Abnormalities in liver functions are common in patients admitted to the intensive care unit (ICU) [1], often considered as the late complications of multiple organ failure [2]. But a recent study demonstrated that cholestasis and liver cell necrosis could occur early and were associated with an increased mortality of the patients in ICU[3]. The common liver dysfunctions in ICU patients can be classified into hypoxic hepatitis and cholestasis[4]. Hypoxic hepatitis, histologically characterized typically by necrosis of the centrilobular liver cells, can be accompanied by a transient but sharp increase of liver enzymes by up to 20 times the normal upper limit[5-6], which often occurs in the event of circulatory, respiratory or heart failure while other conditions that potentially cause increased aminotransferase levels are excluded [7-9]. Hypoxic hepatitis, which is not a rare condition, is frequently accompanied by multiorgan injury and associated with a high mortality[10].
Cholestasis, which is caused by critical illness, is defined as decreased or absent bile flow toward the duodenum caused by either impaired bile formation or an inability to excrete bile through the biliary system[11, 12]. While an elevated bilirubin level to 2-3 mg/dL is thought to be diagnostic of cholestasis, alkaline phosphatase (ALP) and glutamate transpeptidase levels are found to be more sensitive to diagnose cholestasis; bile acid has been shown recently to be an even more sensitive and specific indicator for the diagnosis of cholestasis [13-15]. Such inconsistency in the definition of cholestasis impedes an accurate overall assessment of liver dysfunction in ICU patients. In addition, cholestasis has been shown to lead to increased mortality in sepsis[16].
A previous study showed that withholding parenteral nutrition during the first week of critical illness increased plasma bilirubin level, lowered plasma levels of glutamate transpeptidase and alkaline phosphatase but not bile acid level, and reduced the incidence of gallbladder sludge [17]. In animal studies, fasting in critical illness was found to reduce the markers of liver damage and enhance the transport of bile acids into the blood [18]. These results suggest that hyperbilirubinemia during critical illness does not necessarily indicate cholestasis, and instead it may represent an adaptive response. Some researchers therefore suggested that the cholestatic changes caused by critical diseases may be beneficial or occurred as an adaptive changes of the liver[12]. But so far there have been no large clinical trials to support this hypothesis. In this study, we analyzed the impact of cholestatic changes on the outcomes of ICU patients to test the hypothesis that cholestatic alterations represent primarily adaptive changes in the early stage of critical conditions in ICU patients.
METHODS DatabaseWe performed this retrospective study based on the data of ICU patients extracted from Medical Information Mart for Intensive Care-Ⅲ v1.4 (MIMIC-Ⅲ v1.4) database[19], which is a large, freely accessible database. Because this study is of a retrospective nature and the database conceals identifiable patient information, the need for individual informed consent was waived.
Study designWe defined cholestasis as a bilirubin level >2 mg/dL and elevation of alkaline phosphatase (ALP) to twice the upper limit of the normal range (120 U/L)[12]. Hypoxic hepatitis was defined as a 20-fold increase of transaminase levels relative to the normal upper limit (40 U/L) in the presence of circulatory, respiratory or cardiac failure, with normal bilirubin and alkaline phosphatase levels [5-9, 12]. The patients in the control group all had normal bilirubin, alkaline phosphatase and transaminase levels. We thus divided these patients into control group, hypoxic hepatitis (HH) group and cholestasis (CLD) group. We extracted the data from the database including the demographic data, disease characteristics, and the maximum values of serum biochemical parameters on the first day of admission to ICU. The primary outcome was 28-day case fatality rate (within 28 days of ICU admission), and the secondary outcome was ICU case fatality rate, hospital case fatality rate, length of ICU stay and hospital stay.
Patient selectionAmong the adult patients (aged over 18 years) admitted to ICU for the first time in the years from 2001 to 2011, only those with an ICU stay longer than 24 h were included. The patients with liver cirrhosis, liver cancer, sclerosing cholangitis, hemolytic disease, hereditary hyperbilirubinemia, or pancreatic cancer were excluded. The process of selection of eligible patients is illustrated by the flowchart in Fig. 1.
