南方医科大学学报 ›› 2024, Vol. 44 ›› Issue (7): 1370-1381.doi: 10.12122/j.issn.1673-4254.2024.07.17
• • 上一篇
收稿日期:2024-04-16
出版日期:2024-07-20
发布日期:2024-07-25
通讯作者:
谢海棠
E-mail:wanghongzhe66@163.com;15104758818@163.com
作者简介:王洪哲,主治医师,E-mail: wanghongzhe66@163.com
基金资助:
Hongzhe WANG1( ), Haitang XIE1(
), Haitang XIE1( ), Wulan XU1, Ming Li2
), Wulan XU1, Ming Li2
Received:2024-04-16
Online:2024-07-20
Published:2024-07-25
Contact:
Haitang XIE
E-mail:wanghongzhe66@163.com;15104758818@163.com
摘要:
目的 探讨尿石素A(UA)治疗呼吸道合胞病毒(RSV)引起的新生小鼠肺部感染的疗效及作用机制。 方法 将Babl/c小鼠(5~7 d)随机分为5组(n=10):正常对照(Control)组、RSV感染(RSV)组、低剂量UA(UA-L)组、中剂量UA(UA-M)组、高剂量UA(UA-H)组。BEAS-2B细胞同样分为Control组、RSV组、UA-L组、UA-M组和UA-H组。除正常对照组外,其余4组小鼠或细胞均给予RSV感染。RSV感染2 h后,UA-L组、UA-M组和UA-H组小鼠分别腹腔注射2.5、5和10 mg/kg的UA,1次/d,连续注射2周;UA-L组、UA-M组和UA-H组细胞分别用2.5、5和10 µmol/L的UA处理48 h。HE染色评价肺组织病理学改变。收集支气管肺泡灌洗液用于炎症细胞计数和炎症因子检测。炎症、细胞活力、凋亡和自噬分别采用酶联免疫吸附实验、CCK-8实验、末端脱氧核苷酸转移酶介导的dUTP缺口末端标记实验、流式细胞术、Western blotting和免疫荧光染色检测。qRT-PCR检测miR-136和Sirt1 mRNA的表达,双荧光素酶报告基因系统验证miR-136和Sirt1的相互关系。 结果 与Control组相比,RSV感染组小鼠肺组织病理评分、病毒荷载量、TUNEL阳性细胞数、LC3-II/I、Beclin-1和miR-136表达及BALF中总细胞数、炎症细胞数和炎症因子含量显著升高(P<0.0001),而p62和Sirt1表达显著减少(P<0.0001);与RSV组相比,UA处理则呈剂量依赖性逆转上述检测指标值(P<0.05)。与Control组相比,RSV组BEAS-2B凋亡细胞数、LC3B阳性细胞数及miR-136表达明显增多,Sirt1表达减少(P<0.01);与RSV组相比,UA处理剂量依赖性地减弱上述指标的改变(P<0.01)。与miR-NC组相比,miR-136组Sirt1-WT的荧光素酶活性显著降低,Sirt1表达减少(P<0.001),Sirt1-MUT的荧光素酶活性不变(P>0.05)。与RSV+UA组相比,RSV+UA+miR-136组和RSV+UA+Ex527组炎症因子含量和凋亡细胞数明显增加(P<0.05),LC3B表达显著减少(P<0.0001);miR-136与Ex527共处理进一步改变上述指标值(P<0.05)。 结论 UA通过活化miR-136介导的Sirt1信号通路减轻RSV诱导的新生小鼠肺部感染。
王洪哲, 谢海棠, 徐乌兰, 李明. 尿石素A通过活化miR-136介导的Sirt1信号通路减轻呼吸道合胞病毒诱导的新生小鼠肺部感染[J]. 南方医科大学学报, 2024, 44(7): 1370-1381.
