2. 瑞典卡罗林斯卡医学院医学生物化学和生物物理学系医学炎症研究所,瑞典 斯德哥尔摩 SE-17177
2. Medical Inflammation Research, Department of Medical Biochemistry and Biophysics, Karolinska Institute, SE-17177, Stockholm, Sweden
瓜氨酸化蛋白参与了类风湿性关节炎(RA)的发病过程,在关节炎性环境中,PAD酶被激活[1],将精氨酸转化为瓜氨酸,产生新抗原,激活表达和分泌抗瓜氨酸蛋白抗体(ACPAs)的自身免疫B细胞,进而诱发自身免疫反应[2]。尽管在RA患者的血清与关节滑膜液中均可检测到ACPAs[3],但炎症在这其中的作用尚未明确,或者说,目前还没有直接的证据证明人源的类风湿因子(RF)和ACPAs是诱导关节炎的发生的主要因素。
胶原诱导关节炎(CIA)直接证明了CII参与自身免疫性关节炎的发生与发展[4]。小鼠CII三螺旋分子上鉴定的主要B细胞表位C1(氨基酸残基358-369),是CII的主要免疫显性表位之一[5-7]。且在一些早期RA患者中可以检测到C1表位抗体[8-10],提示C1特异性种系编码的自身抗体可能参与关节炎的发病机制。课题组前期已经制备了一系列针对CII的瓜氨酸C1表位的特异性ACC1和ACC4单克隆抗体。ACC1可识别多肽中第365位的瓜氨酸[11],可结合CII三重螺旋C1表位、变性的CII三重螺旋C1表位、天然CII或变性的CII。ACC4则是识别多肽第360位的瓜氨酸,特异性结合CII-α单链瓜氨酸化的C1表位[12]。许多实验研究也报道了瓜氨酸化抗原存在于软骨和滑膜中,但有关瓜氨酸化在RA发生发展中的作用机制仍有许多尚待解决的问题,如导致瓜氨酸化抗体产生的最初抗原是什么,这些瓜氨酸化抗原又是在何时、何部位、如何打破自身耐受,蛋白瓜氨酸化在RA的始动环节和病变进展过程中扮演重要的角色,这些问题的进一步研究可更深入地了解RA的发病机制,并对RA的早期诊断和正确治疗具有重要意义。因此,本研究中我们采用LPS、热灭活细菌,单克隆抗体等刺激因子在体内外诱导不同物种来源的关节软骨蛋白发生瓜氨酸化,利用ACC1和ACC4单克隆抗体的抗原识别位点的特异性,检测不同物种之间关节软骨蛋白瓜氨酸化表位的表达。
本研究中我们还建立类风湿关节炎动物模型,观察瓜氨酸化表位在动物关节软骨蛋白中的表达,探讨瓜氨酸表位的表达是否足以诱导ACPAs反应,以更全面地了解蛋白质的瓜氨酸化在RA发病各环节中的作用,可能为寻找RA的治疗靶点提供了新方向,并为RA早期诊断、早期治疗提供实验室依据。
1 材料和方法 1.1 材料 1.1.1 试剂M2139、CIIC1、CIIC2、UL1、ACC1、ACC4、L243和Hy2.15单克隆抗体在瑞典卡罗林斯卡医学院生产并纯化(表 1、2),Goat anti-mouse IgG-HRP(Southern Biotech),过氧化物酶E2886(Sigma),HRP AffiniPure Goat Anti-Rabbit IgG(H+L)(Abbkine),胎牛血清(PAN),DMEM高糖培养基(Gibco),硫酸卡那霉素(Biosharp),L-抗坏血酸(Sigma),BCA蛋白质浓度测定试剂盒(碧云天),OTC冷冻包埋剂(世泰),Collagenase(胶原蛋白酶C6885)(Sigma),LPS(Ecoli 055B5)(Sigma),大肠杆菌(ATCC25922)(广东微生物研究所),金黄色葡萄球菌(广东微生物研究所),苏木精-伊红染色液(Leagene),DAB试剂盒(Vectorlabs)。
| 表 1 ACC1/ACC4单克隆抗体信息 Tab.1 Summary of ACC1/ACC4 mAb specifi cities |
| 表 2 单克隆抗体特征 Tab.2 Characteristic features of the anti-CII monoclonal antibodies |
SPF级SD大鼠(200~300 g)(3只)购自南方医科大学实验动物中心;8~10周龄雄性BD(BALB/c×DBA/1 F1)小鼠(7只)、B10Q.ncf1小鼠(5只)和DBA/1小鼠(15只)体质量约25 g,由东莞南方医科大学松山湖实验动物科技园,并饲养于东莞南方医科大学松山湖SPF级实验动物中心,其他未特殊说明的动物均购自南方医科大学实验动物中心。所有动物均饲养于常规的屏障设施中,气候控制的环境12 h,喂普通饲料和水自由采食。本研究符合南方医科大学实验动物伦理委员会所制定的伦理学标准。
1.1.3 人血清样本收集5例RA患者和1例健康志愿者的血清标本,进行免疫组化和液相芯片(Luminex)多重检测。本研究所采集的RA血清样本均经上海光华中西医结合医院伦理委员会批准(2014-03)。