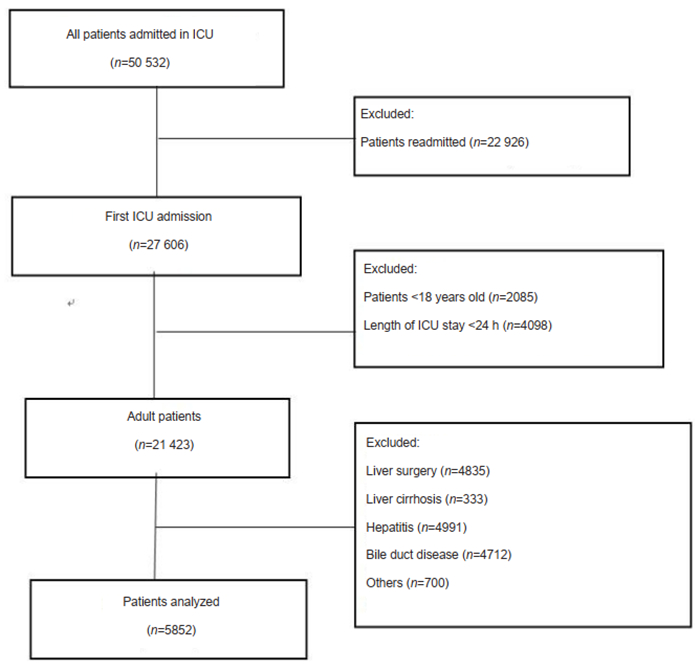
|
Fig.1 Flow chart of case selection. |
Statistical software SPSS 20.0 was used for statistical analysis of the data. The descriptive data are presented as median and quartiles. Chi-square test or Fisher's exact text was used for comparison of the categorical variables among the groups. The continuous variables with normal distribution and homogeneity are presented as Mean ± SD and analyzed with One-Way ANOVA for pairwise comparison. The continuous variables with non-normal distribution are expressed as median with interquartile range. Logistics regression was used to identify the independent risk factors for death, and the results are presented as mortality odds ratio (OR) with 95% confidence intervals (CI). The cumulative survival time of the patients at 30 days, 60 days and 90 days was compared by Kaplan-Meier curve analysis. A P value < 0.05 was considered to indicate a statistically significant difference.
RESULTS Disease distribution characteristicsA total of 5852 patients were included in the analysis. The patients were admitted in ICU due to cardiovascular system diseases (18%), diseases in the digestive system (13%), nervous system (11%), urinary system (8%), and respiratory system (17%), infectious diseases (17%), or other diseases including electrolyte disturbance, trauma, drug overdose (12%); 4% of the patients were admitted for hematologic diseases. The distribution of the diseases was relatively consistent in the overall patients, and their specific diseases are listed in Tab. 1.
| Tab.1 Distribution of specific diseases for ICU admission in the overall patients |
According to the definitions specified previously, the patients were divided into CLD group (n=1869), HH group (n=1046) and control group (n=2937). The average age of the overall patients was 66.1±15.9 years, and 41.5% of the patients were male. No significant differences were found in age (P=0.18) or gender distribution (P=0.56) among the 3 groups. In each of the 3 groups, over 80% of the patients were admitted in ICU in an emergency setting. The types of ICU admission were mostly medical ICU, and the proportion of patients admitted in medical ICU was higher in HH group than in the other 2 groups. Sequential organ failure assessment (SOFA) scores did not differ significantly among the 3 groups. The proportions of patients with cardiovascular and respiratory diseases was significantly higher in HH group and CLD group than in the control group. A higher proportion of patients in the CLD group were admitted due to infectious diseases and required inotropic support.