Hongzhe WANG, Haitang XIE, Wulan XU, Ming Li. Urolithin A alleviates respiratory syncytial virus-induced lung infection in neonatal mice by activating miR-136-mediated Sirt1 signaling[J]. Journal of Southern Medical University, 2024, 44(7): 1370-1381.
| Gene | Primer sequence(5′-3′) |
|---|---|
| mmu-miR-136 | Forward:5'-ACACTCCAGCTGGGACTCCATTTGTTTTGATGA-3' |
| Reverse:5'-CTCAACTGGTGTCGTGGA-3' | |
| hsa-miR-136 | Forward:5'-GCGCACTCCATTTGTTTTGAT-3' |
| Reverse:5'-GTGCAGGGTCCGAGGT-3' | |
| mmu-U6 | Forward:5'-CTCGCTTCGGCAGCACA-3' |
| Reverse:5'-AACGCTTCACGAATTTGCGT-3' | |
| hsa-U6 | Forward:5'-AAAGCAAATCATCGGACGACC-3' |
| Reverse:5'-GTACAACACATTGTTTCCTCGGA-3' | |
| mmu-Sirt1 | Forward:5'-GACGCTGTGGCAGATTGTTA-3' |
| Reverse:5'-GGAATCCCACAGGAGACAGA-3' | |
| hsa-Sirt1 | Forward:5'-TGCCGGAAACAATACCTCCA-3' |
| Reverse:5'-AGACACCCCAGCTCCAGTTA-3' | |
| mmu-GAPDH | Forward:5'-GGCCTCCAAGGAGTAAGAAA-3' |
| Reverse:5'-GCCCCTCCTGTTATTATGG-3' | |
| hsa-GAPDH | Forward:5'-TGTGGGCATCAATGGATTTGG-3' |
| Reverse:5'-ACACCATGTATTCCGGGTCAAT-3' |
表1 qRT-PCR引物序列
Tab.1 Primer sequences for qRT-PCR
| Gene | Primer sequence(5′-3′) |
|---|---|
| mmu-miR-136 | Forward:5'-ACACTCCAGCTGGGACTCCATTTGTTTTGATGA-3' |
| Reverse:5'-CTCAACTGGTGTCGTGGA-3' | |
| hsa-miR-136 | Forward:5'-GCGCACTCCATTTGTTTTGAT-3' |
| Reverse:5'-GTGCAGGGTCCGAGGT-3' | |
