肝细胞癌(HCC)是临床常见的一种恶性肿瘤,致死率在全球癌症中居于第3位[1-3]。HCC恶性程度高,易于复发转移,且以肝内血行转移即门静脉播散为主要转移途径。其中,HCC细胞与门静脉血管内皮细胞粘附、并穿越内皮细胞及基底膜是转移的关键环节。
肝素酶基因于1999年由Vlodavsky等几个小组克隆鉴定,定位于4q21.3,cDNA全长1758 bp,编码由543个氨基酸组成的糖苷内切酶,是迄今发现的唯一能特异性识别、切断细胞外基质(ECM)和基底膜中硫酸肝素蛋白多糖(HSPGs)硫酸肝素(HS)侧链的基质降解酶[4-5]。研究表明,肝素酶在多数恶性肿瘤包括HCC中表达升高[6-8];肝素酶通过降解HSPGs,并与其它蛋白水解酶(如基质金属蛋白酶和纤溶酶原激活物)协同破坏、降解ECM和基底膜;HS释放与其结合的血管内皮细胞生长因子(VEGF),诱发血管生成[8-11];或启动相关信号转导通路,促进肿瘤生长和转移[12-15]。HS模拟剂[16-17]、肝素[18]、肝素酶抑制剂[19-20]或RNAi[21-22]可抑制肝细胞癌或荷瘤裸鼠癌转移。由上可见,有关肝素酶研究主要集中于基质降解、新血管生成及相关信号传导引起的转移等,而缺乏与内皮细胞相互作用方面的研究[23-24]。
课题组前期研究证明,HCC病人癌组织高表达肝素酶,肝素酶表达阳性者有较高的肿瘤侵袭性[25];肝素酶促进HCC细胞与血管内皮细胞粘附及跨内皮迁移[26];HCC细胞裸鼠腹腔注射可诱导门静脉微瘤栓和肝转移灶形成,并见门静脉侵犯,肝素酶抑制剂或RNAi可抑制或显著减少上述现象[27]。但其具体机制并不清楚。本研究,我们首次发现HCC细胞通过肝素酶诱导微血管内皮细胞凋亡,进而促进癌细胞跨内皮迁移,现报告如下。
1 材料和方法 1.1 主要材料人正常脐静脉内皮细胞株HUVECs、正常肝细胞MIHA、肝癌细胞HepG2,BEL-7402(上海中科院细胞库),肝癌HCCLM3细胞(复旦大学肝癌研究所)。4周龄雌性BALB/c裸鼠(上海灵畅生物科技有限公司)。Trizol、M-MLV、Rnase Inhibitor和dNTPs(Promega),oligo dT(上海生工公司),Bulge-LoopTM miRNA(广州锐博),SYBR Master Mixture(TAKARA)。兔抗人肝素酶多克隆抗体(Abcam),鼠抗人甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH)多克隆抗体(Proteintech),细胞膜荧光探针DiD标记溶液、Annexin V-FITC/PI双染法细胞凋亡检测试剂盒(AAT Bioquest)。PVDF膜(Millipore)。ECL Plus发光试剂盒、BCA蛋白浓度测定试剂盒(广州碧云天公司)。慢病毒载体(LV-肝素酶-RNAi-1249-2,含有肝素酶RNAi序列TTTATGTGGCTGGATAAAT)及阴性对照载体(LV-肝素酶-Control,含有阴性对照序列TTCTCCGAACGTGTCACGT),均由上海吉凯基因技术公司基于GV115载体(元件顺序:hU6-MCS-CMVEGFP)的构建、筛选并鉴定。CCK8试剂盒(日本同仁化学研究所)。电镜固定液(Servicebio),812包埋剂(SPI)。
1.2 方法 1.2.1 肝癌细胞肝素酶表达的检测根据GenBank人肝素酶基因登陆号(NM_001098540.2),以β-actin为内参照(NM_001101),设计并合成PCR引物,肝素酶正向引物:5'-TCCTCATCCTCCTGGGTTCTC-3',反向引物:5'-AGGGCCATTCCAACCGTAACT-3';β-actin正向引物:5'-CACCCAGCACAATGAAGATCAAGAT-3',反向引物:5'-CCAGTTTTTAAATCCTGAGTCAAGC-3'。按说明书Trizol法提取总RNA,逆转录合成cDNA;按20 μL配置PCR反应体系,PCR扩增条件:95 ℃预变性10 s;95 ℃变性5 s,56 ℃退火20 s,72 ℃延伸10 s,共40个循环。根据荧光阈值(Ct值),以2-△△Ct表示肝素酶相对表达量。实验重复3次。
收集生长状态良好的目的细胞,提取蛋白,每种样品取等量蛋白质经10% SDS-PAGE,转膜,封闭,分别与一抗和二抗孵育。以GAPDH为内参照。实验重复3次。选取肝素酶表达高的肝癌细胞用于后续实验。
