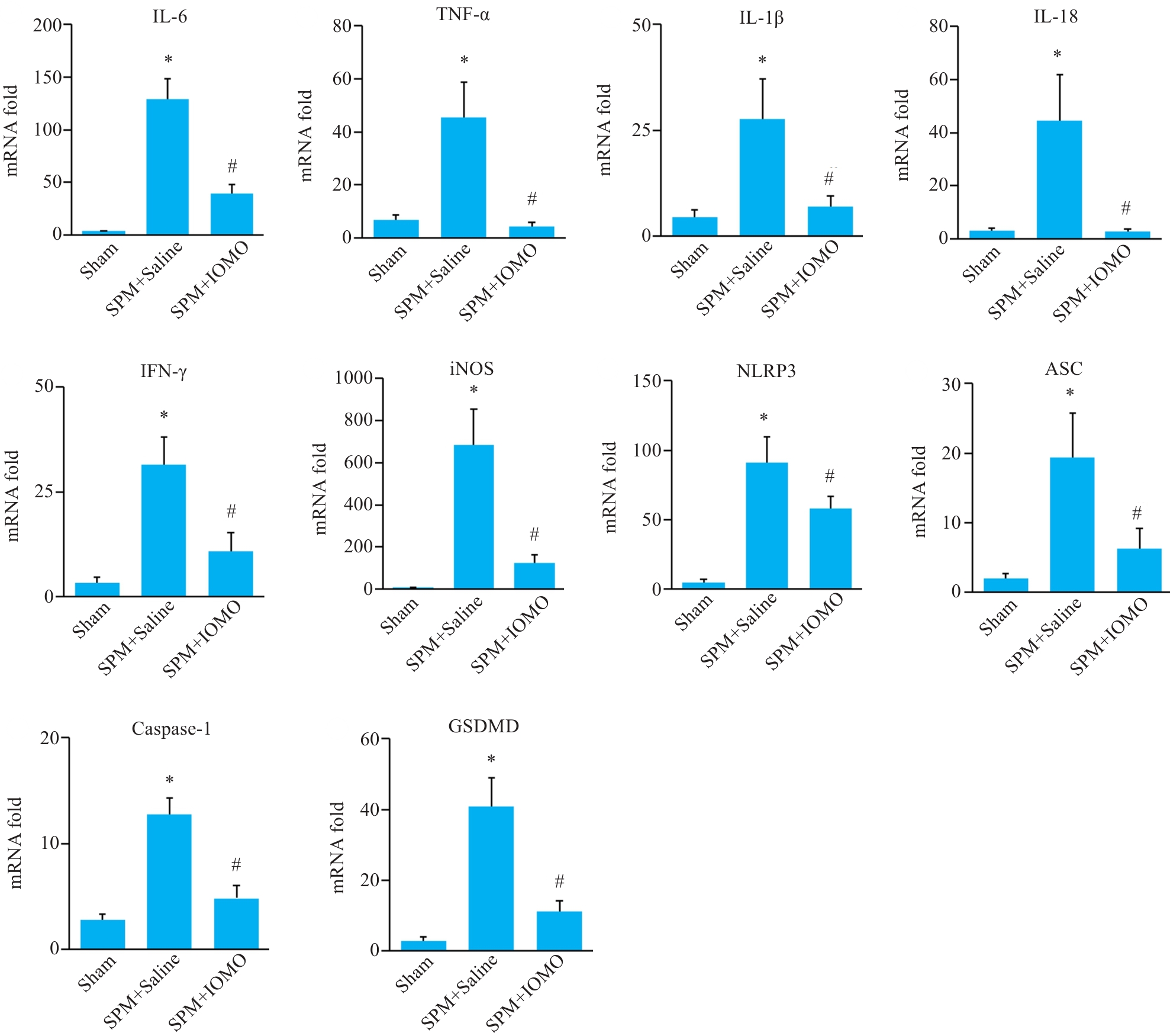
图5 IOMO对SPM小鼠脑皮质组织炎症相关分子mRNA水平的影响
Fig.5 Effects of IOMO on mRNA levels of inflammation-related molecules mRNA levels in the cerebral cortex of SPM mice (Mean±SD, n=4). *P<0.05 vs Sham group, #P<0.05 vs SPM+Saline group.
