2. 广州赛莱拉干细胞科技股份有限公司,广东 广州 510080;
3. 南方医科大学第三附属医院呼吸与危重症科,广东 广州 510000;
4. 南方医科大学第三附属医院广东省骨与关节退行性疾病重点实验室,广东 广州 510000
2. Guangzhou Saliai Stem Cell Science and Technology Company Limited, Guangzhou 510080, China;
3. Department of Respiratory and Critical Care Medicine, Guangzhou 510000, China;
4. Guangdong Provincial Key Laboratory of Bone and Joint Degeneration Diseases, Guangzhou 510000, China
特发性肺纤维化(IPF)是一种慢性、进行性、间质性纤维化的肺疾病,其特征在于肺上皮细胞损伤、成纤维细胞的活化和细胞外基质沉积,常发生在老年人中、确诊后的中位生存期仅为2~5年,五年生存率为20%~ 25%[1-3]。目前临床治疗方法包括抗氧化剂、抗纤维化剂、细胞因子抑制剂等,但效果并不理想,均不能逆转IPF的进展[4]。因此,开发新的有效治疗方法至关重要。吡非尼酮和尼达尼布可明显减缓肺部用力肺活量(FVC)下降速率的能力,已被批准用于IPF。然而这两种药物的治疗效果有限,且可能会引起严重的胃肠道耐受性问题[5-7],并且在使用中已发现其引起的肾毒性[8]。研究表明,IPF的发生、发展与上皮-间质转化(EMT)、转化生长因子β1(TGF-β1)有关,且TGF-β1可以促进EMT过程,导致肺泡上皮细胞(AEC)转化为迁移和/或侵袭性间充质细胞[9-10],从而促进IPF的发展[11]。EMT是肺泡上皮细胞失去细胞-细胞间黏附和基底极性,并获得迁移、侵袭和产生细胞外基质成分的间质特性。EMT是一个复杂的过程,涉及一个大型相互作用系统,在这些作用的最前沿是TGF-β1。EMT过程的失调可形成促纤维化微环境,从而促进肺纤维化的发展[12-13],靶向EMT诱导剂在纤维化病情中具有治疗潜力[14]。
间充质干细胞(MSCs)可来源于骨髓、脐带血、胎盘和脂肪组织等,其可调节炎症反应、修复损伤组织并分化为不同的组织[15-16]。已经发现MSCs具有抗纤维化的作用[17]。然而其安全性、稳定性等仍需考量,与其他组织来源的MSCs相比,人脐带间充质干细胞(hUCMSCs)更容易获得,具有增殖、免疫抑制作用,并且在临床应用中无伦理问题[15],最近的研究表明,MSC的治疗益处取决于其细胞外囊泡(EVs),根据大小和生物生成机制将其分为3种亚型:外泌体(50~150 nm)、微囊泡(100~1000 nm)和凋亡小体(500~5000 nm),其中外泌体稳定性更强[18],且因其不存在细胞治疗引发的安全性等问题而成为研究热点[19]。研究发现外泌体可缓解肺纤维化的发展[20]。但关于hUCMSC-EXOs对IPF的发展及EMT过程的作用尚未见研究。因此本实验选用人脐带间充质干细胞来源的外泌体(hUCMSCEXOs)作为研究对象探究其对肺纤维化的作用。
本研究将采用博来霉素诱导的小鼠肺纤维化模型及TGF-β1诱导的肺泡上皮细胞(A549细胞)EMT细胞模型探讨hUCMSC-EXOs对EMT过程的影响及其对IPF的作用。
1 材料和方法 1.1 主要试剂和仪器TGF-β1酶联免疫吸附试剂盒购自武汉武汉伊莱瑞特公司,波形蛋白(vimentin)抗体、E-钙粘蛋白(Ecadherin)抗体(ABclonal),Smad2/3、p-Smad2/3抗体(北京博奥森公司),Synergy HTX多功能酶标仪(BioTek),电泳电转移系统(Bio-Rad),倒置显微镜(Zeiss)。
1.2 hUCMSC-EXOs的分离与鉴定脐带获自南方医科大学第三附属医院出生的正常新生儿,已经医院研究伦理委员会批准。在收集之前,签署知情同意书。将脐带基质切成2.0 mm2的组织块。加入胶原酶Ⅱ至终浓度为0.05%,并在37 ℃下搅拌消化30 min。加入PBS,并将混合物过滤并离心。然后将沉淀物重悬于DMEM/F12完全培养基中,并以1×106细胞/cm2的密度接种在T-25 cm2培养瓶中。将第3代细胞培养上清液置于4 ℃,1096 r/min 10 min,2831 r/min 20 min的条件下,弃去沉淀物以除去细胞。然后,以6330 r/min离心30 min,弃去沉淀,除去亚细胞组分。6330 r/min 70 min,弃去上清液,最后得到的沉淀为外泌体,用30 mL PBS溶液重悬沉淀,混合,然后6330 r/min 70 min,悬浮于1 mL PBS溶液中,纯化的EXOs溶液放置细胞加入eppendorf管中,并保存在-80 ℃的冰箱中。通过Western blotting、粒径分析(NTA)和透射电子显微镜(TEM)鉴定。