| mmu-U6 | Forward:5'-CTCGCTTCGGCAGCACA-3' |
| Reverse:5'-AACGCTTCACGAATTTGCGT-3' | |
| hsa-U6 | Forward:5'-AAAGCAAATCATCGGACGACC-3' |
| Reverse:5'-GTACAACACATTGTTTCCTCGGA-3' | |
| mmu-Sirt1 | Forward:5'-GACGCTGTGGCAGATTGTTA-3' |
| Reverse:5'-GGAATCCCACAGGAGACAGA-3' | |
| hsa-Sirt1 | Forward:5'-TGCCGGAAACAATACCTCCA-3' |
| Reverse:5'-AGACACCCCAGCTCCAGTTA-3' | |
| mmu-GAPDH | Forward:5'-GGCCTCCAAGGAGTAAGAAA-3' |
| Reverse:5'-GCCCCTCCTGTTATTATGG-3' | |
| hsa-GAPDH | Forward:5'-TGTGGGCATCAATGGATTTGG-3' |
| Reverse:5'-ACACCATGTATTCCGGGTCAAT-3' |
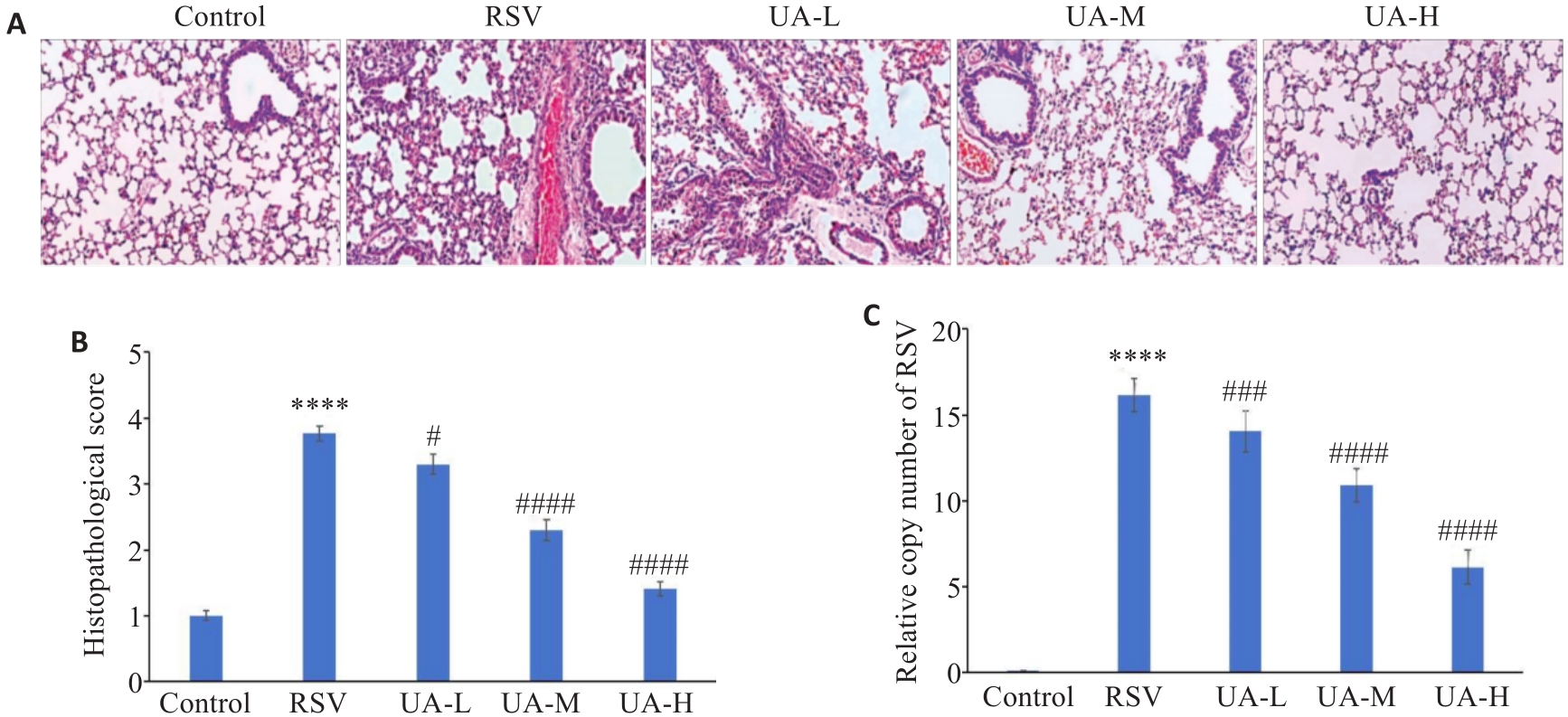
图1 UA减轻RSV诱导的小鼠肺损伤
Fig.1 UA alleviates RSV-induced lung injury in neonatal mice. A: HE staining showing lung tissue injury in the mice (Original magnification: ×200). B: Pathological score based on HE staining results. C: Quantification of the relative copy number of RSV in mouse lung tissues. ****P<0.0001 vs Control group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

图2 UA降低RSV诱导的小鼠肺部炎症反应
Fig.2 UA reduces RSV-induced lung inflammation in neonatal mice. A-D: Total cell (A), lymphocyte (B), macrophage (C) and neutrophil (D) counts in mouse BALF. E-L: Contents of TNF-α (E), IL-1β (F), IL-4 (G), IL-5 (H), IL-6 (I), IL-13 (J), IFN-γ (K) and CXCL1 (L) in lung tissues of mice detected by ELISA. ****P<0.0001 vs Control group; #P<0.05, ##P<0.01, ####P<0.0001 vs RSV group.