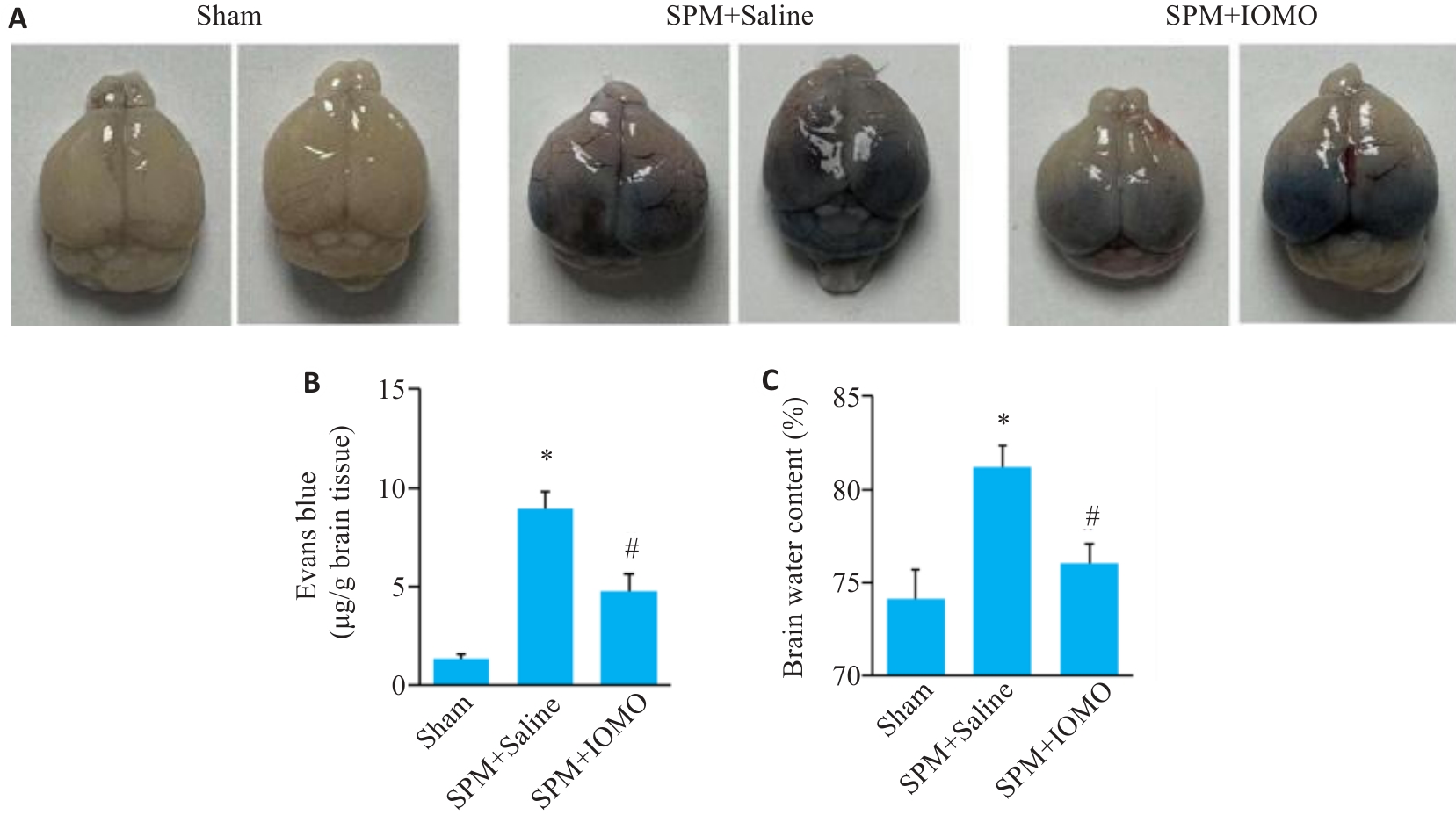
图6 IOMO对SPM小鼠脑组织含水量和BBB通透性的影响
Fig.6 Effects of IOMO on brain water content and BBB permeability of SPM mice (Mean±SD, n=6). A: Evans blue (EB) staining. B: Statistics of the results of EB staining. C: Statistics of brain water content. *P<0.05 vs Sham group,#P<0.05 vs SPM+Saline group.
