2. 重庆医科大学附属第二医院肝胆外科,重庆 400010
2. Department of Hepatobiliary Surgery, Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China
肝脏缺血再灌注损伤(HIRI)是一种广泛存在于肝叶切除、肝移植等各种肝脏手术中的病理生理过程,由其导致的肝脏组织及肝功能损伤极大程度的影响手术的预后和疾病的转归[1-4]。近年来伴随对缺血再灌注损伤机制的研究,发现细胞焦亡是肝脏发生缺血再灌注损伤的机制之一[5],与其他器官的缺血再灌注损伤过程中细胞焦亡经典途径的激活不同,在肝脏手术中,由于肝门部的阻断导致肠系膜静脉淤血,肠道内革兰阴性菌入血并于开放肝门时经门静脉移位入肝所致的内毒素血症可激活肝脏的缺血再灌注过程中除细胞焦亡经典途径以外,由感染引起的与caspase-11相关的细胞焦亡非经典途径也发挥了重要作用[6-9]。因此,对细胞焦亡非经典途径的研究是肝脏缺血再灌注损伤的重要部分。
异氟醚是临床上常用的卤代类吸入麻醉药,因其对肝脏的保护作用而被广泛运用于各种肝脏手术中。吸入麻醉药在肝肾与缺血再灌注损伤相关的手术中的给药方式分为预处理给药、全程给药、后给药。在异氟醚相关研究中,不同实验分别以这3种给药方式在肝脏缺血再灌注模型中进行异氟醚对肝脏保护作用的研究,结果证明这3种给药方式均可达到异氟醚的肝脏保护作用[10-13],但是否通过细胞焦亡非经典途径起到效果,并没有相关研究结论,因此我们将对异氟醚对细胞焦亡非经典途径的作用作为研究重点。而随着近年来麻醉学的发展,预处理的给药方式因具有以较低给药量即能达到预期麻醉效果,可防止药物蓄积,提高患者舒适度等优势被越来越多的使用于临床麻醉中[14-15],因此,关于预处理这一给药方式的研究更具有临床意义。
本文旨在明确异氟醚经预处理给药对缺血再灌注损伤过程中肝脏的保护作用,并探究这一保护作用与非经典细胞焦亡途径间的关系。为临床肝脏缺血再灌注手术提供更合理的麻醉方案和实验依据。
1 材料和方法 1.1 主要设备及试剂主要设备:小动物麻醉机(深圳瑞沃德生命科技有限公司)。主要试剂:小鼠IL-1β及IL-18 ELISA检测试剂盒(武汉博士德生物工程有限公司),BCA蛋白浓度测定试剂盒及RIPA裂解液(碧云天试剂公司),兔抗caspase-11单克隆抗体(Affinity Biosciences),兔抗Gasdermin D单克隆抗体(Abcam),兔抗GAPDH单克隆抗体(Abcam)。
1.2 实验动物雄性C57BL/6小鼠,8~12周,体质量22±1.5 g,共30只,购自重庆医科大学实验动物中心,实验符合重庆医科大学实验动物伦理保护标准及国家相关法律规定。将动物随机分为3组,每组10只。假手术组(control组,n=10),乙醚吸入麻醉后行上腹正中切口开腹,然后缝合;异氟醚组(isoflurane组,n=10),将小鼠置于小动物麻醉机的麻醉诱导箱中,给予1.4%异氟醚吸入30 min,然后进行肝脏缺血再灌注手术;手术组(I/R组,n=10),乙醚吸入麻醉后行肝脏缺血再灌注手术。
1.3 HIRI模型建立小鼠术前12 h禁食,自由饮水,麻醉后经上腹正中切口进腹,分离肝脏周围韧带,暴露肝门,用血管夹夹闭门静脉造成肝脏缺血,30 min后松开血管夹,恢复肝脏灌流,缝合关腹,2 h后取标本。
1.4 肝组织损伤评估取肝叶组织于10%甲醛液中固定后常规切片和HE染色,于光镜下观察肝组织病变情况并以Suzuki's病理评分标准作为评价坏死、淤血、空泡样变的半定量方法。Suzuki's病理评分标准:0分:无坏死、淤血及肝细胞空泡样变;1分:极轻微的淤血及细空泡样变(< 10%),偶见单个细胞坏死;2分:轻度淤血及细胞空泡样变(11%~30%),轻度组织坏死(< 30%);3分:中重度淤血、细胞空泡样变、组织坏死(31%~60%)[16]。检测血清中转氨酶ALT/AST的水平。