1.2.2 慢病毒感染实验分为Control组和RNAi组。贴壁的肝素酶高表达肝癌细胞以2×105/孔铺到12孔板中,24 h后用含有6 μg/mL polybrene的2 mL新鲜培养基替换原培养基。使用微量移液器按MOI=20,分别加入40 μL浓度为1×108 TU/mL慢病毒悬液,RNAi组用LV-肝素酶-RNAi-1249-2慢病毒载体,Control组用阴性对照载体,37 ℃孵育12 h。加入2 mL新鲜培养基以稀释polybrene,继续培养24 h,用新鲜培养基替换含有病毒的培养基继续培养。48 h后荧光显微镜下观察肝癌细胞的感染效率并拍照,将细胞传代并用于其后实验。分别用qRT-PCR及Western blot法检测转染细胞肝素酶表达,检测方法同上,实验重复3次。
1.2.3 跨内皮迁移试验将浓度5×105/mL的HUVECs细胞加入24孔板Transwell小室(孔径8 μm),每孔200 μL,形成致密单层。按说明书用DiD标记溶液制备细胞膜荧光探针工作溶液,用DiD工作溶液进行内皮细胞染色,荧光显微镜拍照。同样设Control组和RNAi组,调整转染72 h的肝癌细胞浓度为6×105/mL,依次加入上室,每孔200 μL(即每孔细胞1.2×105个);下室均加入含20%胎牛血清的DMEM培养基。每组3个复孔。将Transwell小室于37 ℃、5% CO2下培养24 h。取出Transwell板,吸净各孔培养基。下室加入10%的CCK-8染液400 μL,继续孵育1 h。依次吸取200 μL到另一96孔板;酶标仪测定各孔A450 nm值,分别用各组A450 nm值减去单纯内皮细胞组A450 nm值,以排除内皮细胞本身迁移入下室的影响,差值间接反映肝癌细胞迁移率,实验重复3次。
1.2.4 内皮细胞存活率检测将浓度2×103/mL的HUVECs细胞加入Transwell小室下室(孔径3 μm),每孔600 μL。同样设Control组和RNAi组,调整转染72 h的肝癌细胞密度为1.2×105/mL,依次加入上室,每孔200 μL(即每孔1.2×105个细胞);另设上室不加肝癌细胞,仅加等体积培养液作为空白对照,每组3个复孔。将Transwell小室于37 ℃、5% CO2下非接触共培养24 h。下室每孔加入20 μL的CCK-8溶液,继续孵育1 h。依次吸取200 μL到另一96孔板。酶标仪测定各孔A450 nm值,分别用各组A450 nm值减去空白对照组A450 nm值,以排除培养液的影响,差值间接反映内皮细胞存活率。
1.2.5 流式细胞仪凋亡检测分别收集上述各组非接触共培养24 h之下室内皮细胞悬液200 μL至另一6孔板,继续培养24 h。待细胞生长至覆盖率约为70%,1300 r/min离心5 min,弃上清。分别用4 ℃预冷的D-Hanks(pH=7.2~7.4)、1×binding buffer 200 μL洗涤细胞沉淀各1次,1300 r/min离心3 min,收集细胞。Buffer 200 μL重悬细胞。加入10 μL的Annexin V-FITC/PI染色液,室温避光10~15 min。根据细胞量,补加400 μL buffer,流式细胞仪检测。
1.2.6 内皮细胞形态观察细胞刮收集上述各组非接触共培养之内皮细胞,与培养液一起离心,弃上清,制片固定,荧光显微镜观察内皮细胞形态。另取部分离心细胞,2.5%戊二醛固定液固定细胞团块2 h。0.1 mol/L的PBS洗涤3次,1%锇酸固定2 h,0.1 mol/L的磷酸缓冲盐溶液(PBS)洗涤3次,梯度浓度酒精、丙酮脱水,812环氧树脂浸透包埋,超薄切片,醋酸铀及枸橼酸铅染色,透射电镜下观察拍照。
1.2.7 动物实验动物实验经皖南医学院弋矶山医院伦理委员会批准。按照我们先期方法[27],建立裸鼠肝癌模型。即每只裸鼠腹腔注射2×106/200 µL的HCCLM3细胞200 µL,SPF环境下饲养、观察,同样设Control组和RNAi组。6周后,断头处死,解剖观察,取肝脏标本,制作切片,荧光显微镜和普通光学显微镜观察。
1.3 统计分析实验结果用均数±标准差表示, 用SigmaStat 3.5软件进行方差分析、q检验和t检验;计数资料用χ2检验或精确概率法检验。以P<0.05为差异有统计学意义。
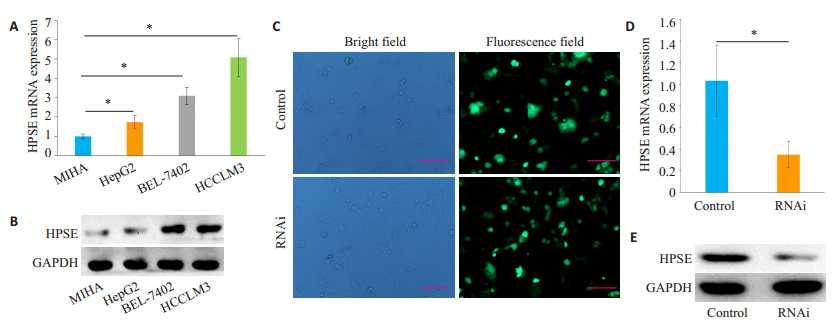
2 结果 2.1 肝素酶在肝癌细胞中的表达由qRT-PCR和Western blot结果可知,肝素酶基因mRNA和蛋白在3种肝癌细胞中均有不同程度表达升高(P<0.05),其中以HCCLM3细胞表达最高(P<0.05,图 1A、B)。据此,选取肝素酶表达最高的HCCLM3细胞用于后续研究。
|