图3 UA抑制RSV诱导的细胞凋亡
Fig.3 UA treatment inhibits RSV-induced apoptosis in BEAS-2B cells. A: CCK-8 assay for assessing viability of BEAS-2B cells treated with 2.5, 5, 10, 20 and 40 µmol/L UA for 24 or 48 h. B: Flow cytometry for analyzing apoptosis of BEAS-2B cells infected with RSV for 2 h followed by treatment with 2.5, 5 or 10 µmol/L UA for 48 h. C: Quantification of the percentage of apoptotic cells. D: TUNEL assay for detecting cell apoptosis in the lung tissues of the mice (400×). E: Number of TUNEL-positive cells. *P<0.05, **P<0.01, ****P<0.0001 vs Control group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

图4 UA促进RSV感染诱导的自噬
Fig.4 UA promotes RSV infection-induced autophagy in BEAS-2B cells. A: Western blotting for detecting LC3-II/I, p62, and Beclin-1 expressions in lung tissues of mice. B-D: Quantification of LC3-II/I (B), p62 (C), and Beclin-1 (D) expressions. E, F: Immunofluorescence assay for detecting LC3B expression in BEAS-2B cells infected with RSV for 2 h followed by treatment with UA (2.5, 5 and 10 µmol/L) for 48 h (×400). ****P<0.0001 vs Control group; #P<0.05, ####P<0.0001 vs RSV group.

图5 UA通过抑制miR-136增加Sirt1的表达
Fig.5 UA increases Sirt1 expression by inhibiting miR-136 in RSV-infected BEAS-2B cells. A: Expressions of miR-136 in lung tissues of mice and BEAS-2B cells detected by qRT-PCR. B, C: Sirt1 mRNA (B) and protein (C) levels analyzed by qRT-PCR and Western blotting. D: Prediction of the binding site of miR-136 to 3'-UTR of Sirt1 mRNA using miRanda, targetScan and RNAInter bioinformatics databases. E: Dual-luciferase reporter assay of BEAS-2B cells co-transfected with miR-136 (or miR-NC) and Sirt1-WT or Sirt1-MUT. F-H: Sirt1 mRNA and protein expressions determined by qRT-PCR (F) and Western blotting (G, H) analysis in BEAS-2B cells transfected with miR-136 or miR-NC. I: Western blotting for detecting protein expressions of Sirt1 in RSV (100TCID50)-infected BEAS-2B cells treated with 10 µmol/L UA, transfected with miR-136, or both. ***P<0.001, ****P<0.0001 vs Control or miR-NC group; #P<0.05, ###P<0.001, ####P<0.0001 vs RSV group.

图6 UA通过活化miR-136介导的Sirt1信号通路抑制RSV诱导的炎症和凋亡并促进自噬
Fig.6 UA inhibits RSV-induced inflammation and apoptosis and promotes autophagy in BEAS-2B cells by activating miR-136-mediated Sirt1 signaling pathway. A-H: ELISA measurement of contents of TNF-α (A), IL-1β (B), IL-4 (C), IL-5 (D), IL-6 (E), IL-13 (F), IFN-γ (G) and CXCL1 (H) in RSV (100TCID50)-infected BEAS-2B cells treated with 10 µmol/L UA, UA+miR-136, UA+Sirt1 inhibitor (Ex527), or UA+miR-136+Ex527. I: Cell apoptosis assessed by flow cytometry. J: Percentage of apoptotic cells. K: Immunofluorescence staining for detecting expression of LC3B (×400). L: Quantification of LC3B-positive cells. **P<0.01, ****P<0.0001 vs Control group; ####P<0.0001 vs RSV group; &P<0.05, &&P<0.01, &&&P<0.001, &&&&P<0.0001 vs RSV+UA group; $P<0.05, $$P<0.01, $$$P<0.001, $$$$P<0.0001 vs RSV+UA+miR-136 or RSV+UA+Ex527 group.