1.1.4 主要仪器正置显微镜(NIKON);CO2培养箱(hermo Scientific™)T;生物安全柜(Nuaire);双层小容量全温度恒温摇床(ZWY-2102C),上海智城分析仪器制造有限公司;冷冻切片机(Leica);低温台式离心机(320R),Hettich UNIVERSAL;超纯水仪,Sartorius;液相芯片分析系统(Luminex200),Merck Millpore;ELx508M洗板机(Biotek)。
1.2 实验方法 1.2.1 不同动物关节软骨组织体外诱导实验无菌条件下取新鲜猪、牛、羊、兔、大鼠关节软骨,无菌手术刀将其切成0.5 mm×0.5 mm×5 mm大小的软骨薄片,将薄片置于24孔板中,用含有10%(v/v)胎牛血清(FBS)和25 μg/mL抗坏血酸的200 μL DMEM培养。实验组:不同浓度的脂多糖(LPS)(50、100、200 µg/mL)、热灭活细菌(105、107、109/mL)与LPS联合单克隆抗体(M2139(25 μg/mL)、CIIC1(25 μg/mL)、CIIC2(25 μg/mL)、UL1(25 μg/mL))至不同动物关节软骨薄片,共培养72 h。每24 h更换1次新鲜培养液,并加入相应的刺激物。设置空白对照组,磷酸缓冲盐溶液(PBS)代替所加刺激物。72 h后取出软骨组织薄片,将其埋入OTC中,在液氮中冷冻并在-80 ℃下保存,制成组织冰冻切片用于随后的免疫组化染色。
1.2.2 LPS体内诱导大鼠膝关节炎症雄性SD大鼠(n=3)右膝关节内注射LPS(2、20、200 µg)诱发单关节炎,左膝关节注射等量无菌PBS作为对照。观察大鼠膝关节肿胀程度。3 d后,处死大鼠,取出膝关节软骨,埋入OTC,液氮冷冻后制备冰冻切片,用于随后的免疫组化染色。
1.2.3 胶原性关节炎小鼠模型建立选取BD(n=7)、B10Q.ncf1(n=5)和DBA/1(n=15)3种不同品系,8~10周左右的雄性小鼠。所有小鼠于第0天在尾部皮下注射100 μg/100 μL完全弗氏佐剂牛CII。第21天,小鼠尾根部较完整部位皮下注射50 µL 1 mg/mL的CII-IFA乳化剂,每3 d使观察小鼠前爪和后脚,评分并记录至第60天[13],分析比较各组发病率和疾病严重程度。第60天,处死小鼠,取出爪子,4%多聚甲醛固定,脱钙,加入OTC,-80 ℃下保存,用于免疫组化染色。
1.2.4 免疫组织化收集体外实验动物关节软骨组织薄片,快速冷冻并制成冰冻切片;取健康或炎症性BD小鼠爪子,固定,脱钙,脱水,OTC包埋,制备关节组织冰冻切片(6 μm)。所有冰冻切片在丙酮中固定10 min,低温保存。分别用3% H2O2(10 min)、5% BSA与2%大鼠血清或2%胎牛血清(30 min)和中性过氧化物酶溶液(15 min)封闭切片。100 μL 5 μg/mL生物素化的ACC1、ACC4、Hy2.15或L243单克隆抗体孵育40 min,DAB试剂显影3 min,苏木精染液(H&E)复染,脱水,透明,封片,置于显微镜下观察并拍照;取患者或健康志愿者血清,40倍稀释后,作为一抗,对小鼠患病关节组织进行免疫组化染色观察,免疫组化步骤同上。
1.2.5 液相芯片(Luminex)多重检测收集不同时间点(0、20、60 d)胶原诱导的关节炎(CIA)小鼠血清或RA患者血清,用于Luminex多重检测,健康小鼠血清与健康志愿者血清分别作为阴性对照。所有未偶联的磁珠采用中性亲和素(NeutrAvidin)固定。3% BSA、5%脱脂奶粉、100 µg/mL中性亲和素封闭血清。血清稀释1: 100,放于摇床,600 r/min,室温避光孵育1 h。96孔板中加入多肽偶联磁珠重悬液(每个多肽32个磁珠),将血清样本转移到96孔板上,重悬磁珠并密封板子,放于摇床,600 r/min,室温避光孵育1 h。PBS-T平板清洗,加入羊抗鼠IgG(Goat-mouse IgG)Fc抗体,继续孵育40 min,PBS-T再次清洗,加入60 µL鞘液,重悬磁珠,将板子放入Luminex FlexMap3D仪器,测量其平均荧光强度(MFI)。
1.3 统计分析所有数据用SPSS21.0软件进行分析。GraphPad Prism 5用于绘制关节炎的发病率和严重程度。采用卡方分析和Mann-Whitney U检验(Mann-Whitney U检验)来确定数据的显著性。P≤0.05认为差异有统计学意义。
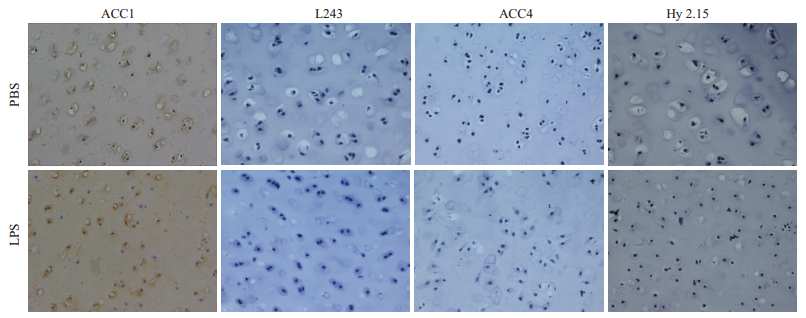
2 结果 2.1 LPS和灭活细菌体外诱导关节软骨蛋白瓜氨酸化表达情况免疫组化结果(图 1)显示,与PBS对照组相比,经LPS刺激后,刺激组关节软骨组织出现较多空的软骨陷窝,部分软骨细胞凋亡,但ACC4抗体染色组未检测到软骨瓜氨酸化的蛋白。ACC1抗体能与天然的三螺旋CII抗原表位发生交叉反应,对软骨组织呈阳性反应[11],因此,在刺激组和对照组中,均发现ACC1抗体结合至猪、牛、羊、兔以及大鼠关节软骨组织。当使用热灭活的革兰氏阴性菌和革兰氏阳性菌进行刺激(数据未显示)时,也得到了类似结果。
|