1.5 肝脏细胞焦亡检测收集各组灌注2 h后的小鼠血清,按照ELISA试剂盒说明书检测血清中IL-1β和IL-18的水平。
各组小鼠肝组织中细胞焦亡相关破细胞膜蛋白GSDMD(Gasdermin D)的表达情况以Western blot检测。用RIPA裂解液和蛋白酶抑制剂的混合物溶解肝脏组织,以BCA法测定裂解液的蛋白浓度。80 V恒压下电泳30 min后转为120 V恒压电泳90 min分离等量不同的组织蛋白,而后转膜到0.45 mm PVDF膜上。经5%脱脂乳封膜1 h后,孵育一抗GSDMD,4 ℃过夜,而后孵育二抗,ECL显色,BioRad系统曝光,Image Lab分析图片。
1.6 Western blot检测肝脏细胞焦亡非经典途径相关的caspase-11的蛋白表达用Western blot检测各组小鼠肝组织caspase-11的蛋白表达。方法同GSDMD。
1.7 免疫荧光检测肝脏细胞焦亡非经典途径相关的caspase-11的蛋白表达取再灌注2 h后的小鼠肝脏组织制备石蜡切片,常规脱蜡复水,修复抗原,用0.1%的Triton 37 ℃孵育10 min进行通透然后山羊血清封闭1 h,加入caspase-11抗体4 ℃封闭过夜,PBS洗3次×3 min,加入山羊抗兔的荧光二抗(绿色)37 ℃避光孵育1 h,用DAPI进行细胞核染色,用抗荧光淬灭剂封片,荧光显微镜下观察,用ImageJ软件进行图片荧光半定量分析。
1.8 统计学分析采用SPSS 23.0软件进行统计学分析处理数据。所有结果均以均数±标准差表示,采用方差分析法分析多组间的差异,两组间的差异用LSD法分析,P < 0.05为差异有统计学意义。
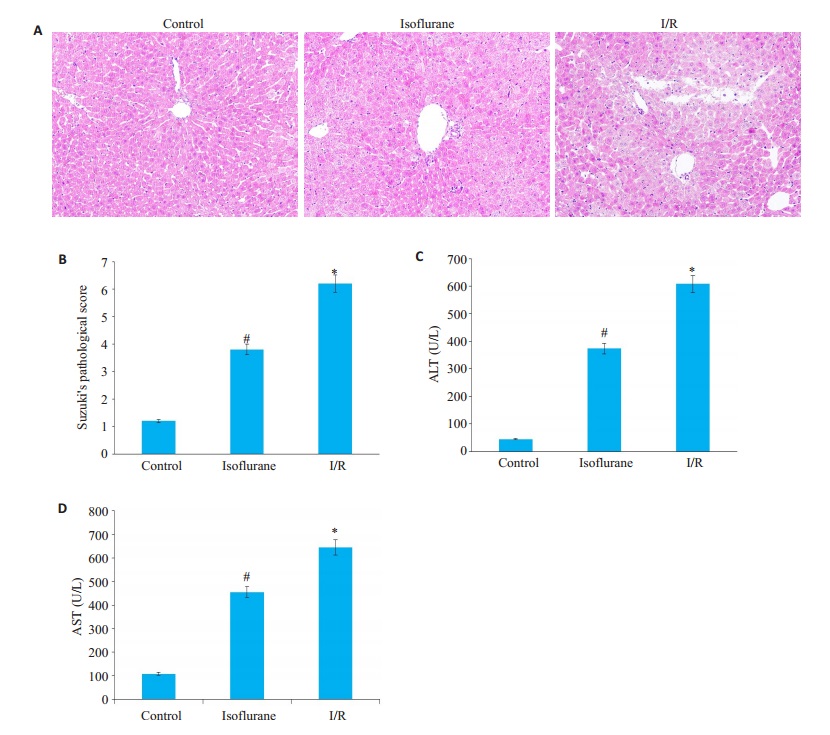
2 结果 2.1 异氟醚预处理对HIRI的保护作用观察各组HE染色切片,control组Suzuki评分1.20±0.91,与此对照,I/R组肝脏再灌注后2 h,汇管区可见大量的炎性细胞聚集伴有广泛肝细胞变性坏死,偶见肝组织结构破坏不完整,Suzuki评分6.20 ± 1.03;isoflurane组肝脏再灌注后2 h,汇管区可见炎性细胞聚集,局部见细胞空泡样变及坏死,肝组织结构基本完整,Suzuki评分3.80±1.23。各组HE染色切片评分,差异均具有统计学意义(P < 0.05,图 1A、B)。
|
图 1 缺血再灌注后的肝脏的损伤情况 Fig.1 liver tissue after ischemia- reperfusion injury. A: HE staining sections of liver tissue after ischemia-reperfusion (Original magnification:×200); B: Data of Suzuki's pathological score (Mean±SD, n= 10). *P < 0.05 vs control group, #P < 0.05 vs I/R; C、D: ALT and AST serum levels. Data were presented as (Mean±SD, n=10). *P < 0.05 vs control group, #P < 0.05 vs I/R group. |
经生化检测各组血清中ALT/AST水平,I/R组小鼠血清中ALT/AST水平明显高于control组小鼠,isoflurane组小鼠血清中ALT/AST水平较I/R组则明显下降。差异均具有统计学意义(P < 0.05,图 1C、D )。
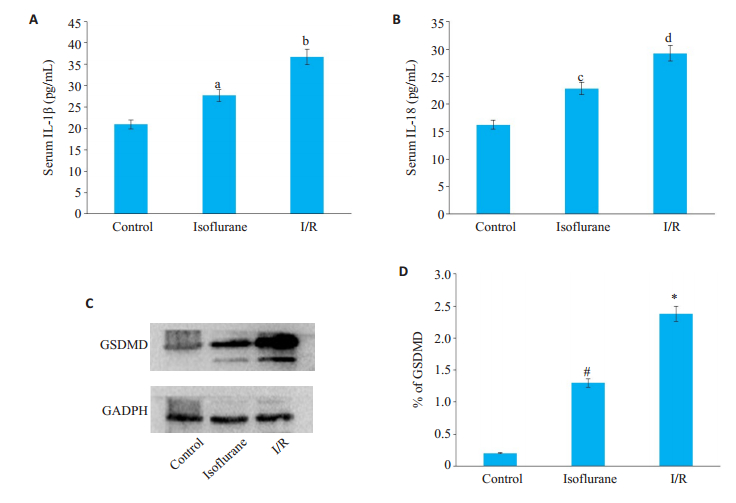