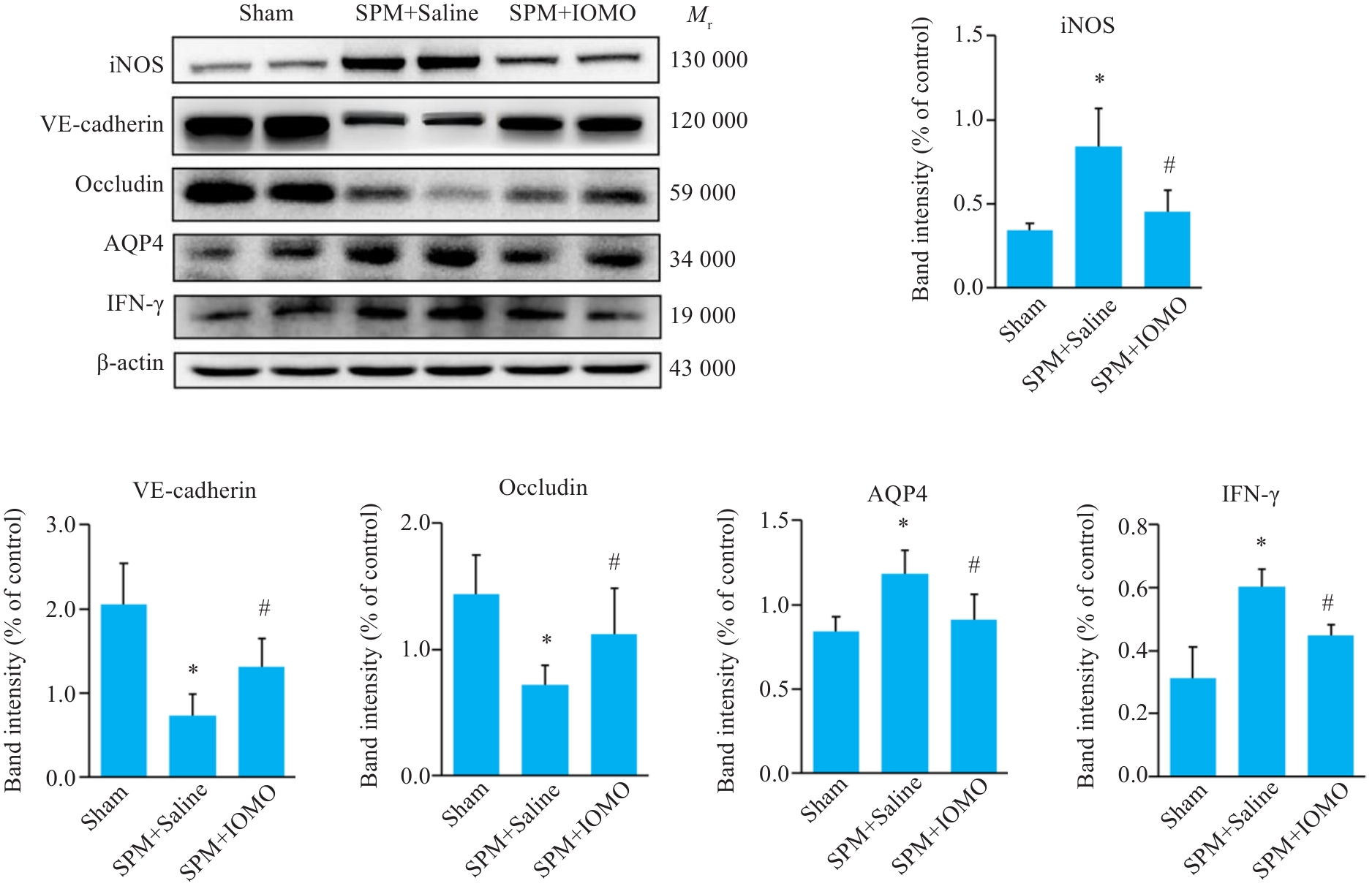
图7 IOMO对SPM小鼠BBB通透性相关分子表达水平的影响
Fig.7 Effects of IOMO on BBB permeability-related molecular levels in SPM mice (Mean±SD, n=6). *P<0.05 vs Sham group,#P<0.05 vs SPM+Saline group.