图 1 LPS体外刺激软骨组织瓜氨酸化蛋白表达情况 Fig.1 Expression of citrullinated protein in the cartilage explants stimulated by LPS in vitro (Immunohistochemical staining, original magnification:×20). Pig cartilage explants were incubated with LPS (200 μg/mL) or PBS for 72 h. Immunohistochemical staining with biotin-ACC1/ACC4/Hy2.15/L243 antibodies.LPS group: The ACC1 antibody stained cartilage of pig (L243 antibody staining as the subtype negative control group for ACC1 antibody); The ACC4 antibody failed to stainpig articular cartilage after stimulation (Hy2.15 antibody staining as the subtype negative control for ACC4 antibody). PBS group: The staining results were the same as those in LPS group. |
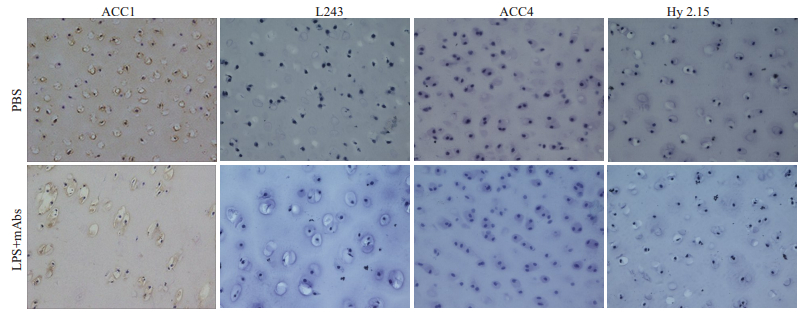
M2139、CIIC1、CIIC2和UL1抗体与LPS组成的4种致病性单克隆抗体混合物作为刺激物加入不同动物关节软骨组织中培养,免疫组化染色结果显示,ACC1抗体可结合至正常软骨以及LPS联合单克隆抗体刺激后的关节软骨,但未能观察到ACC4抗体结合至任何关节软骨蛋白(图 2)。
|
图 2 LPS联合抗CII-mAbs抗体体外刺激关节软骨组织瓜氨酸化蛋白表达情况 Fig.2 Expression of citrullinated protein in the cartilage explants stimulated by LPS and anti-CII mAbs cocktail in vitro (Immunohistochemical staining, ×20). Pig cartilage explants incubated with LPS and 4-monoclonal antibody cocktail (M2139, CIIC1, CIIC2 and UL1) or PBS for 72 h. Immunohistochemical staining with biotin-ACC1/ACC4/Hy2.15/L243 antibodies. LPS+ mAbs group: The ACC1 antibody stained cartilage of pig (L243 antibody staining as the subtype negative control group for ACC1 antibody; The ACC4 antibody failed to stained articular cartilage of pig after stimulation (Hy2.15 antibody staining as the subtype negative control for ACC4 antibody). |
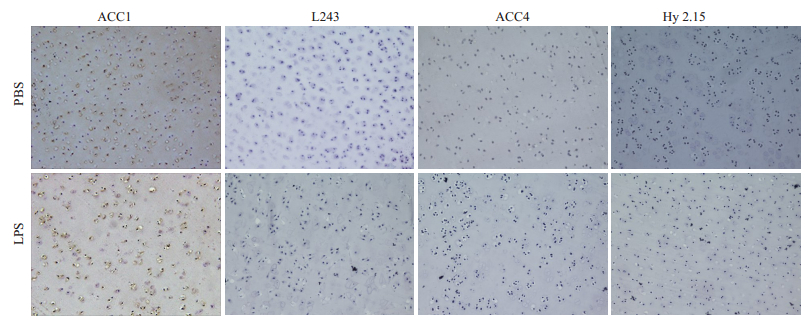
大鼠右膝关节注射不同浓度LPS(2、20、200 µg)。3 d后,取大鼠关节软骨,制成软骨组织冰冻切片。免疫组化染色结果与体外诱导实验结果相一致,均未能观察ACC4抗体结合至关节软骨组织(图 3)。
|
图 3 LPS刺激大鼠体内关节软骨瓜氨酸化蛋白表达情况 Fig.3 Expression of citrullinated protein in the cartilage of rats in vivo after injected LPS. (Immunohistochemical staining, ×20). The staining of the knee joint cartilage of rats for the tright side one, as experimental group(200 μg LPS, 3 days), the knee joint cartilage of rats for the lef side one, as PBS control group. LPS group: The ACC1 antibody stained cartilage of pig (L243 antibody staining as the subtype negative control group for ACC1 antibody). The ACC4 antibody failed to stained articular cartilage of pig after stimulation (Hy2.15 antibody staining as the subtype negative control for ACC4 antibody). |