| Tab.2 Patient characteristics in the 3 groups [Mean±SD or n (%)] |
The test results of serum biochemical parameters on the first day of admission to ICU in the 3 groups are listed in Tab. 3. Univariate analysis showed no significant differences in serum albumin or hemoglobin levels among the 3 groups. Lactic acid (LAC) level in HH group was significantly higher than that in the control group (P=0.00) and CLD group (P=0.03). Of the liver function indicators, total bilirubin, direct bilirubin and indirect bilirubin levels were all significantly higher in CLD group than in the control group and HH group (P < 0.01). Although the total bilirubin level was slightly higher in HH group than in the control group, it was still within the normal range. Alkaline phosphatase (ALP) in the CLD group was higher than that in the other two groups (P < 0.05). The levels of AST and ALT were both significantly higher in HH group than in the other 2 groups (P=0.00). The renal function indicators creatinine (Cr; P=0.001) and blood urea nitrogen (BUN; P=0.002) were also significantly higher in HH group than in the other 2 groups. The international normalized ratio (INR) of HH group was significantly higher than that in the control group (P < 0.01) but comparable with that in CLD group (P=0.81).
| Tab.3 Comparison of serum biochemical parameters on the first day of ICU admission among control, HH and CLD groups (median with interquartile range or Mean±SD) |
As shown in Tab. 4, HH group had the highest 28-day case fatality rate, hospital case fatality rate, and ICU case fatality rate (all P < 0.01) but the shortest average survival (P < 0.01) among the 3 groups. The case fatality rate or the average survival days did not differ significantly between CLD group and the control group.
| Tab.4 Outcomes of the ICU patients in the 3 groups |
We used Kaplan-Meier curve analysis to compare the survival outcomes among the 3 groups. As shown in Fig. 2, the cumulative survival rates were consistently lower in HH group than in the other 2 groups at 30, 60 and 90 days (P < 0.01) but did not differ significantly between CLD group and the control group at 30 days (P=0.619), 60 days (P=0.053), or 90 days (P=0.012). The average survival days of the patients were also the lowest in HH group (P=0.00, Tab. 4).
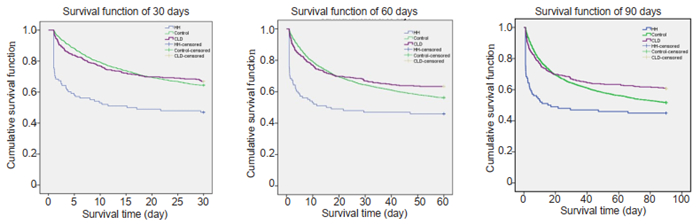
|
Fig.2 Cumulative survival time of HH group, CLD group and control group at 30, 60 and 90 days. |
Logistic regression analysis showed that LAC (OR=1.137, 95% CI: 1.066-1.213), AST (OR 1.093, 95% CI: 1.017-1.166), INR (OR 1.503, 95%CI: 1.158-1.951) were all independent risk factors for 28-day mortality among these patients (Tab. 5).
| Tab.5 Logistic regression analysis of the rsik factors of case fatality rate |
Receiver operating characteristics (ROC) curve analysis (Fig. 3) showed that bilirubin and ALP had a poor ability to predict 28-day case fatality rate. Although AST and ALT had greater areas under the curve (AUC) (0.67 and 0.64, respectively; P=0.00) than bilirubin and ALP, their prediction ability was at a moderate level.