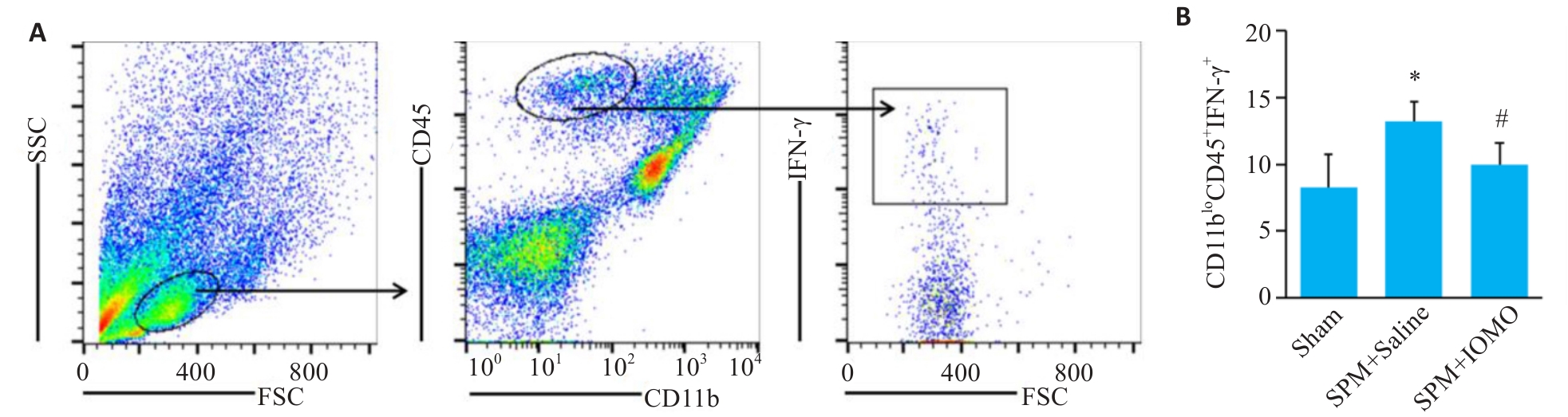
图8 脑组织浸润淋巴细胞IFN-γ水平检测
Fig.8 Assessment of IFN-γ levels in the infiltrating lymphocytes (Mean±SD, n=4). *P<0.05 vs Sham group,#P<0.05 vs SPM+Saline group.
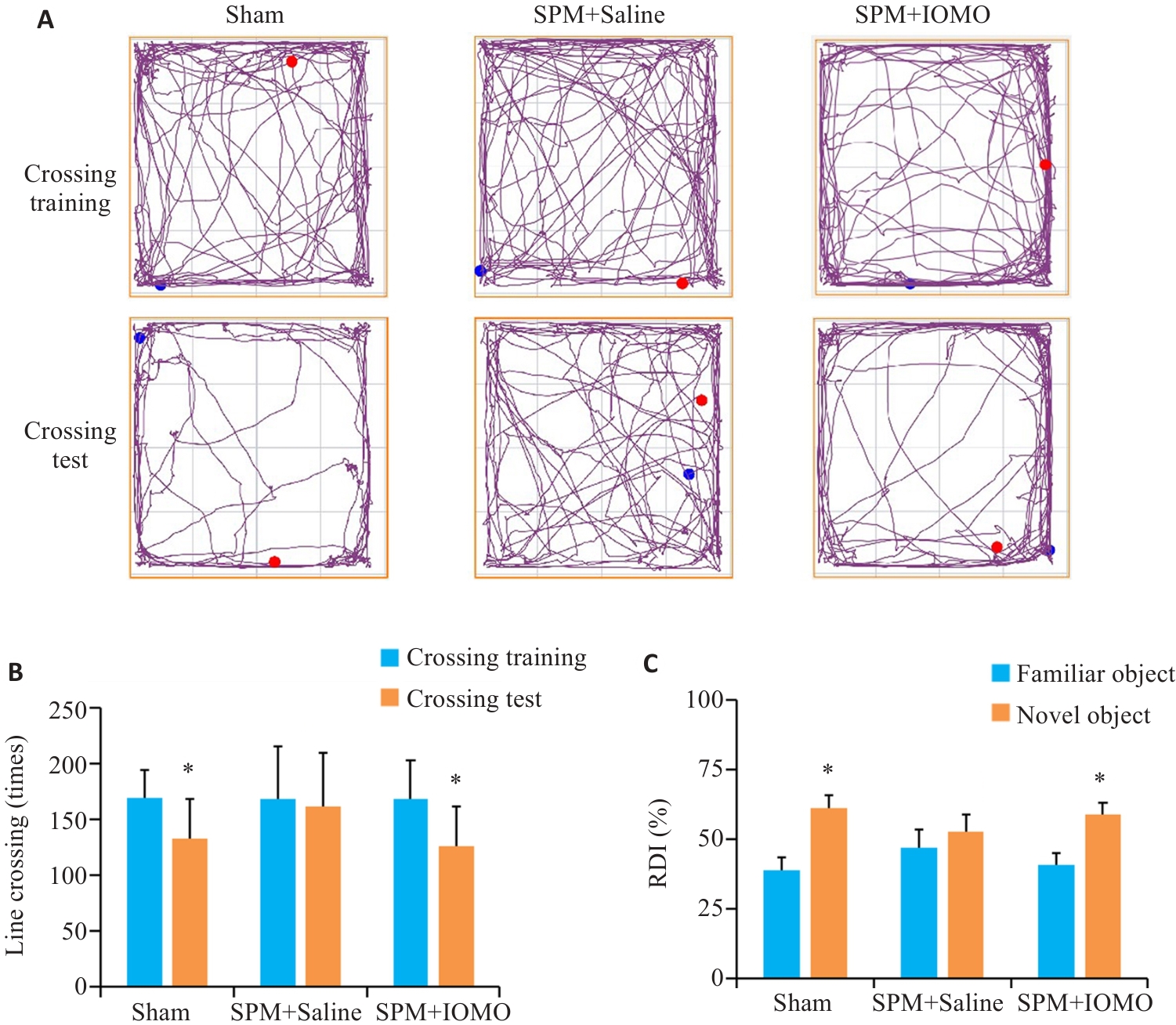
图9 IOMO缓解SPM引起的小鼠学习记忆功能障碍
Fig.9 IOMO alleviates SPM-induced learning and memory dysfunction in mice (Mean±SD, n=8). A, B: Traveling traces and statistics in open field test (*P<0.05 vs Crossing Training). C: Statistics of the results of new object recognition test (*P<0.05 vs familiar group).
| 1 | Glimåker M, Naucler P, Sjölin J. Etiology, clinical presentation, outcome and the effect of initial management in immuno-compromised patients with community acquired bacterial meningitis[J]. J Infect, 2020, 80(3): 291-7. |
| 2 | Mook-Kanamori BB, Geldhoff M, van der Poll T, et al. Pathogenesis and pathophysiology of pneumococcal meningitis[J]. Clin Microbiol Rev, 2011, 24(3): 557-91. |
| 3 | Gil E, Wall E, Noursadeghi M, et al. Streptococcus pneumoniae meningitis and the CNS barriers[J]. Front Cell Infect Microbiol, 2023, 12: 1106596. |
| 4 | Costerus JM, Brouwer MC, Bijlsma MW, et al. Community-acquired bacterial meningitis[J]. Curr Opin Infect Dis, 2017, 30(1): 135-41. |
| 5 | Gao ZQ, Song YJ, Hsiao TY, et al. Machine-learning-assisted microfluidic nanoplasmonic digital immunoassay for cytokine storm profiling in COVID-19 patients[J]. ACS Nano, 2021, 15(11): 18023-36. |
| 6 | Huang K, Zhang P, Zhang ZH, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms[J]. Pharmacol Ther, 2021, 225: 107843. |
| 7 | Zhang JH, Xin HL, Xu YM, et al. Morinda officinalis How.-A comprehensive review of traditional uses, phytochemistry and pharmacology[J]. J Ethnopharmacol, 2018, 213: 230-55. |
| 8 | Wang YH, Yin YY, Yao JQ, et al. Behavioral evaluation of anti-post-traumatic stress disorder effect of inulin-type oligosaccharides of Morinda officinalis[J]. Chin J Pharmacol Toxicol. 2021, 35(1): 27-35. |