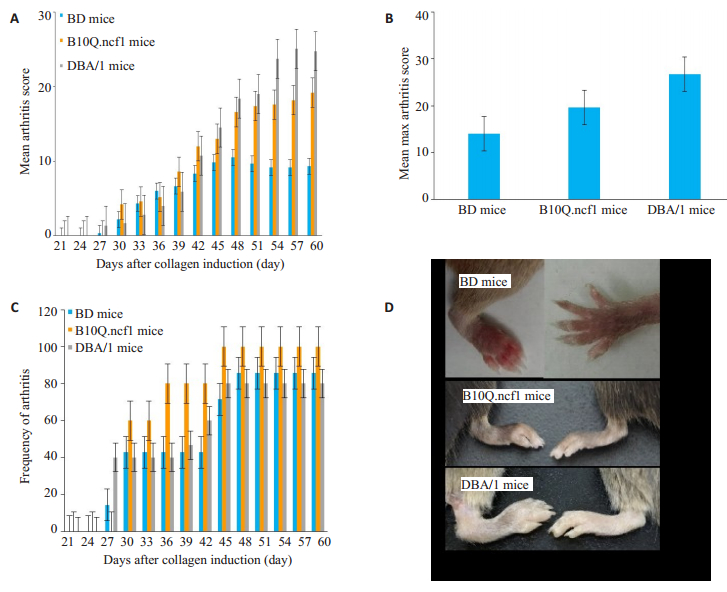
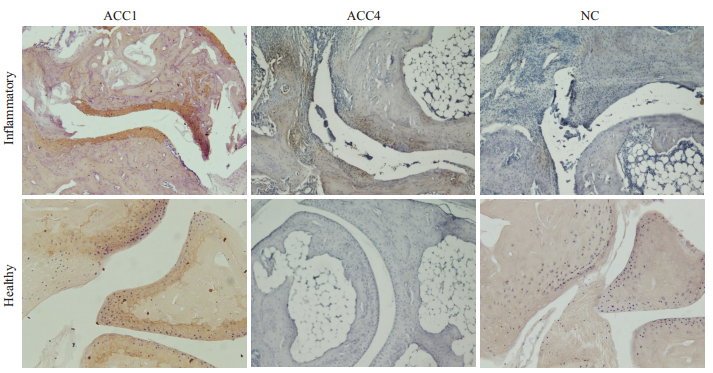
BD(n=7)、B10Q.ncf1(n=5)、DBA/1(n=15)小鼠在加强免疫之后均出现慢性关节炎,BD小鼠关节炎严重程度明显低于DBA/1和B10Q.ncf1小鼠,DBA/1和B10Q.ncf1小鼠的关节炎严重程度在疾病高峰期时存在统计学差异(P<0.05,图 4A)。B10Q.ncf1小鼠疾病发病率更高(图 4B)。染色结果分析表明,ACC1抗体均可结合至健康和炎症关节组织上的软骨蛋白。同时,在ACC4抗体染色结果中,我们发现虽ACC4不结合至正常关节软骨上的蛋白,但可结合至炎症关节软骨周围的滑膜组织(图 5)。
|
图 4 3种不同品系小鼠胶原诱导的关节炎模型 Fig.4 Collagen induced arthritis in 3 different mouse strains. BD (n=7), B1010Q.ncf1 (n=5), DBA/1 (n=15). A: Disease severity of 3 strains of mice (P < 0.05); B: Frequency of arthritis in three strains of mice (P > 0.05); C: The average maximum score of CIA model in three strains of mice (P > 0.05); D: Appearance changes of the healthy joint (right) and diseased joint (left) in three strains of mice. |
|
图 5 小鼠炎症和健康关节组织的瓜氨酸化蛋白表达情况 Fig.5 Expression of citrullinated protein in the inflamed and healthy joint tissues of mice. (Immunohistochemical staining, ×20). Results shown are representative histological pictures of arthritic (n=4) and control (n=3) mice used in the staining of joints with anticitrulline antibodies. A: The ACC1antibody stained inflammatory articular cartilage, the ACC4 stained the pannus tissue close to the eroded cartilage in arthritic mice B: Only ACC1 stained cartilage in healthy mice, but ACC4 was not stained. NC: Negative control group. |
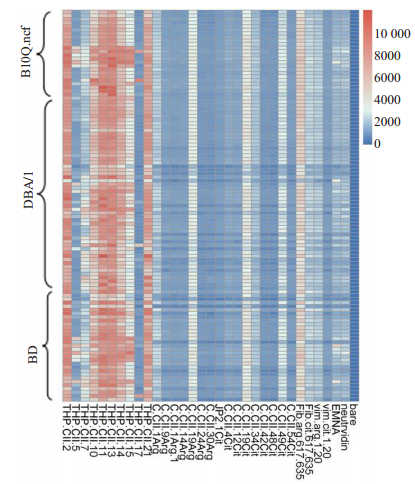
Luminex多重分析了CII诱导的3种不同小鼠品系关节炎小鼠的血清,研究高强度炎症瓜氨酸化蛋白的表达在小鼠血清中ACPA免疫应答。热图分析观察到小鼠血清对各种多肽(包括瓜氨酸化和非瓜氨酸化)的反应性。健康的B10Q.Ncf1,DBA/1和BD小鼠对不同肽段的抗体反应性相对较低。对B10Q.Ncf1和BD小鼠进行CII免疫后,针对三重螺旋CII肽的抗体信号较强。首次免疫后(第0天),B10Q.Ncf1和BD小鼠对THP-CII15和THP-CII17的抗体应答显著增强。在免疫后(第20天)的DBA/1小鼠中,针对THP-CII2,THP-CII11,THP-CII13和THP-CII21肽的抗体的信号增强,这些抗多肽抗体针对CII上不同的三重螺旋B细胞抗原表位。在CIA小鼠血清中无法检测到瓜氨酸表位的抗体(图 6)。
|
图 6 CIA血清中未瓜氨酸化和瓜氨酸CII表位的抗体反应 Fig.6 Antibody response to unmodified and citrullinated CII epitopes present in CIA serum. THP-CII is triple helical CII peptide, C-CII is cyclic alpha chain CII peptide, Fib is fibrinogen peptide, Vim is a specific vimentin peptide. |
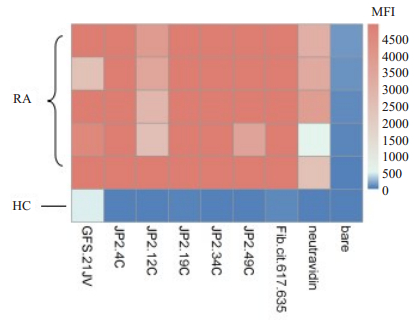
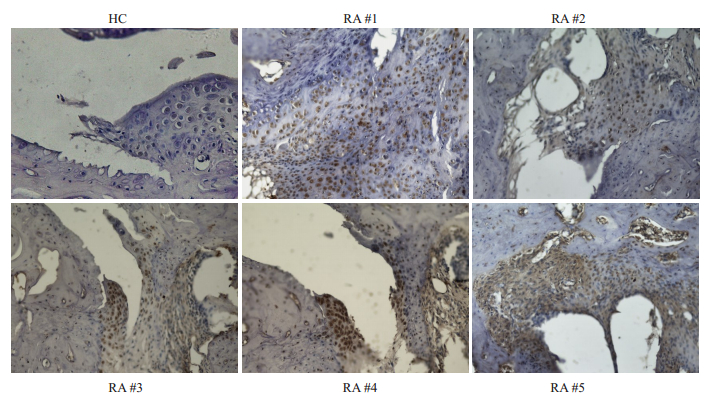