| 1 | Jain H, Schweitzer JW, Justice NA. Respiratory syncytial virus infection in children[M]. StatPearls, 2023. |
| 2 | Toivonen L, Karppinen S, Schuez-Havupalo L, et al. Respiratory syncytial virus infections in children 0-24 months of age in the community[J]. J Infect, 2020, 80(1): 69-75. |
| 3 | Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children[J]. J Allergy Clin Immunol, 2017, 140(4): 895-906. |
| 4 | Jartti T, Bønnelykke K, Elenius V, et al. Role of viruses in asthma[J]. Semin Immunopathol, 2020, 42(1): 61-74. |
| 5 | Kwon YS, Park SH, Kim MA, et al. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults[J]. BMC Infect Dis, 2017, 17(1): 785. |
| 6 | Kuo CH, Tsai ML, Li CH, et al. Altered pattern of macrophage polarization as a biomarker for severity of childhood asthma[J]. J Inflamm Res, 2021, 14: 6011-23. |
| 7 | Vasconcelos LHC, Ferreira SRD, Silva MDCC, et al. Uncovering the role of oxidative imbalance in the development and progression of bronchial asthma[J]. Oxid Med Cell Longev, 2021, 2021: 6692110. |
| 8 | Carande EJ, Pollard AJ, Drysdale SB. Management of respiratory syncytial virus bronchiolitis: 2015 survey of members of the European society for paediatric infectious diseases[J]. J Can Des Mal Infect De La Microbiol Med, 2016, 2016: 9139537. |
| 9 | Cerdá B, Periago P, Espín JC, et al. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds[J]. J Agric Food Chem, 2005, 53(14): 5571-6. |
| 10 | D'Amico D, Andreux PA, Valdés P, et al. Impact of the natural compound urolithin A on health, disease, and aging[J]. Trends Mol Med, 2021, 27(7): 687-99. |
| 11 | Lou LJ, Wang M, He JJ, et al. Urolithin A (UA) attenuates ferroptosis in LPS-induced acute lung injury in mice by upregulating Keap1-Nrf2/HO-1 signaling pathway[J]. Front Pharmacol, 2023, 14: 1067402. |
| 12 | Cao XL, Wan H, Wan H. Urolithin A induces protective autophagy to alleviate inflammation, oxidative stress, and endoplasmic reticulum stress in pediatric pneumonia[J]. Allergol Immunopathol, 2022, 50(6): 147-53. |
| 13 | Wang SY, Qiao JH, Chen YP, et al. Urolithin A inhibits enterovirus 71 replication and promotes autophagy and apoptosis of infected cells in vitro [J]. Arch Virol, 2022, 167(10): 1989-97. |
| 14 | Felekkis K, Touvana E, Stefanou CH, et al. MicroRNAs: a newly described class of encoded molecules that play a role in health and disease[J]. Hippokratia, 2010, 14(4): 236-40. |
| 15 | Liu Z, Fan PP, Chen M, et al. miRNAs and leukotrienes in respiratory syncytial virus infection[J]. Front Pediatr, 2021, 9: 602195. |
| 16 | Zhang X, Huang F, Yang DY, et al. Identification of miRNA-mRNA crosstalk in respiratory syncytial virus- (RSV-) associated pediatric pneumonia through integrated miRNAome and transcriptome analysis[J]. Mediators Inflamm, 2020, 2020: 8919534. |