1.3 动物成年雄性C57 BL/6小鼠(22~25 g,6~8周)购于南方医科大学实验动物中心。将所有小鼠饲养在具有12 h光照/黑暗周期的自动控制的房间中,保证其自由进食。动物实验获得动物伦理委员会的批准,采用随机数字表法将24只雄性小鼠按平均分为4组:空白组、模型组(博来霉素3 mg/kg气管内注射)、外泌体一组(造模第2天给予hUCMSC-EXOs)、外泌体二组(造模第11天给予hUCMSC-EXOs),治疗组小鼠尾静脉注射给予hUCMSC-EXOs 100 μg/250 μL,模型组小鼠给予等体积的生理盐水,造模后21 d取材。
1.4 大体标本和肺指数小鼠麻醉后置于操作台上,用冷的等渗盐水洗涤取下的肺组织,用尺子做参照,拍照,称重,然后立即使用以下公式计算肺指数:肺指数=湿肺质量(g)/小鼠体质量(g)。
1.5 HE染色和Masson染色在室温下,将肺组织置于低聚甲醛中24~48 h,梯度乙醇脱水,包埋在石蜡中,切片(4 μmol/L),脱蜡,水化,然后用HE或Masson染色,按照试剂盒说明书操作。
1.6 细胞培养人肺泡上皮细胞A549细胞系购自ATCC。将细胞培养在含有10%胎牛血清(FBS)、100 U/mL青霉素和100 μg/mL链霉素的RPMI培养基1640(Gibco,美国)中,在5%二氧化碳培养箱中于37 ℃培养。将细胞以104个细胞/孔的密度接种在含有培养基的平底96孔组织培养板中,过夜后加入hUCMSC-EXOs(浓度分别为0、20、40、80 μg/mL)共同孵育24 h,根据CCK8细胞增殖测定试剂盒说明书检测hUCMSC-EXOs的细胞毒性。将细胞以105个细胞/孔的密度接种到6孔板中过夜,并用hUCMSC-EXOs(40 μg/mL)预处理6 h,TGF-β1(2 ng/mL)刺激24 h后收取细胞以进行下一步的实验。
1.7 血清中TGF-β1含量的测定酶联免疫吸附实验(ELISA)测定小鼠外周血血清中TGF-β1的含量,按照试剂盒说明书操作,以450 nm下吸光度值A450 nm计算TGF-β1浓度。
1.8 免疫组织化学法为测量肺组织中vimentin、E-cadherin、p-Smad2的表达水平,常规对样品切片进行脱蜡水化,使用柠檬酸钠缓冲液(pH=6.0)进行抗原修复。用山羊血清白蛋白封闭后,将载玻片与一抗在4 ℃下孵育过夜,将切片与二抗在室温下孵育30 min,用DAB染色并用苏木精复染。
1.9 Western blotting在含有1%苯基甲磺酰氟(PMSF)和2%磷酸酶抑制剂的RIPA裂解缓冲液中溶解细胞,将其裂解为50 μL。通过二辛可宁酸(BCA)测定法定量蛋白质浓度。使用8%~10% SDS/PAGE凝胶在16 V下电泳分离每个样品中的蛋白质60~70 min,低温100 V,90 min下转移到硝酸纤维素膜上。将膜在含5%脱脂奶粉的TBST中封闭,根据预先标记的分子量切割,然后与适当的一抗(1:1000)在4 ℃孵育过夜。用TBST洗涤3次,将膜与辣根过氧化物酶偶联的二抗(1:5000)在室温下孵育1 h。用TBST洗涤3次,膜显影后用GIS凝胶图像分析系统取像。
1.10 统计学分析统计分析采用Graphpad Prism 6.0软软件。多组间比较采用单因素方差分析,P < 0.05认为差异具有统计学意义。
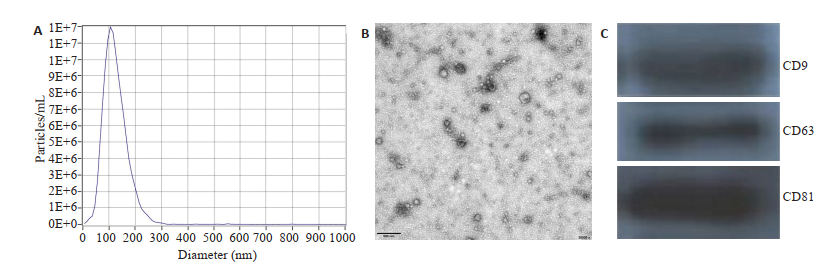
2 结果 2.1 hUCMSC-EXOs的鉴定从hUCMSC培养上清液中分离出hUCMSCEXOs。如图 1使用纳米颗粒示踪分析检测浓度和粒径。透射电子显微镜检测到的典型形状为120 nm。Western blotting鉴定hUCMSC-EXOs表面标记物CD9、CD63和CD81,证明提取到的为hUCMSC-EXOs。
|
图 1 hUCMSC-EXOs的鉴定 Fig.1 Identification of hUCMSC-EXOs. A: NTA of exosomes; B: Transmission electron micrograph (TEM) of the exosomes. Scale bar=200 nm; C: Markers of exosomes assessed by Western blotting. |
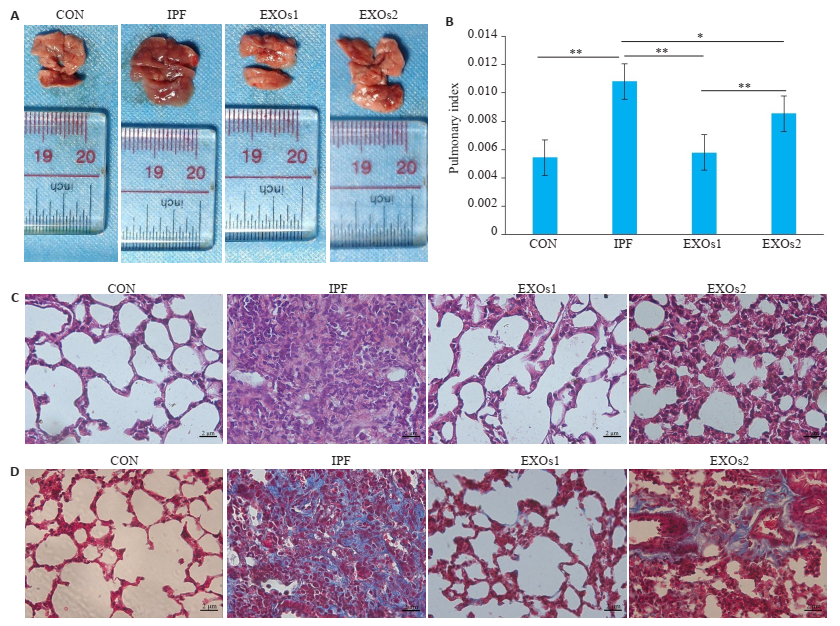