2.2 异氟醚预处理对HIRI过程中的细胞焦亡途径有抑制作用经ELISA检测各组血清中与细胞焦亡相关的炎症因子IL-1β和IL-18的表达情况,与control组相比,I/R组血清中IL-1β与IL-18的表达均明显增高,其差异有统计学意义(P < 0.05)。经异氟醚预给药处理的isoflurane组血清中IL-1β与IL-18的表达较I/R组显著降低,差异有统计学意义(P < 0.05,图 2A、B)。
|
图 2 血清中IL-1β和IL-18的表达水平及肝脏中GSDMD的表达水平 Fig.2 Expression levels of IL-1β and IL-18 in serum and GSDMD in liver tissue. A: Serum levels of IL-1β and IL-18; B: Data are shown as Mean±SD, n=10. bP < 0.05 vs control group, aP < 0.05 vs I/R group; dP < 0.05 vs control group, cP < 0.05 vs I/R group; C: Expression of GSDMD in liver tissue detects by Western blot; D: Data are shown as Mean±SD, n=3. *P < 0.05 vs control group, #P < 0.05 vs isoflurane |
Western blot检测I/R组小鼠肝组织中GSDMD的蛋白表达较control组明显增高(P < 0.05)。而经异氟醚预给药处理的isoflurane组GSDMD的蛋白表达较I/R组显著降低(P < 0.05,图 2C、D)。
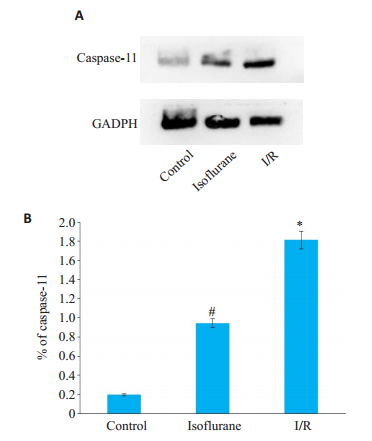
2.3 异氟醚预处理对与非经典细胞焦亡途径相关的caspase-11有抑制作用Western blot实验显示I/R组中caspase-11的蛋白表达较control组明显增高(P < 0.05)。经异氟醚预给药处理的isoflurane组caspase-11的蛋白表达较I/R组显著降低(P < 0.05,图 3)。
|
图 3 Western blot检测肝脏组织中caspase-11的表达 Fig.3 Expression of caspase-11 in liver tissue detects by Western blot. A: Expression of caspase-11 in liver tissue detects by Western Blot; B: Data are shown as Mean ± SD, n=3. *P < 0.05 vs control group, #P < 0.05 vs isoflurane group |
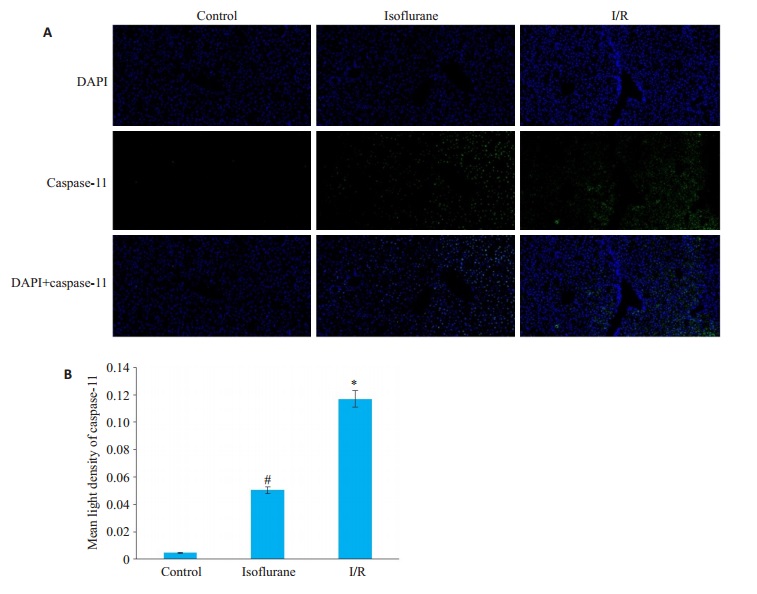
免疫荧光实验表明相较control组中caspase-11在肝脏组织中极少量的表达,I/R组caspase-11的表达增高明显(P < 0.05)。而isoflurane组的表达情况较I/R组降低显著(P < 0.05,图 4)。
|
图 4 免疫荧光检测肝脏组织中caspase-11的表达 Fig.4 Expression of caspase-11 in liver tissue detects by immunofluorescence. A: Expression of caspase-11 in liver tissue detects by immunofluorescence (×200); B: Data are shown as Mean ± SD, n=3. *P < 0.05 vs control group, #P < 0.05 vs I/R group |