| 9 | Zhang HL, Li J, Xia JM, et al. Antioxidant activity and physicochemical properties of an acidic polysaccharide from Morinda officinalis [J]. Int J Biol Macromol, 2013, 58: 7-12. |
| 10 | Shin JS, Yun KJ, Chung KS, et al. Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-κB inactivation[J]. Food Chem Toxicol, 2013, 53: 263-71. |
| 11 | Li ZF, Xu HX, Xu Y, et al. Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation[J]. CNS Neurosci Ther, 2021, 27(12): 1570-86. |
| 12 | Liang J, Liang JW, Hao HR, et al. The extracts of Morinda officinalis and its hairy roots attenuate dextran sodium sulfate-induced chronic ulcerative colitis in mice by regulating inflammation and lymphocyte apoptosis[J]. Front Immunol, 2017, 8: 905. |
| 13 | Yau B, Mitchell AJ, Too LK, et al. Interferon-γ-induced nitric oxide synthase-2 contributes to blood/brain barrier dysfunction and acute mortality in experimental Streptococcus pneumoniae meningitis[J]. J Interferon Cytokine Res, 2016, 36(2): 86-99. |
| 14 | Koopmans MM, Engelen-Lee J, Brouwer MC, et al. Characterization of a Listeria monocytogenes meningitis mouse model[J]. J Neuroinflammation, 2018, 15(1): 257. |
| 15 | Li G, Tang LL, Hou CM, et al. Peripheral injection of tim-3 antibody attenuates VSV encephalitis by enhancing MHC-I presentation[J]. Front Immunol, 2021, 12: 667478. |
| 16 | Waltl I, Käufer C, Gerhauser I, et al. Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage[J]. Brain Behav Immun, 2018, 74: 186-204. |
| 17 | Gao ZF, Xie S, Wang LY, et al. Hypidone hydrochloride (YL-0919) protects mice from meningitis via Sigma1R-STAT1-NLRP3-GSDMD pathway[J]. Int Immunopharmacol, 2024, 128: 111524. |
| 18 | Yau B, Hunt NH, Mitchell AJ, et al. Blood-brain barrier pathology and CNS outcomes in Streptococcus pneumoniae meningitis[J]. Int J Mol Sci, 2018, 19(11): 3555. |
| 19 | van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis[J]. Clin Microbiol Infect, 2016, 22(): S37-62. |