图 1 肝素酶在肝癌细胞中的表达 Fig.1 Expression of heparanase in HCC cell lines. A, B: Heparanase (HPSE) mRNA and protein expression levels in MIHA cell line and 3 HCC cell lines were detected by qRT-PCR and Western blotting, respectively; C: Fluorescence microscopy of HCCLM3 cells transfected with the lentiviral vector (Scale bar: 50 μm); D, E: HPSE mRNA and protein expression levels in transfected HCCLM3 cells detected by qRT-PCR and Western blotting, respectively. *P < 0.05. |
慢病毒感染HCCLM3细胞,72 h后均可见明亮绿色荧光,细胞轮廓清晰,形态正常(图 1C),计数感染率达70%以上。qRT-PCR检测发现,慢病毒感染后,RNAi组肝素酶mRNA表达下降,显著低于Control组(P<0.05,图 1D);Western blot检测肝素酶蛋白表达亦呈相似改变(图 1E)。
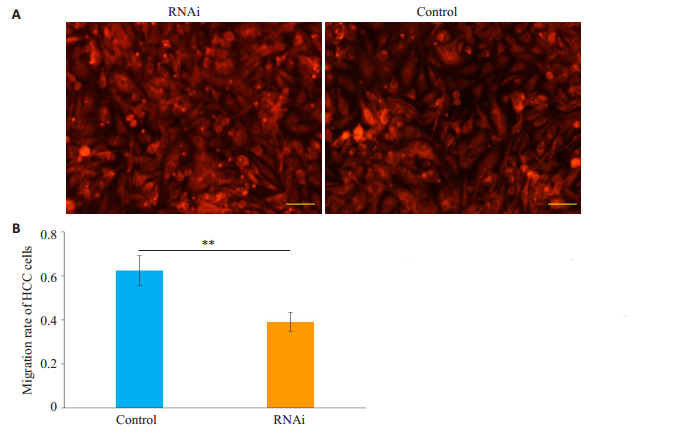
2.3 肝癌细胞跨内皮迁移能力根据实验分组,先行加入Transwell小室(孔径8 μm)的HUVECs,经DiD染色可见,两组内皮细胞均形成致密单层(图 2A)。加入转染72 h的肝癌细胞培养。24 h后下室经CCK-8法检测发现,Control组A450 nm值显著高于组RNAi组(P<0.01,图 2B)。
|
图 2 肝癌细胞跨内皮迁移试验 Fig.2 Trans-endothelial migration assay of hepatocarcinoma cells. A: Human umbilical vein endothelial cells were stained with DiD dye solution (Scale bar: 50 μm); B: Transendothelial migration rates of HCC cells in the two groups detected by CCK-8 assay. **P < 0.01. |
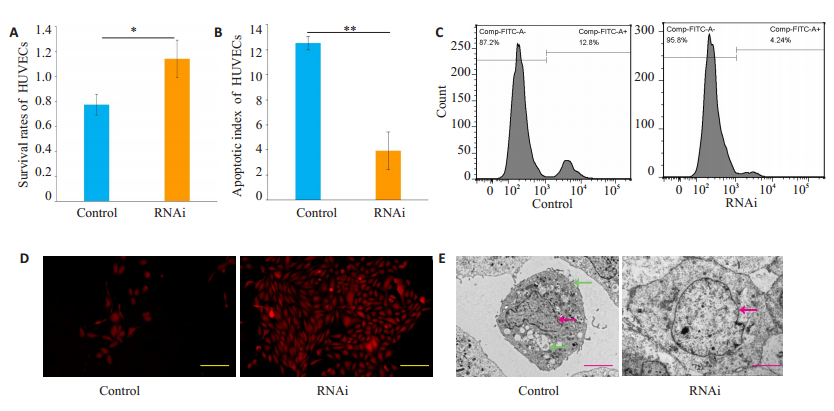
收集非接触共培养之下室HUVECs悬液,CCK-8法检测RNAi组A450 nm值显著高于Control组(P<0.05,图 3A)。Annexin V-FITC/PI双染色,流式细胞仪检测发现,Control组内皮细胞可见明显的早期凋亡和晚期凋亡现象,RNAi组凋亡明显减少;RNAi组凋亡指数(3.94±1.51)明显低于Control组(12.53±0.55)(P<0.05,图 3B、C)。本研究由于DiD标记液仅能标记细胞膜,Control组荧光显微镜仅能观察到细胞皱缩及部分细胞碎片,不能观察胞核和凋亡小体;RNAi组细胞形态相对正常(图 3D)。透射电镜观察,与RNAi组比较,Control组内皮细胞体积变小,胞质浓缩;核质盘绕、凝聚、边缘化,出现空泡和凋亡小体(图 3E)。
|
图 3 血管内皮细胞的凋亡 Fig.3 Apoptosis of microvascular endothelial cells. A: Survival rates of HUVECs in the two groups detected by CCK-8 assay; B: Apoptotic index of HUVECs in the two groups detected by flow cytometry; C: Apoptotic peaks shown by flow cytometry; D, E: Morphology of HUVECs under fluorescence microscopy (scale bars: 50 μm) and transmission electron microscopy (scale bar: 5 μm). Red arrows indicate the nucleus and green the foams. *P < 0.05, **P < 0.05. |
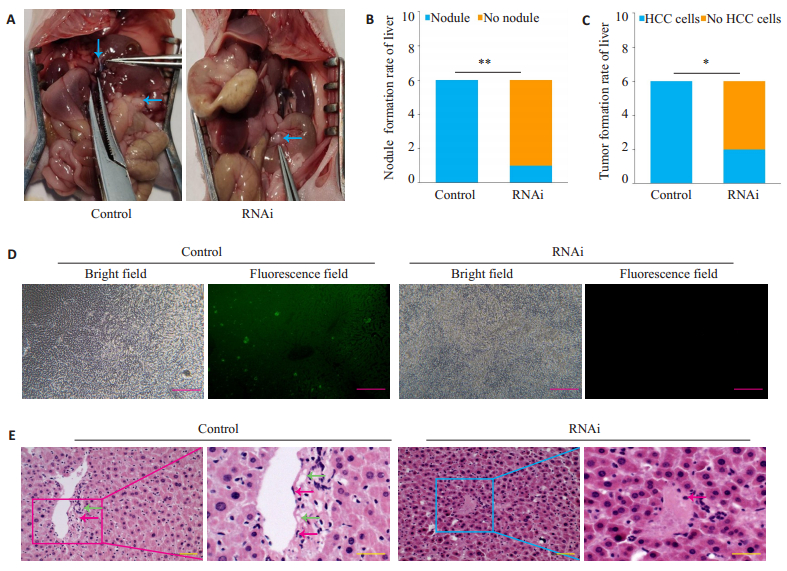
在观察期内,2组裸鼠均存活,成活率100%;饮食、活动和体质量无明显改变。6周后,解剖观察,2组裸鼠腹腔肉眼均见明显腹水及多发性1~2 mm大小不等的癌结节(图 4A);Control组6只肝脏表面均可见癌结节,RNAi组肝脏仅1例见结节形成,Control组肝脏结节率(100%,6/6)显著高于RNAi组(16.7%,1/6)(P<0.01,图 4B)。肝组织荧光显微镜观察:Control组6例肝组织中均出现GFP标记的HCC细胞,镜下成瘤率(100%,6/6)亦明显高于RNAi组(33.3%,2/6)(P<0.05),提示Control组癌细胞夸内皮细胞迁移能力强于RNAi组(图 4C、D)。光镜:Control组肝组织内可见肝癌细胞,MVECs可见坏死,胞浆出现空泡,胞核皱缩;RNAi组肝组织内罕见肝癌细胞,肝血窦淤血,MVECs坏死轻微,无典型空泡和胞核皱缩现象(图 4E)。