静吸复合麻醉是临床上常用的一种全身麻醉方式,因其较全凭静脉麻醉有着减少单一麻醉药物用量、防止药物蓄积,更易控制麻醉深度等优势而被广泛运用[17]。其中,异氟醚作为一种无肝肾毒性的卤代类吸入麻醉药而被广泛接受。评价吸入麻醉药的效价强度时常用最低肺泡有效浓度药物浓度,我们在研究过程中选用异氟醚的最低肺泡有效浓度,即1.4%作为实验浓度。
肝脏缺血再灌注损伤是一种与炎症相关的复杂的病理生理过程,可导致肝功能损害甚至衰竭,影响病程的良性发展[18-20]。近年来,在肝脏缺血再灌注损伤的保护研究中,麻醉剂预处理受到广泛关注。多项研究表明:异氟醚等卤代类吸入麻醉药被证实在缺血前给予一定时间的吸入可减轻后面缺血再灌注造成的损害[10, 12, 14]。在本研究中,通过对肝脏HE染色切片的观察和病理评分以及血清ALT/AST水平检测结果,显示异氟醚预处理对HIRI有保护作用,与文献报道一致。肝脏缺血再灌注损伤的特殊之处在于肝门阻断造成肠系膜静脉淤血,肠道内革兰阴性菌移位入血,再灌注时入血的肠道革兰阴性菌随血液经门静脉入肝并释放内毒素造成的内毒素血症是肝脏缺血再灌注损伤过程中不可避免发生的感染因素[21-25]。因此,在研究通过抑制细胞焦亡途径保护肝脏缺血再灌注损伤时,与感染相关的细胞焦亡非经典途径的抑制情况成为研究重点。多项研究发现:肝脏的缺血再灌注损伤与细胞焦亡途径相关[26-27]。本研究检测到,I/R组血清中IL-1β和IL-18的表达以及肝组织中焦亡相关破细胞膜蛋白GSDMD的表达明显高于control组,证实缺血再灌注激活细胞焦亡的结论,而经异氟醚预处理的isoflurane组中IL-1β和IL-18表达较I/R组显著降低。由此进一步证明异氟醚预处理对肝脏缺血再灌注损伤过程中的细胞焦亡有抑制作用。Caspase-11是细胞焦亡非经典途径的重要启动因子。肝脏缺血再灌注时,革兰阴性菌经门静脉入肝,释放表面的脂多糖(LPS)经TLR通路激活Kupffer细胞这一肝脏固有巨噬细胞,LPS以内吞的形式进入Kupffer细胞,与胞内caspase-11结合并将其活化,由此启动了细胞焦亡非经典途径。活化表达的caspase-11一方面激活下游NLRP3炎症因子,释放IL-1β和IL-18;另一方面活化破细胞膜蛋白GSDMD,破坏细胞膜,释放细胞内容物,导致炎性损伤[28-31]。与其他针对抑制细胞焦亡经典途径对肝脏缺血再灌注损伤产生保护作用的研究不同,本研究结果表明,肝脏缺血再灌注损伤建模后caspase-11的蛋白表达明显增高,结合IL-1β、IL-18与GSDMD的检测结果,说明肝脏缺血再灌注损伤过程中存在显著的细胞焦亡非经典途径活化现象,并对炎性相关损伤的发展结局起了促进作用。在本研究中,给予异氟醚预处理干预后,caspase-11的蛋白表达显著下降,血清中的IL-1β和IL-18降低,肝组织中GSDMD表达降低,说明异氟醚预处理可抑制细胞焦亡非经典途径的激活,并对由此引起的炎性损伤有明显保护作用。
综上所述,在肝脏缺血再灌注前给予一段时间的异氟醚预处理可通过抑制细胞焦亡非经典途径明显减轻缺血再灌注造成的肝脏炎性损伤。但因本研究只对动物模型中组织形态和血清中的炎症因子表达进行了研究,而未进行相关体外实验对这一作用机制进行进一步验证,也未能探究异氟醚预处理对抑制细胞焦亡非经典途径这一作用的有效浓度范围。未来将模拟体内环境建立细胞实验模型,对该机制及有效浓度范围深入研究,为异氟醚预处理的临床应用提供实验依据。
| [1] |
Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury[J]. Curr Pharm Des, 2006, 12(23): 2867-73. DOI:10.2174/138161206777947597 |
| [2] |
Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation[J]. Transplant Proc, 2005, 37(4): 1653-6. |