| 20 | Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis[J]. Cochrane Database Syst Rev, 2015, 2015(9): CD004405. |
| 21 | 钟 亮, 林如琴, 龙北国, 等. 鼠李糖乳杆菌抑制阪崎克罗诺杆菌致脑膜炎的体内外实验[J]. 南方医科大学学报, 2015, 35(8): 1079-83. DOI: 10.3969/j.issn.1673-4254.2015.08.001 |
| 22 | Tunkel AR, Michael Scheld W. Corticosteroids for everyone with meningitis[J]? N Engl J Med, 2002, 347(20): 1613-5. |
| 23 | Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy[J]. Trends Neurosci, 2002, 25(1): 38-45. |
| 24 | 王 轶, 郭婷婷, 尹勇玉, 等. 菊淀粉型巴戟天寡糖对慢性应激模型小鼠抑郁、焦虑样行为的改善及对调节性T细胞数量的调节作用[J]. 中国药理学与毒理学杂志, 2022, 36(6): 426-34. DOI: 10.3867/j.issn.1000-3002.2022.06.004 |
| 25 | Yang X, Chen DL, Yang J, et al. Effects of oligosaccharides from Morinda officinalis on gut microbiota and metabolome of APP/PS1 transgenic mice[J]. Front Neurol, 2018, 9: 412. |
| 26 | 孔庆梅, 舒 良, 张鸿燕, 等. 巴戟天寡糖胶囊治疗抑郁症的临床疗效与安全性[J]. 中国临床药理学杂志, 2011, 27(3): 170-3. |
| 27 | 呼亚玲. 巴戟天寡糖胶囊治疗轻中度抑郁症急性发作期的临床研究[J]. 中西医结合心脑血管病杂志, 2018, 16(7): 970-1, 992. |
| 28 | 李囿松, 呼亚玲, 申 静, 等. 巴戟天寡糖胶囊治疗广泛人群下急性发作期轻中度抑郁症的Ⅳ期临床研究[J]. 中药药理与临床, 2022, 38(4): 136-9. |
| 29 | Sharief MK, Ciardi M, Thompson EJ. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-alpha but not interleukin-1 beta[J]. J Infect Dis, 1992, 166(2): 350-8. |
| 30 | Barichello T, Generoso JS, Simões LR, et al. Interleukin-1β receptor antagonism prevents cognitive impairment following experimental bacterial meningitis[J]. Curr Neurovasc Res, 2015, 12(3): 253-61. |
| 31 | Klein RS, Hunter CA. Protective and pathological immunity during central nervous system infections[J]. Immunity, 2017, 46(6): 891-909. |
| 32 | Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis[J]. J Biol Chem, 2005, 280(14): 13906-12. |
| 33 | Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease[J]. Pharmacol Rev, 2005, 57(2): 173-85. |