收集临床已确诊的RA患者血清,利用瓜氨酸化CII相关多肽,液态芯片(Luminex)技术检测患者血清中对瓜氨酸化多肽的反应(图 7),热图分析观察患者对各个多肽反应的差异性,从而挑选出具有高滴度的抗瓜氨酸化特异性表位(JP2-4-Cit、JP2-19-Cit、JP2-34-Cit)抗体的RA患者血清,即高滴度的ACPAs患者血清,同时ELSA检测血清中抗天然二型胶原蛋白抗体水平(OD值表示)。RA患者具体信息如表 3。将患者血清40倍稀释后,作为一抗,对小鼠患病关节组织进行免疫组化染色观察。小鼠关节病变组织的免疫组化结果表明,与正常对照组相比,发现5位RA患者血清明显地结合至小鼠炎症关节滑膜组织(图 8)。
|
图 7 瓜氨酸化多肽抗体在RA患者和健康者血清中的表达 Fig.7 The expression of citrullinated peptide antibody in RA patients and healthy human. Sera from patients (n=5) and a healthy human were used for analysis. In heat map, Y-axis represents the different serum samples of human and the abscissa represents peptides. The band color indicates the strong reaction of the sample to the polypeptide. From blue to red indicates that the reaction is weak to strong. JP2.49Cit, JP2.4Cit, JP2.34Cit, JP2.12Cit and JP2.19Cit are citrullinated polypeptides, GFS.21JV is a polypeptide fragment of the triple helical structure of CII.Fib. cit.617.635 is citrullinated fibrinogen (unpublished polypeptide sequences). |
| 表 3 RA患者性别、年龄、病程、DAS28计分、瓜氨酸化多肽平均荧光强度值(MFI)以及抗天然二型胶原蛋白抗体水平(OD) Tab.3 The information about gender, age, symptom duration, DAS28, MIF and OD of RA |
|
图 8 高ACPAs滴度RA患者血清结合至关节软骨组织 Fig.8 Serum of RA patients with high ACPAs titer binds to the articular cartilage (Immunohistochemical staining, ×40). HC: Healthy human serum immunohistochemically stained mouse joint inflammatory tissue, as control group; Immunohistochemically stained joint inflammatory tissue of mice with RA#1, RA#2, RA#3, RA#4, RA#5 sera, respectively.The serum from five RA patients significantly bound to the inflammatory joints compared to healthy controls (HC). Inflamed mouse joints bound to autoantibodies present in the RA sera. |
本研究的结果表明,胶原诱导的关节炎在发生发展过程中,可诱导软骨蛋白发生瓜氨酸化,但并不能通过在体外单独分离软骨组织诱导蛋白瓜氨酸化。CIA小鼠关节炎组织中观察到明显的瓜氨酸化迹象,但其血液中并没有检测到抗瓜氨酸化CII抗体的免疫应答。
RA患者的关节软骨和滑膜组织中大量存在瓜氨酸化的蛋白。抗RA瓜氨酸化蛋白抗体(ACPAs)由IgG,IgA和IgM组成[14],但亲和力低[15]。ACPA出现在RA病程的早期,甚至出现在临床疾病发作的数年之前[16-18],ACPA与早期RA患者关节炎的临床放射学进展有关[19]。超过70%的RA患者血清中ACPAs含量较高[20],据报道,疾病发展之前ACPA的产生与遗传(HLA共享表位)和环境(吸烟)因素有关[21-22]。ACPA抗原表位的扩展发生在关节炎发作之前,且与RA早期的疾病发展有关,尤其是对瓜氨酸化烯醇化酶肽1(CEP-1),纤维蛋白原(Fibβ36-52)和丝聚蛋白的抗体反应[23]。然而,从RA患者的血清和滑液中纯化的ACPA抗体可与来自α-烯醇化酶、波形蛋白、纤维蛋白原和CII的瓜氨酸肽结合[24]。体外细胞实验的报告表明,ACPA在RA中具有致病作用,其主要通过促进滑膜组织来源的成纤维细胞发生迁移、激活血小板和肥大细胞[25]以及通过趋化因子依赖机制激活破骨细胞[26]。尽管RA血清中ACPA的存在可用于预测RA的发展[27],但尚未阐明它们在RA发病中的作用(致病性,保护性或调节性)。