从大体标本观察得知,空白组肺组织色泽及条索状回沟等状况良好,模型组肺组织颜色较深,水肿程度较高,肺组织表面可见大小不一的结节样改变、且弹性下降、不易剥离,外泌体治疗组小鼠肺组织色泽及条索状回沟、结节改变、肺水肿和充血等的情况有不同程度的改善(图 2A、B)。此外,模型组肺系数显著高于空白组(P < 0.05),而治疗组hUCMSC-EXOs可减轻肺纤维化所致的肺指数升高,其中第2天给予hUCMSCEXOs的效果优于第11天给予hUCMSC-EXOs(P < 0.01);HE和Masson染色显示肺组织切片病理学变化和胶原蛋白沉积,hUCMSC-EXOs可以明显减轻肺纤维化小鼠肺组织的炎症改变、肺泡实质变化和胶原沉积(图 2C、D)。
|
图 2 hUCMSC-EXOs肺纤维化小鼠肺系数和病理改变的影响 Fig.2 Effect of hUCMSC-EXOs on pulmonary index and pathological changes in mice with pulmonary fibrosis. A: Gross specimen of every group; B: The pulmonary index of every group, *P < 0.05, **P < 0.01; C: HE staining (Original magnification: ×400), scale bar=2 μmol/L; D: Masson trichrome staining (×400), scale bar=2 μmol/L. CON: Control group, IPF: Model group, EXOs1: hUCMSC-EXOs were given on the 2nd day after modeling, EXOs2: hUCMSC-EXOs were given on the 21th day after modeling. |
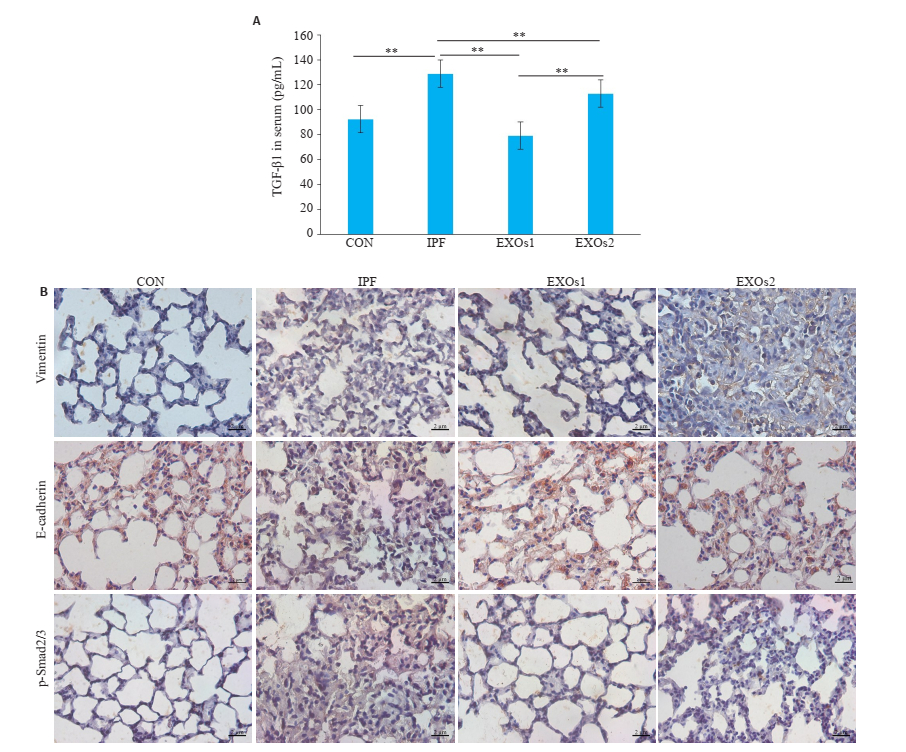
通过ELISA检测血清中TGF-β1的表达,与空白组相比,模型组TGF-β1水平显着升高(P < 0.01);与模型组相比,hUCMSC-EXOs治疗组TGF-β1水平显着降低(P < 0.01,图 3A)。肺切片免疫组化法分析EMT过程的生物标志物vimentin和E-cadherin,结果发现与模型组相比,hUCMSC-EXOs可降低vimentin、p-Smad2/3的表达,促进E-cadherin的表达(图 3B)。
|
图 3 hUCMSC-EXOs对TGF-β1、vimentin、E-cadherin和p-Smad2/3表达的影响 Fig.3 Effect of hUCMSC-EXOs on expressions of TGF-β1, vimentin, E-cadherin and p-Smad2/3. A: The expression of TGF-β1 was detected by ELISA, **P < 0.01; B: The expression of vimentin, E-cadherin and p-Smad2/3 was detected by immunohistochemistry (×400), scale bar: 2 μmol/L. CON: Control group, IPF: Model group, EXOs1: hUCMSC-EXOs were given on the second day after modeling, EXOs2: hUCMSC-EXOs were given on the twenty-first day after modeling. |
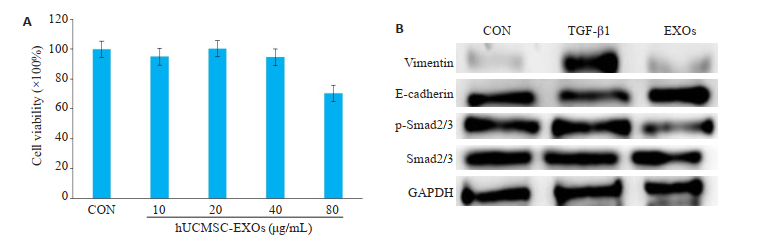
首先观察hUCMSC-EXOs对A549细胞的细胞毒性,在0、20、40、80 μg/mL的浓度下,hUCMSC-EXOs对A549细胞没有明显的细胞毒性(P > 0.05,图 4A)。进一步研究hUCMSC-EXOs对TGF-β1诱导的A549细胞EMT的影响,Western blotting结果显示,与对照组相比,TGF-β1可促进下游Smad2/3蛋白磷酸化,表现在E-cadherin的下调和vimentin的上调,而这一作用可被hUCMSC-EXOs逆转(图 4B、C)。
|