| 34 | Barichello T, Generoso JS, Simões LR, et al. Role of oxidative stress in the pathophysiology of pneumococcal meningitis[J]. Oxid Med Cell Longev, 2013, 2013: 371465. |
| 35 | Meli DN, Christen S, Leib SL. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway[J]. J Infect Dis, 2003, 187(9): 1411-5. |
| 36 | Braun JS, Hoffmann O, Schickhaus M, et al. Pneumolysin causes neuronal cell death through mitochondrial damage[J]. Infect Immun, 2007, 75(9): 4245-54. |
| 37 | Hupp S, Heimeroth V, Wippel C, et al. Astrocytic tissue remodeling by the meningitis neurotoxin pneumolysin facilitates pathogen tissue penetration and produces interstitial brain edema[J]. Glia, 2012, 60(1): 137-46. |
| 38 | Coutinho LG, Grandgirard D, Leib SL, et al. Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis[J]. BMC Infect Dis, 2013, 13: 326. |
| 39 | Hoegen T, Tremel N, Klein M, et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release[J]. J Immunol, 2011, 187(10): 5440-51. |
| 40 | Häusler KG, Prinz M, Nolte C, et al. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages[J]. Eur J Neurosci, 2002, 16(11): 2113-22. |
| 41 | Too LK, Ball HJ, McGregor IS, et al. The pro-inflammatory cytokine interferon-gamma is an important driver of neuropathology and behavioural sequelae in experimental pneumococcal meningitis[J]. Brain Behav Immun, 2014, 40: 252-68. |
| 42 | Liu CY, Zhou F, Pan DY, et al. Study on effect of melatonin on protection of brains of mice with Cryptococcus neoformans meningitis[J]. Chin J Nosocomiol, 2020, 30(7): 961-966. |
| 43 | Winkler F, Koedel U, Kastenbauer S, et al. Differential expression of nitric oxide synthases in bacterial meningitis: role of the inducible isoform for blood-brain barrier breakdown[J]. J Infect Dis, 2001, 183(12): 1749-59. |
| 44 | Chi LD, Chen LX, Zhang JW, et al. Development and application of bio-sample quantification to evaluate stability and pharmacokinetics of inulin-type fructo-oligosaccharides from Morinda Officinalis[J]. J Pharm Biomed Anal, 2018, 156: 125-32. |