软骨特异性分子Ⅱ型胶原(CⅡ)被认为是RA诱导或维持关节炎症的致病性自身免疫反应的靶点。PAD酶可将CII上的许多精氨酸位点催化为瓜氨酸[27]。探索针对瓜氨酸化靶抗原的自身免疫反应已成为了解RA发病机制的重要关键。因此,鉴定关节软骨蛋白的瓜氨酸化位点以及了解ACPA的产生机制,对更好地了解RA具有重要意义。在这种情况下,与瓜氨酸化CII表位结合的特异性抗体如ACC1和ACC4单克隆抗体[12, 29]的产生和表征使我们能够研究可诱导瓜氨酸表位在关节软骨中表达的条件。
在我们当前的研究中,使用LPS或LPS与致病性抗CII单克隆抗体的混合物诱导软骨炎症,以诱导关节软骨中瓜氨酸化蛋白的表达。我们发现,LPS引发的急性炎症不足以诱导关节软骨中蛋白质表位的瓜氨酸化。最重要的是,当在体内诱导强烈而持续的炎症时(如胶原蛋白诱发的关节炎),我们检测到了瓜氨酸化表位的存在。同时,CIA小鼠中仅瓜氨酸化表位的表达不足以诱导ACPA免疫应答,但若存在其他强的二级信号,可能发生ACPA免疫反应。众所周知,ACPA+ RA患者在临床发展为关节炎之前发生强烈的炎症反应,瓜氨酸化蛋白表位的表达和随后的ACPA产生应早于该疾病的临床发展。特异性结合瓜氨酸化的CII肽抗体可能仅是构成RA血清中存在的部分抗体的,且这些抗体可能在不同程度上与瓜氨酸化的CII表位发生特异性反应。已有研究证明,在与瓜氨酸化CII直接作用的抗体中,存在与瓜氨酸表位广泛交叉反应的抗体。另一方面,在CIA中,由于抗体对三重螺旋CII的反应占主导地位,我们检测不到的ACPA组分,这可能在模仿人类RA的动物模型中,与缺少人类遗传因素(如特定MHC等位基因)或环境因素(如吸烟或牙周炎)等其他因素相关。
综上所述,体内强烈且持续的炎症会促进关节软骨中瓜氨酸化表位的表达,并且除了存在瓜氨酸化表位以外,B细胞或浆母细胞产生ACPA应答还需要其他的免疫信号。
| [1] |
Muller S, Radic M. Citrullinated Autoantigens: From Diagnostic Markers to Pathogenetic Mechanisms[J]. Clin Rev Allergy Immunol, 2015, 49(2): 232-9. DOI:10.1007/s12016-014-8459-2 |
| [2] |
Umeda N, Matsumoto I, Sumida T. The pathogenic role of ACPA in rheumatoid arthritis[J]. Nihon Rinsho Meneki Gakkai Kaishi, 2017, 40(6): 391-5. DOI:10.2177/jsci.40.391 |
| [3] |
Brink M, Hansson M, Mathsson L, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis[J]. Arthritis Rheum, 2013, 65(4): 899-910. DOI:10.1002/art.37835 |
| [4] |
Li Y, Tong D, Liang P, et al. Cartilage-binding antibodies initiate joint inflammation and promote chronic erosive arthritis[J]. Arthritis Res Ther, 2020, 22(1): 120. DOI:10.1186/s13075-020-02169-0 |
| [5] |
Holmdahl R, Rubin K, Klareskog L, et al. Characterization of the antibody response in mice with type Ⅱ collagen-induced arthritis, using monoclonal anti-type Ⅱ collagen antibodies[J]. Arthritis Rheum, 1986, 29(3): 400-10. DOI:10.1002/art.1780290314 |
| [6] |
Schulte S, Unger C, Mo JA, et al. Arthritis-related B cell epitopes in collagen Ⅱ are conformation-dependent and sterically privileged in accessible sites of cartilage collagen fibrils[J]. J Biol Chem, 1998, 273(3): 1551-61. DOI:10.1074/jbc.273.3.1551 |