|
图 4 组织学观察 Fig.4 Histological observation of the tumor tissues in nude mice. A: Intraperitoneal cancer nodules (blue arrows) in the two groups of nude mice; B: Rate of cancer nodule formation in the liver in the two groups; C: Comparison of liver tumor formation rates; D: Liver tissues of nude mice under fluorescence microscopy (scale bar: 50 μm); E: Liver tissues of nude mice under light microscopy (HE staining, scale bar: 50 μm). Red arrows indicate the nucleus and green ones the foams). *P < 0.01, **P < 0.05. |
HCC肝内门静脉播散,癌细胞必须突破内皮细胞屏障。但癌细胞体积显著大于生物大分子,不可能经正常的细胞缝隙穿越内皮细胞。其能否穿越内皮细胞屏障,取决于以下3个因素:①癌细胞自身形态改变,如变细长或扁平状态利于通过;②内皮细胞形态改变,如细胞皱缩、细胞间隙增大最宽时,有利于癌细胞穿越;③内皮细胞出现坏死[28]或凋亡时,相当于细胞间隙极度增大,更有利于癌细胞穿越。根据既往研究结果,HCC细胞和肝癌组织高表达肝素酶,而肝素酶又有促肝癌转移的特性,肝素酶可诱导肝癌细胞跨内皮迁移[25-27],我们推测,HCC细胞是否通过肝素酶引发MVECs坏死或凋亡而促进癌细胞穿越血管呢?这是个创新而又富有挑战性的课题。
本研究我们首先筛选出高表达肝素酶的肝癌细胞;然后用慢病毒RNAi载体降低其肝素酶表达水平。Transwell小室跨内皮试验,证实肝素酶可促肝癌细胞跨内皮迁移。继之,我们用两种细胞非接触共培养,CCK-8法检测发现Control组内皮细胞存活率下降,RNAi组内皮细胞存活率上升,提示肝癌细胞肝素酶可降低血管内皮细胞存活率,促进其坏死或凋亡。Annexin V-FITC/PI双染流式细胞仪检测共培养之下室内皮细胞发现,Control组内皮细胞可见明显的早期和晚期凋亡现象,RNAi组凋亡指数明显下降,提示肝癌细胞肝素酶可以诱导内皮细胞凋亡,RNAi可以逆转之。透射电镜观察,Control组内皮细胞体积变小,胞质浓缩,空泡形成;核质盘绕、边聚化,出现凋亡小体,证明肝素酶可介导体外血管内皮细胞凋亡。动物实验表明高表达肝素酶的肝癌细胞经腹腔注射后,除在腹腔形成肿瘤外,还可在肝脏形成转移瘤,而RNAi组肝癌细胞则较少形成肝转移瘤。镜下观察可见Control组肝组织MVECs坏死、体积缩小、胞核皱缩等现象,进一步证实HCC细胞肝素酶可诱导MVECs凋亡,相当于内皮细胞间隙显著扩大,粘附于微血管处的HCC细胞更易于穿越微血管屏障,而进入新的部位形成转移灶。
细胞凋亡于1972年为Kerr首先观察[29],是一种依赖于天冬氨酸特异性半胱氨酸蛋白酶(caspase-8)和细胞色素C的程序性细胞死亡,形成的凋亡小体被巨噬细胞或相邻细胞吞噬。肝素酶一般抑制癌细胞凋亡,HS模拟剂、肝素酶抑制剂或基因沉默则促进癌细胞凋亡[30-33];肝素酶还可以促进淋巴管内皮细胞增殖[34],对阿尔茨海默病、糖尿病和缺血再灌注损伤心肌细胞有保护作用[35]。而肝素酶介导肝癌MVECs凋亡可能是肝癌细胞跨内皮迁移、促进肝内转移的重要原因,则是本研究首次报道。其机制可能为粘附于MVECs表面的肝癌细胞,通过肝素酶降解内皮细胞表面HSPGs,释放HS,而后者具有促凋亡作用;肝素酶本身也可上调肿瘤坏死因子-α(TNF-α)等细胞因子表达[36];此外,HSPGs降解后,其胞外区脱落,其结合的细胞因子如TNF-α得以释放[37],从而触发凋亡。
肝素酶一个重要功能是促进血管增生[8-11],本研究则发现肝素酶可诱导MVECs凋亡,二者似乎矛盾。肝素酶究竟发挥何种作用,我们认为,要根据其具体环境及其底物HSPGs上结合何种细胞因子进行具体分析,而不应教条化。通常所述的肝素酶促进血管增生, 是指肝素酶作用于癌组织微环境ECM中的HSPGs,该处HSPGs主要结合有VEGF,当肝素酶降解HSPGs后主要释放VEGF,从而促进新生血管形成。而本研究主要是癌细胞与MVECs相互作用,内皮细胞表面HSPGs结合的细胞因子可能主要是TNF-α,肝素酶作用后主要释放TNF-α,后者再启动内皮细胞凋亡程序。因此,肝素酶两种看似相反的功能,实际上是统一的,也体现了肝素酶功能的复杂性,值得进一步研究。