| 45 | Zhang ZW, Gao CS, Zhang H, et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota[J]. Acta Pharm Sin B, 2022, 12(8): 3298-312. |
| 46 | 李新翼, 刘玉杰, 邓克崇, 等. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-10. |
| [1] | 王静娴, 任自敬, 周佩洋. S1PR5激动与过表达通过调控氧化应激增强脑微血管内皮细胞屏障功能抵抗氧糖剥夺/复氧复糖损伤[J]. 南方医科大学学报, 2025, 45(7): 1451-1459. |
| [2] | 赵倩, 张振, 周星琦, 荣翔宇, 刘雪柔, 赵新丽, 王豪杰, 庞金龙, 李姗姗, 李娴. 川芎增强替莫唑胺对小鼠脑转移黑色素瘤脑的抑制作用[J]. 南方医科大学学报, 2024, 44(6): 1088-1097. |
| [3] | 许光明, 高安迪, 丛 斌. 束缚应激通过激活Rho/ROCK通路诱导大鼠杏仁核血脑屏障的损伤[J]. 南方医科大学学报, 2024, 44(3): 411-419. |
| [4] | 李新翼, 刘玉杰, 邓克崇, 胡义奎. 调节肠道菌群可改善卒中后大鼠的神经功能和抑郁症状[J]. 南方医科大学学报, 2024, 44(2): 405-410. |
| [5] | 牛雯雯, 荣翔宇, 赵 倩, 刘雪柔, 徐廉松, 李姗姗, 李 娴. 酒川芎增强阿美替尼对EGFR突变型非小细胞肺癌脑移植瘤的抑制作用[J]. 南方医科大学学报, 2023, 43(3): 375-382. |
| [6] | 李 晒, 李 丽, 闵思敏, 刘赛赛, 秦志文, 熊志尚, 徐建国, 王博文, 丁渡山, 赵士弟. 大豆异黄酮可减轻大鼠脑缺血/再灌注损伤:基于抑制铁死亡及炎症级联反应[J]. 南方医科大学学报, 2023, 43(2): 323-330. |
| [7] | 李 帆, 朱艳艳, 孙晓明, 胡慧娟, 周苗苗, 白怡雪, 胡 浩. 邻苯二甲酸二乙基己酯可能通过损伤血脑屏障诱导小鼠焦虑样行为和学习记忆障碍[J]. 南方医科大学学报, 2022, 42(8): 1237-1243. |
| [8] | 袁 巧, 谢丽英, 陈朝俊. 黄芪多糖减轻大脑中动脉闭塞模型大鼠的血脑屏障损伤:基于抑制P2X7R通道[J]. 南方医科大学学报, 2022, 42(11): 1705-1711. |
| [9] | 李 静, 郑 晶, 王敏达, 张 艳, 江益凡, 张小凤, 郭 普. 抑制CD96可促进NK细胞分泌IFN-γ并减轻小鼠肺部的衣原体感染[J]. 南方医科大学学报, 2020, 40(07): 930-935. |
| [10] | 李杰,闫堃,杨屹,李华,王志东,徐心. 白介素-17通过拮抗γ-干扰素的作用促进小鼠肝癌细胞的生长与增殖[J]. 南方医科大学学报, 2019, 39(01): 1-. |
| [11] | 吴碧娟,周杏子,孙婧雯,谭翠雯,吴新荣. 生物素靶向显影探针Biotin-S-S-Rhodol对乳腺癌细胞的靶向显影作用[J]. 南方医科大学学报, 2017, 37(01): 124-. |
| [12] | 曾庆,何肖龙,肖汉森,杜蕾,李雨静,陈乐程,田慧文,黄胜和,曹虹. 鼠李糖乳杆菌培养上清液通过抑制NF-κB通路预防大肠杆菌性脑膜炎[J]. 南方医科大学学报, 2017, 37(01): 24-. |
| [13] | 黄健,黄美容,吴凯峰,朱杰华,黎兵,闵迅. spd1672基因敲除显著影响肺炎链球菌毒力[J]. 南方医科大学学报, 2015, 35(08): 1197-. |
| [14] | 钟亮,林如琴,龙北国,吴娴波,范宏英. 鼠李糖乳杆菌抑制阪崎克罗诺杆菌致脑膜炎的体内外实验[J]. 南方医科大学学报, 2015, 35(08): 1079-. |
| [15] | 张立科,邱嘉文,梁晓路,黄宝怡,李妍,杜蕾,龙敏,罗军,黄胜和,曹虹. CD44在新生隐球菌体外感染血脑屏障中对单核细胞迁移的影响[J]. 南方医科大学学报, 2015, 35(04): 468-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||