| [7] |
Holmdahl R, Bailey C, Enander I, et al. Origin of the autoreactive anti-type Ⅱ collagen response. Ⅱ. Specificities, antibody isotypes and usage of V gene families of anti-type Ⅱ collagen B cells[J]. J Immunol, 1989, 142(6): 1881-6. |
| [8] |
Burkhardt H, Sehnert B, Bockermann R, et al. Humoral immune response to citrullinated collagen type Ⅱ determinants in early rheumatoid arthritis[J]. Eur J Immunol, 2005, 35(5): 1643-52. DOI:10.1002/eji.200526000 |
| [9] |
Snir O, Widhe M, Hermansson M, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients[J]. Arthritis Rheum, 2010, 62(1): 44-52. DOI:10.1002/art.25036 |
| [10] |
Cao D, Khmaladze I, Jia H, et al. Pathogenic autoreactive B cells are not negatively selected toward matrix protein collagen Ⅱ[J]. J Immunol, 2011, 187(9): 4451-8. DOI:10.4049/jimmunol.1101378 |
| [11] |
Uysal H, Bockermann R, Nandakumar K S, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type Ⅱ in experimental arthritis[J]. J Exp Med, 2009, 206(2): 449-62. DOI:10.1084/jem.20081862 |
| [12] |
Ge C, Xu B, Liang B, et al. Structural Basis of Cross-Reactivity of Anti-Citrullinated Protein Antibodies[J]. Arthritis Rheumatol, 2019, 71(2): 210-21. |
| [13] |
Raposo B, Vaartjes D, Ahlqvist E, et al. System A amino acid transporters regulate glutamine uptake and attenuate antibodymediated arthritis[J]. Immunology, 2015, 146(4): 607-17. DOI:10.1111/imm.12531 |
| [14] |
Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis[J]. Arthritis Res Ther, 2011, 13(1): R13. DOI:10.1186/ar3237 |
| [15] |
Suwannalai P, Scherer HU, van der Woude D, et al. Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens[J]. Ann Rheum Dis, 2011, 70(2): 373-9. DOI:10.1136/ard.2010.135509 |
| [16] |
Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis[J]. Arthritis Rheum, 2003, 48(10): 2741-9. DOI:10.1002/art.11223 |
| [17] |
Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors[J]. Arthritis Rheum, 2004, 50(2): 380-6. DOI:10.1002/art.20018 |
| [18] |
Johansson L, Pratesi F, Brink M, et al. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis[J]. Arthritis Res Ther, 2016, 18(1): 127. |