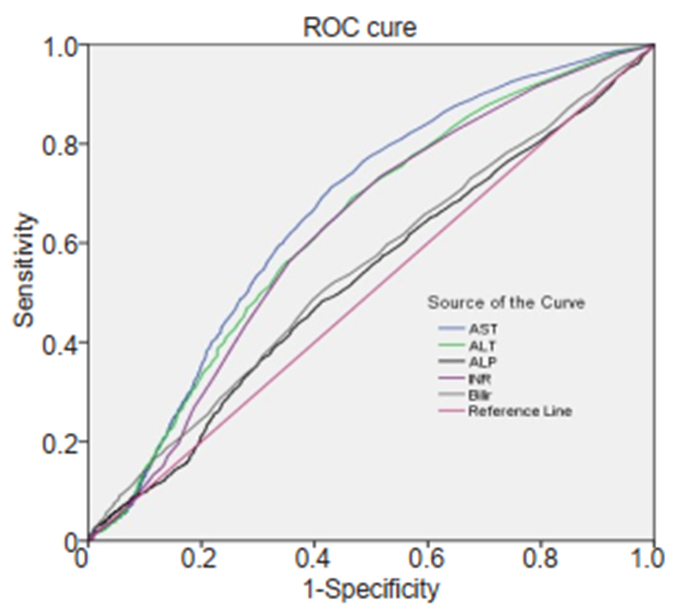
|
Fig.3 Area under ROC curve for different indicators for predicting 28-day case fatality. ALP: Alkaline phosphatase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; INR: International normalized ratio; Bilir: Total bilirubin. |
We found that in critically ill patients, abnormal liver function indicators could be detected as early as the first day of ICU admission and were associated with poor prognosis and increased mortality of the patients[20]. Our results also appear to support the hypothesis that the early abnormalities in the indicators of cholestasis may represent a biochemical epiphenomenon in critically ill patients, and can even be beneficial to the prognosis of patients[21].
Our results demonstrate high incidences of both hypoxic hepatitis and cholestasis in critically ill patients. The incidence of hypoxic hepatitis was 17.9% in the ICU patients, consistent with previous studies where its incidence was reported to range from 10% to 20%[6, 22]. Clinically, hypoxic hepatitis occurs often in ischemic and hypoxic conditions (as in circulatory failure and respiratory failure) [23, 24] and is associated with an increased mortality of the patients. We found that the patients in HH group had a 28-day case fatality rate of 46%, a hospital case fatality rate of 40% and an ICU case fatality rate of 35.7%. The hospital case fatality rate of these patients was higher than those in previous studies[25], possibly due to an greater mean age of the patients, a higher percentage of infections by drug-resistant bacteria and greater numbers of patients with sepsis, severe diseases or organ failure [26, 27]. We noted, however, a shorter ICU stay of the patients in HH group as compared with the other two groups, likely as a result of the high ICU mortality in HH group, where the patients had a shorter survival time and a greater number of early deaths.
A previous report documents an incidence of cholestasis in critically ill patients as high as 54%[16], as compared with 31.9% in our finding. This high incidence of cholestasis, however, did not cause significant increases in 28-day mortality, hospital mortality or ICU mortality as compared with the control group. In the study by Nagaer et al [28], elevated direct bilirubin level was reported to result in reduced 28-day survival rate among postoperative patients. Liver dysfunction was an independent risk factor for death, and the higher the bilirubin level, the greater the risk [20]. The inconsistency in the results may arise from different diagnostic criteria for liver dysfunction: some studies defined a bilirubin level greater than 2 mg/dL as the criteria for diagnosis of liver dysfunction.
Previous preclinical and clinical studies reported changes in liver transporters and nuclear receptors in the early stage of critical illness, especially in sepsis[29, 30]. In patients with sepsis, the protein expressions of NTCP, OATP and BSEP are down-regulated, while the MRP3 and MRP4 transporters are up-regulated in the liver to cause nuclear receptor shifts into the cytoplasm, leading to the loss of the feedback inhibition effect [11]. The overall effect is that bile salt uptake by the hepatocytes is decreased and they are increasingly transferred to the blood circulation, while their synthesis is not inhibited[16, 31]. In a rabbit model of burns, the trend of transporter changes was similar between the fasting group and the parenteral feeding group, but fasting was found to reduce the markers of liver cell damage and enhance the transport of bile acids to the blood[18]. Such increases of circulating bilirubin and changes in transporter levels in critically ill patients may be interpreted as an incidental biochemical response or an adaptive change[21], which is supported by our findings.
We performed logistic regression analysis and identified LAC, AST, and INR as independent risk factors for 28-day death in the ICU patients, but the markers of cholestasis did not show significant correlation with this outcome. This suggests that the early elevation of markers of cholestasis may not necessarily reflect actual impairment of the liver function.