图 4 hUCMSC-EXOs对TGF-β1刺激后的A549细胞中vimentin、E-cadherin和p-Smad2/3表达的影响 Fig.4 Effect of hUCMSC-EXOs on expressions of vimentin, E-cadherin and p-Smad2/3 in A549 cells stimulated by TGF-β1. A: The CCK8 assay showed the effects of different concentration of hUCMSC-EXOs on the cytotoxicity of A549 cells, there was no significant difference (P > 0.05), n=3; B: The level of vimentin, E-cadherin, p-Smad2/3 was detected by western blotting, CON: Control group; TGF-β1: A549 cells were stimulated by TGF-β1 for 20 min (2 ng/mL); EXOs: hUCMSC-EXOs was administered for 24 h (40 μg/mL). |
目前对IPF的治疗有限,迫切需要新的有效治疗方法。抗CCN抗体pamrevlumab(FG3019)刚刚成功完成治疗IPF II期临床试验[21];GLPG1690完成了Ⅱ期临床试验证明了其对IPF的有效性[22];吡非尼酮和尼达尼布已被批准作为IPF的单一疗法[23],与安慰剂相比,两者均可降低疾病进展速度,但并未逆转或停止IPF的发展,并且患者在治疗期间仍出现肺功能的下降[24-25]。MSCs在肺纤维化模型中显示出治疗作用,可降低IPF的死亡率,其可通过调节单核细胞表型来预防和逆转小鼠肺纤维化,减少肺部胶原沉积,并降低支气管肺泡灌洗液中的炎性标志物[26-27];人MSCs条件培养基可抑制AEC的凋亡[28];MSCs可通过STC1对AEC产生作用[29]。但其对EMT的作用尚未发现。
研究发现,直接给予MSCs可能会导致转入已突变或受损的DNA、MSC因其直径太大而无法通过毛细血管、无法迁移至目标器官、MSC数量可因微环境的变化而迅速降低、微环境的改变也会影响MSCs的治疗作用或促进MSCs产生不必要的分化[30-31]。而外泌体则因其较高的安全性、较低的免疫原性、无法直接形成肿瘤等优势成为无细胞疗法的研究热点。此外,外泌体中的组成也可通过MSCs的体外预处理来调节,从而能够基于疾病产生有效治疗疾病的新产品,进一步作为无细胞治疗疾病的新疗法[32]。本研究证实,hUCMSC-EXOs可抑制小鼠肺纤维化的发展,为肺纤维化的治疗提供了新的治疗方向,其可能是IPF的一种新的治疗策略。
本研究通过肺纤维化动物模型观察hUCMSCEXOs对IPF的作用。博来霉素是一种糖肽类抗生素,其在细胞中可引起单链和双链DNA断裂并由此中断细胞周期而起作用,DNA裂解后产生超氧化物和氢氧根自由基,从而引起炎症反应、肺毒性及肺纤维化[33]。研究表明,气管内注射博来霉素造模更加稳定可靠,且与临床IPF的病理机制更加贴近,因此我们使用气管内注射博来霉素诱导的小鼠肺纤维化模型研究hUCMSC-EXOs对IPF影响的体内研究,结果显示,hUCMSC-EXOs可缓解IPF的进展。Mansouri等[19]证明,外泌体可通过调节巨噬细胞表型抑制肺纤维化程度。EMT可能通过上皮细胞和间充质细胞之间旁分泌信号的失调来形成促纤维化微环境[14],我们的结果发现hUCMSC-EXOs可抑制肺纤维化小鼠肺中的EMT过程。
为了进一步明确hUCMSC-EXOs抑制EMT的作用机制,本研究随后建立体外EMT细胞模型,探讨了hUCMSC-EXOs对EMT过程的影响,首先,我们检测了hUCMSC-EXOs对于A549细胞增殖的影响,结果发现,hUCMSC-EXOs在10、20、40、80 μg/mL浓度下,其对A549细胞的增殖无明显影响,进一步的研究发现,hUCMSC-EXOs抑制EMT过程是通过TGF-β1/Smad2/3信号通路实现的。
综上,hUCMSC-EXOs可通过抑制TGF-β1/Smad2/3信号通路激活的EMT过程缓解小鼠肺纤维化,且在造模第二天时给予效果更好,因造模后1~3 d为博来霉素因其的炎症反应阶段[34],猜测可能与hUCMSC-EXOs免疫抑制的作用相关,需要进一步的研究。而hUCMSC-EXOs作为一种无细胞疗法,安全性较高,是IPF一种潜在的治疗手段。
| [1] |
Somogyi V, Chaudhuri N, Torrisi SE, et al. The therapy of idiopathic pulmonary fibrosis: what is next[J]. ? Eur Respir Rev, 2019, 28(153): 190021. DOI:10.1183/16000617.0021-2019 |