本研究还观察到一种有趣的现象,即肝素酶作用的内皮细胞同时有经典凋亡和坏死性凋亡(未发表资料)。有研究发现小鼠肾单位发育过程中各部位程序性死亡有明显差异,既有经典凋亡,也有坏死性凋亡和副凋亡[38]。缺血性神经元死亡细胞中同样具有凋亡和坏死的特征[39]。凋亡和坏死性凋亡均需要TNF-α启动,但前者多依赖于caspase激活,后者发生于caspase-8抑制时。本研究检测之凋亡可能与一些不依赖于caspase的凋亡诱导因子和促凋亡基因Bax激活有关,也可能有p38MAPK通路参与[40],有别于经典的caspase-8凋亡途径[41];此外,文献报道,凋亡和坏死性凋亡相互关联并可相互转化[42]。
综上所述,肝癌细胞可通过肝素酶诱导微血管内皮细胞凋亡促进肝癌门静脉播散和转移。
| [1] |
Yarchoan M, Agarwal P, Villanueva A, et al. Recent developments and therapeutic strategies against hepatocellular carcinoma[J]. Cancer Res, 2019, 79(17): 4326-30. DOI:10.1158/0008-5472.CAN-19-0803 |
| [2] |
Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma[J]. Cancer Treat Rev, 2020, 82: 101946. DOI:10.1016/j.ctrv.2019.101946 |
| [3] |
Pascual S, Miralles C, Bernabe JM, et al. Surveillance and diagnosis of hepatocellular carcinoma: a systematic review[J]. World J Clin Cases, 2019, 7(16): 2269-86. DOI:10.12998/wjcc.v7.i16.2269 |
| [4] |
Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis[J]. Nat Med, 1999, 5(7): 793-802. DOI:10.1038/10518 |
| [5] |
Hulett MD, Freeman C, Hamdorf BJ, et al. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis[J]. Nat Med, 1999, 5(7): 803-9. DOI:10.1038/10525 |
| [6] |
Arvatz G, Weissmann M, Ilan N, et al. Heparanase and cancer progression: New directions, new promises[J]. Hum Vaccin Immunother, 2016, 12(9): 2253-6. DOI:10.1080/21645515.2016.1171442 |
| [7] |
Goldberg R, Meirovitz A, Abecassis A, et al. Regulation of heparanase in diabetes-associated pancreatic carcinoma[J]. Front Oncol, 2019, 9: 1405. DOI:10.3389/fonc.2019.01405 |
| [8] |
Lv B, Zhang B, Hu XY, et al. Heparanase regulates in vitro VEGF-C expression and its clinical significance to pancreatic ductal cell adenocarcinoma[J]. Oncol Lett, 2016, 11(2): 1327-34. DOI:10.3892/ol.2016.4085 |
| [9] |
Lv Q, Wu KJ, Liu FL, et al. Interleukin-17A and heparanase promote angiogenesis and cell proliferation and invasion in cervical cancer[J]. Int J Oncol, 2018, 53(4): 1809-17. |