There are some limitations in this study. Firstly, we did not analyze the relationship between the dynamic evolution of liver-related indicators and the patients' outcomes, which needs to be addressed in future studies. Secondly, as less than 100 patients had elevations of both transaminase and bilirubin levels, we did not evaluate the effect of simultaneous elevations of transaminase, bilirubin and ALP on the patients' outcomes.
ConclusionAlthough cholestatic liver dysfunction has a higher incidence than hypoxic hepatitis in ICU patients, it does not increase the mortality of the patients, suggesting that cholestatic liver dysfunction probably signals the early adaptation of the liver to a critical disease.
| [1] |
Thomson SJ, Cowan ML, Johnston I, et al. 'Liver function tests' on the intensive care unit:a prospective, observational study[J]. Intensive Care Med, 2009, 35(8): 1406-11. DOI:10.1007/s00134-009-1511-7 |
| [2] |
Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care.Results of a prospective, multicentre study[J]. Intensive Care Med, 1999, 25(7): 686-96. DOI:10.1007/s001340050931 |
| [3] |
Saloojee A, Skinner DL, Loots E, et al. Hepatic dysfunction:a common occurrence in severely injured patients[J]. Injury, 2017, 48(1): 127-32. DOI:10.1016/j.injury.2016.08.017 |
| [4] |
Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients[J]. Curr Opin Crit Care, 2013, 19(2): 128-32. DOI:10.1097/MCC.0b013e32835ec9e6 |
| [5] |
Henrion J, Schapira M, Luwaert R, et al. Hypoxic hepatitis:clinical and hemodynamic study in 142 consecutive cases[J]. Medicine, 2003, 82(6): 392. DOI:10.1097/01.md.0000101573.54295.bd |
| [6] |
Fuhrmann V, Kneidinger N, Herkner H, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit[J]. Intensive Care Med, 2011, 37(8): 1302-10. |
| [7] |
Waseem N, Chen PH. Hypoxic hepatitis:a review and clinical update[J]. J Clin Transl Hepatol, 2016, 4(3): 263-68. |
| [8] |
Gibson PR, Dudley FJ. Ischemic hepatitis:clinical features, diagnosis and prognosis[J]. Aust N Z J Med, 2010, 14(6): 822-25. |
| [9] |
Fuhrmann V, Kneidinger N, Herkner H. Hypoxic hepatitis:underlying conditions and risk factors for mortality in critically ill patients[J]. Intensive Care Med, 2009, 35(8): 1397-405. DOI:10.1007/s00134-009-1508-2 |
| [10] |
Raurich JM, Llompart-Pou JA, Ferreruela M, et al. Hypoxic hepatitis in critically ill patients:incidence, etiology and risk factors for mortality[J]. J Anesth, 2011, 25(1): 50-56. DOI:10.1007/s00540-010-1058-3 |
| [11] |
Jenniskens M, Langouche L, Vanwijngaerden YM, et al. Cholestatic liver (dys) function during sepsis and other critical illnesses[J]. Intensive Care Med, 2016, 42(1): 16-27. |
| [12] |
Jenniskens M, Langouche L, Van den Berghe G. Cholestatic alterations in the critically ill:some new light on an old problem[J]. Chest, 2018, 153(3): 733-43. DOI:10.1016/j.chest.2017.08.018 |
| [13] |
Wulkan RW, Leijnse B. Alkaline phosphatase and cholestasis[J]. Ann Clin Biochem, 1986, 23(4): 405-12. DOI:10.1177/000456328602300405 |
| [14] |
Bulle F, Mavier P, Zafrani ES, et al. Mechanism of gamma-glutamyl transpeptidase release in serum during intrahepatic and extrahepatic cholestasis in the rat:a histochemical, biochemical and molecular approach[J]. Hepatology, 2010, 11(4): 545-50. |
| [15] |