| [2] |
Grimminger F, Günther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis[J]. Eur Respir J, 2015, 45(5): 1426-33. DOI:10.1183/09031936.00149614 |
| [3] |
Mathai SK, Schwartz DA. Translational research in pulmonary fibrosis[J]. Transl Res, 2019, 209: 1-13. DOI:10.1016/j.trsl.2019.02.001 |
| [4] |
Chioma OS, Drake WP. Role of microbial agents in pulmonary fibrosis[J]. Yale J Biol Med, 2017, 90(2): 219-27. |
| [5] |
Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline[J]. Am J Respir Crit Care Med, 2015, 192(2): e3-19. |
| [6] |
Nathan SD, Albera C, Bradford WZ, et al. Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis[J]. Lancet Respir Med, 2017, 5(1): 33-41. DOI:10.1016/S2213-2600(16)30326-5 |
| [7] |
Cerri S, Monari M, Guerrieri A, et al. Real-life comparison of pirfenidone and nintedanib in patients with idiopathic pulmonary fibrosis: a 24-month assessment[J]. Respir Med, 2019, 159: 105803. DOI:10.1016/j.rmed.2019.105803 |
| [8] |
Inoue D, Nishi H, Honda K, et al. Renal thrombotic microangiopathy during nintedanib treatment for idiopathic pulmonary fibrosis[J]. Clin Nephrol, 2020, 93(1): 47-50. DOI:10.5414/CN109900 |
| [9] |
Quinn C, Wisse A, Manns ST. Clinical course and management of idiopathic pulmonary fibrosis[J]. Multidiscip Respir Med, 2019, 14: 35. DOI:10.1186/s40248-019-0197-0 |
| [10] |
Han Q, Lin LJ, Zhao BL, et al. Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis by regulating epithelialmesenchymal transition[J]. Biochem Biophys Res Commun, 2018, 500(4): 839-45. DOI:10.1016/j.bbrc.2018.04.148 |
| [11] |
Liu G, Wang YH, Yang LW, et al. Tetraspanin 1 as a mediator of fibrosis inhibits EMT process and Smad2/3 and beta-catenin pathway in human pulmonary fibrosis[J]. J Cell Mol Med, 2019, 23(5): 3583-96. DOI:10.1111/jcmm.14258 |
| [12] |
Hutchinson J, Fogarty A, Hubbard R, et al. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review[J]. Eur Respir J, 2015, 46(3): 795-806. DOI:10.1183/09031936.00185114 |
| [13] |