| [19] |
Sun M, Rethi B, Krishnamurthy A, et al. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts[J]. Ann Rheum Dis, 2019, 78(12): 1621-31. DOI:10.1136/annrheumdis-2018-214967 |
| [20] |
van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibodies: the past, the present and the future[J]. Nat Rev Rheumatol, 2011, 7(7): 391-8. DOI:10.1038/nrrheum.2011.76 |
| [21] |
Kokkonen H, Brink M, Hansson M, et al. Associations of antibodies against citrullinated peptides with human leukocyte antigen-shared epitope and smoking prior to the development of rheumatoid arthritis[J]. Arthritis Res Ther, 2015, 17: 125. DOI:10.1186/s13075-015-0638-x |
| [22] |
Hensvold AH, Magnusson PK, Joshua V, et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins[J]. Ann Rheum Dis, 2015, 74(2): 375-80. DOI:10.1136/annrheumdis-2013-203947 |
| [23] |
van der Woude D, Rantapaa-Dahlqvist S, Ioan-Facsinay A, et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis[J]. Ann Rheum Dis, 2010, 69(8): 1554-61. DOI:10.1136/ard.2009.124537 |
| [24] |
Ossipova E, Cerqueira CF, Reed E, et al. Affinity purified anticitrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint[J]. Arthritis Res Ther, 2014, 16(4): R167. DOI:10.1186/ar4683 |
| [25] |
Habets K L, Trouw L A, Levarht E W, et al. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis[J]. Arthritis Res Ther, 2015, 17: 209. DOI:10.1186/s13075-015-0665-7 |
| [26] |
Kuhn KA, Kulik L, Tomooka B, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism[J]. Ann Rheum Dis, 2016, 75(4): 730-8. DOI:10.1136/annrheumdis-2015-208094 |
| [27] |
Klareskog L, Ronnelid J, Lundberg K, et al. Immunity to citrullinated proteins in rheumatoid arthritis[J]. Annu Rev Immunol, 2008, 26: 651-75. DOI:10.1146/annurev.immunol.26.021607.090244 |
| [28] |
Haag S, Schneider N, Mason D E, et al. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type Ⅱ collagen[J]. Arthritis Rheumatol, 2014, 66(6): 1440-9. DOI:10.1002/art.38383 |
| [29] |
Ytterberg AJ, Joshua V, Reynisdottir G, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation[J]. Ann Rheum Dis, 2015, 74(9): 1772-7. DOI:10.1136/annrheumdis-2013-204912 |
 2020, Vol. 40
2020, Vol. 40