| 17 | Atherton LJ, Jorquera PA, Bakre AA, et al. Determining immune and miRNA biomarkers related to respiratory syncytial virus (RSV) vaccine types[J]. Front Immunol, 2019, 10: 2323. |
| 18 | Eilam-Frenkel B, Naaman H, Brkic G, et al. MicroRNA 146-5p, miR-let-7c-5p, miR-221 and miR-345-5p are differentially expressed in Respiratory Syncytial Virus (RSV) persistently infected HEp-2 cells[J]. Virus Res, 2018, 251: 34-9. |
| 19 | Zhuang SH, Tang QY, Chen P, et al. Bivalirudin exerts antiviral activity against respiratory syncytial virus-induced lung infections in neonatal mice[J]. Acta Pharm, 2022, 72(3): 415-25. |
| 20 | Yang YS, Liu Y, Wang YW, et al. Regulation of SIRT1 and its roles in inflammation[J]. Front Immunol, 2022, 13: 831168. |
| 21 | Fukuda Y, Akimoto K, Homma T, et al. Virus-induced asthma exacerbations: SIRT1 targeted approach[J]. J Clin Med, 2020, 9(8): 2623. |
| 22 | Owczarczyk AB, Schaller MA, Reed M, et al. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus-induced immune responses[J]. J Immunol, 2015, 195(4): 1637-46. |
| 23 | Elesela S, Morris SB, Narayanan S, et al. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells[J]. PLoS Pathog, 2020, 16(2): e1008319. |
| 24 | Li HB, Niu XX, Shi HJ, et al. circHECTD1 attenuates apoptosis of alveolar epithelial cells in acute lung injury[J]. Lab Invest, 2022, 102(9): 945-56. |
| 25 | Martínez I, Lombardía L, Herranz C, et al. Cultures of HEp-2 cells persistently infected by human respiratory syncytial virus differ in chemokine expression and resistance to apoptosis as compared to lytic infections of the same cell type[J]. Virology, 2009, 388(1): 31-41. |
| 26 | Sun Y, López CB. Respiratory syncytial virus infection in mice and detection of viral genomes in the lung using RT-qPCR[J]. Bio-protocol, 2016, 6(10): e1819. |
| 27 | Ruiz-Galiana J, Cantón R, De Lucas Ramos P, et al. Respiratory syncytial virus: a new era[J]. Rev Esp Quimioter, 2024, 37(2): 134-48. |
| 28 | Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis[J]. Lancet, 2022, 399(10340): 2047-64. |
| 29 | Chatterjee A, Mavunda K, Krilov LR. Current state of respiratory syncytial virus disease and management[J]. Infect Dis Ther, 2021, 10(): 5-16. |
| 30 | Zhang XL, Zhang X, Hua W, et al. Expert consensus on the diagnosis, treatment, and prevention of respiratory syncytial virus infections in children[J]. World J Pediatr, 2024, 20(1): 11-25. |
| 31 | Zou G, Cao SS, Gao Z, et al. Current state and challenges in respiratory syncytial virus drug discovery and development[J]. Antiviral Res, 2024, 221: 105791. |
| 32 | Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults[J]. N Engl J Med, 2023, 388(7): 595-608. |