Nieto, M A, Huang RY, Jackson RA, et al. Emt: 2016[J]. Cell, 2016, 166: 21-45. DOI:10.1016/j.cell.2016.06.028 |
| [14] |
Sakata J, Utsumi F, Suzuki S, et al. Inhibition of ZEB1 leads to inversion of metastatic characteristics and restoration of paclitaxel sensitivity of chronic chemoresistant ovarian carcinoma cells[J]. Oncotarget, 2017, 8(59): 99482-94. DOI:10.18632/oncotarget.20107 |
| [15] |
Jiang LR, Zhang SQ, Hu HZ, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages[J]. Biochem Biophys Res Commun, 2019, 508(3): 735-41. DOI:10.1016/j.bbrc.2018.11.189 |
| [16] |
Tzouvelekis A, Toonkel R, Karampitsakos T, et al. Mesenchymal stem cells for the treatment of idiopathic pulmonary fibrosis[J]. Front Med (Lausanne), 2018, 5: 142. |
| [17] |
Huang K, Kang XW, Wang XY, et al. Conversion of bone marrow mesenchymal stem cells into type Ⅱ alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats[J]. Mol Med Rep, 2015, 11(3): 1685-92. |
| [18] |
Qiu GG, Zheng GP, Ge MH, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles[J]. Stem Cell Res Ther, 2019, 10(1): 359. DOI:10.1186/s13287-019-1484-6 |
| [19] |
Mansouri N, Willis GR, Fernandez-Gonzalez A, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes[J]. JCI Insight, 2019, 4(21): e128060. DOI:10.1172/jci.insight.128060 |
| [20] |
Li T, Xia MX, Gao YY, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy[J]. Expert Opin Biol Ther, 2015, 15(9): 1293-306. DOI:10.1517/14712598.2015.1051528 |
| [21] |
Leask A. Breathe, breathe in the air: the anti-CCN2 antibody pamrevlumab (FG-3019) completes a successful phase Ⅱ clinical trial for idiopathic pulmonary fibrosis[J]. J Cell Commun Signal, 2019, 13(4): 441-2. |
| [22] |
Maher TM, van der Aar EM, van de Steen O, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial[J]. Lancet Respir Med, 2018, 6(8): 627-35. DOI:10.1016/S2213-2600(18)30181-4 |
| [23] |
Flaherty KR, Fell CD, Huggins JT, et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis[J]. Eur Respir J, 2018, 52(2): 1800230. DOI:10.1183/13993003.00230-2018 |