| [10] |
Hoß SG, Grundmann M, Benkel T, et al. Pro-angiogenic effects of latent heparanase and thrombin receptor-mediated pathways-do they share a common ground in melanoma cells?[J]. Thromb Haemost, 2018, 118(10): 1803-14. DOI:10.1055/s-0038-1669922 |
| [11] |
Poupard N, Badarou P, Fasani F, et al. Assessment of heparanasemediated angiogenesis using microvascular endothelial cells: identification of λ-carrageenan derivative as a potent anti angiogenic agent[J]. Mar Drugs, 2017, 15(5): E134. DOI:10.3390/md15050134 |
| [12] |
Hermano E, Goldberg R, Rubinstein AM, et al. Heparanase accelerates obesity-associated breast cancer progression[J]. Cancer Res, 2019, 79(20): 5342-54. DOI:10.1158/0008-5472.CAN-18-4058 |
| [13] |
Liu X, Zhou ZH, Li W, et al. Heparanase promotes tumor growth and liver metastasis of colorectal cancer cells by activating the p38/ MMP1 Axis[J]. Front Oncol, 2019, 9: 216. DOI:10.3389/fonc.2019.00216 |
| [14] |
Batool T, Fang JP, Barash U, et al. Overexpression of heparanase attenuated TGF-β-stimulated signaling in tumor cells[J]. FEBS Open Bio, 2017, 7(3): 405-13. DOI:10.1002/2211-5463.12190 |
| [15] |
Barash U, Spyrou A, Liu P, et al. Heparanase promotes glioma progression via enhancing CD24 expression[J]. Int J Cancer, 2019, 145(6): 1596-608. DOI:10.1002/ijc.32375 |
| [16] |
Lanzi C, Cassinelli G. Heparan sulfate mimetics in cancer therapy: the challenge to define structural determinants and the relevance of targets for optimal activity[J]. Molecules, 2018, 23(11): E2915. DOI:10.3390/molecules23112915 |
| [17] |
Lanzi C, Zaffaroni N, Cassinelli G. Targeting heparan sulfate proteoglycans and their modifying enzymes to enhance anticancer chemotherapy efficacy and overcome drug resistance[J]. Curr Med Chem, 2017, 24(26): 2860-86. |
| [18] |
Djaafar S, Dunand-Sautier I, Gonelle-Gispert C, et al. Enoxaparin attenuates mouse colon cancer liver metastases by inhibiting heparanase and interferon-γ-inducible chemokines[J]. Anticancer Res, 2016, 36(8): 4019-32. |
| [19] |