| 33 | Larrosa M, González-Sarrías A, García-Conesa MT, et al. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities[J]. J Agric Food Chem, 2006, 54(5): 1611-20. |
| 34 | Espín JC, Larrosa M, García-Conesa MT, et al. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far[J]. Evid Based Complement Alternat Med, 2013, 2013: 270418. |
| 35 | Kujawska M, Jodynis-Liebert J. Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection[J]. World J Gastroenterol, 2020, 26(23): 3170-81. |
| 36 | Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health[J]. Food Res Int, 2011, 44(5): 1150-60. |
| 37 | Jiao PF, Wang YR, Ren GF, et al. Urolithin A exerts a protective effect on lipopolysaccharide-induced acute lung injury by regulating HMGB1-mediated MAPK and NF-κB signaling pathways[J]. Naunyn Schmiedebergs Arch Pharmacol, 2024, [Online ahead of print]. |
| 38 | Kim S, Kim J, Song Y, et al. Unripe Rubus occidentalis, ellagic acid, and urolithin A attenuate inflammatory responses in IL-1β-stimulated A549 cells and PMA-stimulated differentiated HL-60 cells[J]. Nutrients, 2023, 15(15): 3364. |
| 39 | Carvajal JJ, Avellaneda AM, Salazar-Ardiles C, et al. Host components contributing to respiratory syncytial virus pathogenesis[J]. Front Immunol, 2019, 10: 2152. |
| 40 | Schmidt ME, Varga SM. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection[J]. Cytokine, 2020, 133: 154481. |
| 41 | Clementi N, Ghosh S, De Santis M, et al. Viral respiratory pathogens and lung injury[J]. Clin Microbiol Rev, 2021, 34(3): e00103-20. |
| 42 | O'Donnell DR, Milligan L, Stark JM. Induction of CD95 (Fas) and apoptosis in respiratory epithelial cell cultures following respiratory syncytial virus infection[J]. Virology, 1999, 257(1): 198-207. |
| 43 | Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective[J]. Cell, 2019, 176(1/2): 11-42. |
| 44 | Li JJ, Liu ML, Lv JN, et al. Polysaccharides from Platycodonis Radix ameliorated respiratory syncytial virus-induced epithelial cell apoptosis and inflammation through activation of miR-181a-mediated Hippo and SIRT1 pathways[J]. Int Immunopharmacol, 2022, 104: 108510. |
| 45 | Zhang Y, Shao LY. Decreased microRNA-140-5p contributes to respiratory syncytial virus disease through targeting Toll-like receptor 4[J]. Exp Ther Med, 2018, 16(2): 993-9. |
| 46 | Huang Z, Liu XX, Wu X, et al. MiR-146a alleviates lung injury caused by RSV infection in young rats by targeting TRAF-6 and regulating JNK/ERKMAPK signaling pathways[J]. Sci Rep, 2022, 12(1): 3481. |
| 47 | Wang SS, Ling YT, Yao YY, et al. Luteolin inhibits respiratory syncytial virus replication by regulating the miR-155/SOCS1/STAT1 signaling pathway[J]. Virol J, 2020, 17(1): 187. |
| 48 | Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation[J]. Inflammation, 2020, 43(5): 1589-98. |