| [24] |
Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial[J]. Lancet Respir Med, 2020, 8(2): 147-57. DOI:10.1016/S2213-2600(19)30341-8 |
| [25] |
Flaherty KR, Brown KK, Wells AU, et al. Design of the pf-ild trial: A double-blind, randomised, placebo-controlled phase iii trial of nintedanib in patients with progressive fibrosing interstitial lung disease[J]. BMJ open Respir Res, 2017, 4(1): e000212. DOI:10.1136/bmjresp-2017-000212 |
| [26] |
Limper AH. Safety of iv human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis[J]. Chest, 2017, 151(5): 951-52. DOI:10.1016/j.chest.2016.12.015 |
| [27] |
Ghadiri M, Young PM, Traini D. Cell-based therapies for the treatment of idiopathic pulmonary fibrosis (ipf) disease[J]. Expert Opin Biol Th, 2016, 16(3): 375-87. DOI:10.1517/14712598.2016.1124085 |
| [28] |
Bernard O, Jeny F, Uzunhan Y, et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating hif and ros hypoxic signaling[J]. Am J Physiol-lung C, 2018, 314(3): L360-71. DOI:10.1152/ajplung.00153.2017 |
| [29] |
Ono M, Ohkouchi S, Kanehira M, et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1[J]. Mol Ther, 2015, 23(3): 549-60. DOI:10.1038/mt.2014.217 |
| [30] |
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy[J]. Stem Cells, 2017, 35(4): 851-8. DOI:10.1002/stem.2575 |
| [31] |
Volarevic V, Markovic BS, Gazdic M, et al. Ethical and safety issues of stem cell-based therapy[J]. Int J Med Sci, 2018, 15(1): 36-45. DOI:10.7150/ijms.21666 |
| [32] |
Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome[J]. Stem Cells Transl Med, 2020, 9(1): 28-38. |
| [33] |
Della Latta V, Cecchettini A, Del Ry S, et al. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions[J]. Pharmacol Res, 2015, 97: 122-30. DOI:10.1016/j.phrs.2015.04.012 |
| [34] |
Chaudhary NI, Schnapp A, Park JE, et al. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model[J]. Am J Resp Crit Care, 2006, 173(7): 769-76. DOI:10.1164/rccm.200505-717OC |
 2020, Vol. 40
2020, Vol. 40