Horvatits T, Drolz A, Rutter K, et al. Circulating bile acids predict outcome in critically ill patients[J]. Ann Intensive Care, 2017, 7(1): 48. |
| [16] |
Giovannini I, Chiarla C, Giuliante F, et al. Sepsis-induced cholestasis[J]. Hepatology, 2008, 47(1): 361. |
| [17] |
Vanwijngaerden YM, Langouche L, Brunner R, et al. Withholding parenteral nutrition during critical illness increases plasma bilirubin but lowers the incidence of biliary sludge[J]. Hepatology, 2014, 60(1): 202-10. |
| [18] |
Vanwijngaerden YM, Langouche L, Derde S, et al. Impact of parenteral nutrition versus fasting on hepatic bile acid production and transport in a rabbit model of prolonged critical illness[J]. Shock, 2014, 41(1): 48-54. DOI:10.1097/SHK.0000000000000046 |
| [19] |
Johnson AE, Pollard TJ, Shen L, et al. MIMIC-Ⅲ, a freely accessible critical care database[J]. Sci Data, 2016, 3: 160035. DOI:10.1038/sdata.2016.35 |
| [20] |
Kramer L, Jordan B, Druml W, et al. Incidence and prognosis of early hepatic dysfunction in critically ill patients--a prospective multicenter study[J]. Crit Care Med, 2007, 35(4): 1099-104. DOI:10.1097/01.CCM.0000259462.97164.A0 |
| [21] |
Jenniskens M, Güiza F, Oorts M, et al. On the role of illness duration and nutrient restriction in cholestatic alterations that occur during critical illness[J]. Shock, 2017, 50(2): 187-98. |
| [22] |
Raurich JM, Pérez O, Llompart-Pou JA, et al. Incidence and outcome of ischemic hepatitis complicating septic shock[J]. Hepatol Res, 2009, 39(7): 700-5. DOI:10.1111/j.1872-034X.2009.00501.x |
| [23] |
Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy:pathophysiology and prognosis[J]. Internal Med, 2007, 46(14): 1063-70. DOI:10.2169/internalmedicine.46.0059 |
| [24] |
Henrion J. Hypoxic hepatitis:the point of view of the clinician[J]. Acta Gastroenterol Belg, 2007, 70(2): 214-6. |
| [25] |
Aboelsoud MM, Javaid AI, Al-Qadi MO, et al. Hypoxic hepatitis-its biochemical profile, causes and risk factors of mortality in critically-ill patients:a cohort study of 565 Patients[J]. J Crit Care, 2017, 41: 9-15. DOI:10.1016/j.jcrc.2017.04.040 |
| [26] |
Herrán-Monge R, Muriel-Bombín A, García-García MM, et al. Epidemiology and changes in mortality of sepsis after the implementation of Surviving Sepsis Campaign Guidelines[J]. Intensive Care Med, 2019, 34(9): 740-50. DOI:10.1177/0885066617711882 |
| [27] |
Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock:The CUB-Réa Network[J]. Am J Respir Crit Care Med, 2003, 168(2): 165-72. DOI:10.1164/rccm.2201087 |
| [28] |
Nagaer M, Egi M, Kubota K, Makino S, et al. Association of direct bilirubin level with postoperative outcome in critically ill postoperative patients[J]. Korean J Anesthesiol, 2018, 71(1): 30-6. DOI:10.4097/kjae.2018.71.1.30 |
| [29] |
Elfaki DA, Bjornsson E, Lindor KD. Review article:nuclear receptors and liver disease-current understanding and new therapeutic implications[J]. Aliment Pharmacol Ther, 2009, 30(8): 816-25. DOI:10.1111/j.1365-2036.2009.04104.x |
| [30] |
Geier A, Dietrich CG, Voigt S, et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis[J]. Hepatology, 2003, 38(2): 345-54. DOI:10.1053/jhep.2003.50317 |
| [31] |
Strnad P, Tacke F, Koch A, et al. Liver-guardian, modifier and target of sepsis[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(1): 55-66. DOI:10.1038/nrgastro.2016.168 |
 2020, Vol. 40
2020, Vol. 40