Singh P, Blatt A, Feld S, et al. The heparanase inhibitor PG545 attenuates colon cancer initiation and growth, associating with increased p21 expression[J]. Neoplasia, 2017, 19(3): 175-84. DOI:10.1016/j.neo.2016.12.001 |
| [20] |
Weissmann M, Bhattacharya U, Feld S, et al. The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action[J]. Matrix Biol, 2019, 77: 58-72. DOI:10.1016/j.matbio.2018.08.005 |
| [21] |
Jin H, Cui M. Gene silencing of heparanase results in suppression of invasion and migration of gallbladder carcinoma cells[J]. Biosci Biotechnol Biochem, 2018, 82(7): 1116-22. DOI:10.1080/09168451.2018.1456316 |
| [22] |
Liu XY, Tang QS, Chen HC, et al. Lentiviral miR30-based RNA interference against heparanase suppresses melanoma metastasis with lower liver and lung toxicity[J]. Int J Biol Sci, 2013, 9(6): 564-77. DOI:10.7150/ijbs.5425 |
| [23] |
Coombe DR, Gandhi NS. Heparanase: a challenging cancer drug target[J]. Front Oncol, 2019, 9: 1316. DOI:10.3389/fonc.2019.01316 |
| [24] |
Vlodavsky I, Singh P, Boyango I, et al. Heparanase: From basic research to therapeutic applications in cancer and inflammation[J]. Drug Resist Updat, 2016, 29: 54-75. DOI:10.1016/j.drup.2016.10.001 |
| [25] |
Chen XP, Liu YB, Rui J, et al. Heparanase mRNA expression and point mutation in hepatocellular carcinoma[J]. World J Gastroenterol, 2004, 10(19): 2795-9. DOI:10.3748/wjg.v10.i19.2795 |
| [26] |
Chen XP, Jiang W, Yue CF, et al. Heparanase contributes to trans-endothelial migration of hepatocellular carcinoma cells[J]. J Cancer, 2017, 8(16): 3309-17. DOI:10.7150/jca.20159 |
| [27] |
陈晓鹏, 岳朝辅, 卢林明, 等. 肝素酶介导的裸鼠肝癌门静脉微瘤栓形成[J]. 第三军医大学学报, 2016, 38(21): 2297-302. |
| [28] |
Alarcón CR, Tavazoie SF. Cancer: Endothelial-cell killing promotes metastasis[J]. Nature, 2016, 536(7615): 154-5. DOI:10.1038/nature19465 |
| [29] |
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics[J]. Br J Cancer, 1972, 26(4): 239-57. DOI:10.1038/bjc.1972.33 |
| [30] |
李琨琨, 吴慧丽. 肝素酶抑制剂OGT2115通过阻断Wnt/β-catenin通路抑制HT29结肠癌细胞增殖并促进其凋亡[J]. 细胞与分子免疫学杂志, 2017, 33(10): 1371-5. |
| [31] |