| 49 | DiNicolantonio JJ, McCarty MF, O'Keefe JH. Nutraceutical activation of Sirt1: a review[J]. Open Heart, 2022, 9(2): e002171. |
| 50 | Peng XP, Li XH, Li Y, et al. The protective effect of oleanolic acid on NMDA-induced MLE-12 cells apoptosis and lung injury in mice by activating SIRT1 and reducing NF‑κB acetylation[J]. Int Immunopharmacol, 2019, 70: 520-9. |
| 51 | Liu XL, Shao KQ, Sun TY. SIRT1 regulates the human alveolar epithelial A549 cell apoptosis induced by Pseudomonas aeruginosa lipopolysaccharide[J]. Cell Physiol Biochem, 2013, 31(1): 92-101. |
| 52 | Liu ZH, Meng YL, Miao Y, et al. Artesunate reduces sepsis-mediated acute lung injury in a SIRT1-dependent manner[J]. Bioimpacts, 2023, 13(3): 219-28. |
| 53 | Liu JY, Lv XJ, Dong WJ, et al. The role of SIRT1 in autophagy in lipopolysaccharide-induced mouse type II alveolar epithelial cells[J]. Inflammation, 2018, 41(6): 2222-35. |
| 54 | Velagapudi R, Lepiarz I, El-Bakoush A, et al. Induction of autophagy and activation of SIRT-1 deacetylation mechanisms mediate neuroprotection by the pomegranate metabolite urolithin A in BV2 microglia and differentiated 3D human neural progenitor cells[J]. Mol Nutr Food Res, 2019, 63(10): e1801237. |
| [1] | 周凤敏, 郭艳菊, 陈 宁. 运动诱导的Irisin表达改善2型糖尿病大鼠的肾脏损伤[J]. 南方医科大学学报, 2024, 44(4): 675-681. |
| [2] | 叶红伟, 张钰明, 云 琦, 杜若丽, 李 璐, 李玉萍, 高 琴. 白藜芦醇可减轻高糖诱导的心肌细胞肥大:基于促进SIRT1表达维持线粒体稳态[J]. 南方医科大学学报, 2024, 44(1): 45-51. |
| [3] | 辛 辰, 王笑影, 李 响, 陈 宇, 王 雪, 宁佳曦, 杨 适, 王忠琼. LncRNA SOX2OT靶向SIRT1/自噬通路增强胆管癌细胞5-FU耐药[J]. 南方医科大学学报, 2024, 44(1): 187-193. |
| [4] | 郭晶晶, 张文龙, 梁 飘, 张龙军, 彭凌音, 闵钰琦, 潘小珍, 杨志英, 邓华菲. 葛根素减轻LPS诱导的小鼠急性肾损伤:基于调节SIRT1/NF-κB信号通路[J]. 南方医科大学学报, 2023, 43(7): 1248-1253. |
| [5] | 辛 辰, 王笑影, 李 响, 陈 宇, 王 雪, 宁佳曦, 杨 适, 王忠琼. 沉默SIRT1降低胆管癌细胞对 5-氟尿嘧啶的耐药:基于抑制FOXO1/Rab7自噬通路[J]. 南方医科大学学报, 2023, 43(3): 454-459. |
| [6] | 秦 娜, 黄 林, 董 瑞, 李 芬, 唐叙恒, 曾振华, 王兴民, 杨 翃. 虎杖苷减轻大鼠创伤性颅脑损伤后的肠损伤:基于激活Sirt1介导的SOD2和HMGB1去乙酰化抑制氧化应激和炎症反应[J]. 南方医科大学学报, 2022, 42(1): 93-100. |
| [7] | 肖 珊, 马郁文, 李 婧, 张彦红, 何 泓, 方春香, 王万铭. Angiotensin II通过氧化应激引起巨噬细胞AMPK/SIRT1能量信号紊乱[J]. 南方医科大学学报, 2021, 41(3): 384-390. |
| [8] | 葛鑫宇, 郭 芳, 范 俊, 陈宝田, 于 林, 任 静, 李纪强, 卢成林, 莫嘉文, 李树基, 袁乐欣, 胡号应, 刘 赟, 周 晓, 崔 娟, 朱志敏, 曹 雄. 柴胡桂枝汤通过sirt1-p53信号通路产生抗抑郁作用[J]. 南方医科大学学报, 2021, 41(3): 399-405. |
| [9] | 吴宇翔,辛道,刘灿,王峰. SIRT1通过调控Snail表达参与食管癌EC-9706和Eca-109细胞的上皮间质转化[J]. 南方医科大学学报, 2018, 38(11): 1325-. |
| [10] | 蔡迎迎,邹少洲,范存霞,吴春艳,方舒,李萍,薛耀明,关美萍. Exendin-4通过调控Sirt1/PGC1α延缓糖尿病小鼠心肌损伤[J]. 南方医科大学学报, 2018, 38(05): 520-. |
| [11] | 蒋文玲 杨占秋 陈文 肖红 罗宪玲. 空心莲子草有效成分抗呼吸道合胞病毒作用的实验研究[J]. 南方医科大学学报, 2007, 27(01): 62-. |
| [12] | 张其威1, 游上游2, 孙继民2, 吴旗2, 余春华2, 张楚瑜1. 呼吸道合胞病毒实时荧光定量PCR检测[J]. 南方医科大学学报, 2005, 25(07): 847-852. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||