Song TY, Spillmann D. Transcriptomic analysis reveals cell apoptotic signature modified by heparanase in melanoma cells[J]. J Cell Mol Med, 2019, 23(7): 4559-68. |
| [32] |
Cassinelli G, Favini E, Dal Bo L, et al. Antitumor efficacy of the heparan sulfate mimic roneparstat (SST0001) against sarcoma models involves multi-target inhibition of receptor tyrosine kinases[J]. Oncotarget, 2016, 7(30): 47848-63. |
| [33] |
Song YN, Hu B, Qu HJ, et al. Novel 1, 3-N, O-Spiroheterocyclic compounds inhibit heparanase activity and enhance nedaplatin-induced cytotoxicity in cervical cancer cells[J]. Oncotarget, 2016, 7(24): 36154-67. DOI:10.18632/oncotarget.8959 |
| [34] |
Yu SJ, Lv H, Zhang H, et al. Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway[J]. Biochem Biophys Res Commun, 2017, 485(2): 432-9. |
| [35] |
Wang FL, Pulinilkunnil T, Flibotte S, et al. Heparanase protects the heart against chemical or ischemia/reperfusion injury[J]. J Mol Cell Cardiol, 2019, 131: 29-40. DOI:10.1016/j.yjmcc.2019.04.008 |
| [36] |
Zhang Y, Wang ZQ, Liu J, et al. Cell surface-anchored syndecan-1 ameliorates intestinal inflammation and neutrophil transmigration in ulcerative colitis[J]. J Cell Mol Med, 2017, 21(1): 13-25. |
| [37] |
Zhang Y, Wang ZQ, Liu J, et al. Suppressing syndecan-1 shedding ameliorates intestinal epithelial inflammation through inhibiting NF-κB pathway and TNF-Α[J]. Gastroenterol Res Pract, 2016, 2016. |
| [38] |
邵姝元, 刘玉玲, 郭敏, 等. 小鼠肾单位发育过程中的经典凋亡和坏死性凋亡[J]. 第三军医大学学报, 2012, 34(9): 874-8. |
| [39] |
Unal-Cevik I, Kilinç M, Can A, et al. Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia[J]. Stroke, 2004, 35(9): 2189-94. DOI:10.1161/01.STR.0000136149.81831.c5 |
| [40] |
曾海龙, 黄志秋, 张艺能, 等. p38MAPK/eNOS信号通道在胰高血糖素样肽-1抑制AGEs诱导的人脐静脉内皮细胞凋亡中的作用[J]. 南方医科大学学报, 2016, 36(1): 116-9, 139. |
| [41] |
高宏, 文楠, 徐雪松, 等. 内质网应激介导的Kupffer细胞源性TNF-α经TNFR/caspase 8途径诱导肝星状细胞凋亡[J]. 南方医科大学学报, 2020, 40(5): 632-9. |
| [42] |
Han WD, Xie JS, Li L, et al. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis[J]. Apoptosis, 2009, 14(5): 674-86. DOI:10.1007/s10495-009-0334-x |
 2020, Vol. 40
2020, Vol. 40