| [3] |
Bavarsad K, Riahi MM, Saadat S, et al. Protective effects of curcumin against ischemia-reperfusion injury in the liver[J]. Pharmacol Res, 2019, 141: 53-62. DOI:10.1016/j.phrs.2018.12.014 |
| [4] |
Osman AS, Osman AH, Kamel MM. Study of the protective effect of ischemic and pharmacological preconditioning on hepatic ischemic reperfusion injury induced in rats[J]. JGH Open, 2017, 1(3): 105-11. DOI:10.1002/jgh3.12018 |
| [5] |
Yuan JY, Najafov A, Py BF. Roles of caspases in necrotic cell death[J]. Cell, 2016, 167(7): 1693-704. DOI:10.1016/j.cell.2016.11.047 |
| [6] |
Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease[J]. Nature, 2012, 481(7381): 278-86. DOI:10.1038/nature10759 |
| [7] |
Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection[J]. Nat Rev Immunol, 2017, 17(3): 151-64. DOI:10.1038/nri.2016.147 |
| [8] |
Ali JM, Davies SE, Brais RJ, et al. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes[J]. Liver Transpl, 2015, 21(4): 487-99. DOI:10.1002/lt.24072 |
| [9] |
Yi YS. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses[J]. Immunology, 2017, 152(2): 207-17. |
| [10] |
Lv X, Yang LQ, Tao KM, et al. Isoflurane preconditioning at clinically relevant doses induce protective effects of heme oxygenase-1 on hepatic ischemia reperfusion in rats[J]. BMC Gastroenterol, 2011, 11: 31. DOI:10.1186/1471-230X-11-31 |
| [11] |
Zhang HF, Xiong XX, Liu J, et al. Emulsified isoflurane protects against transient focal cerebral ischemia injury in rats via the PI3K/ Akt signaling pathway[J]. Anesth Analg, 2016, 122(5): 1377-84. DOI:10.1213/ANE.0000000000001172 |
| [12] |
Wang ZM, Lv H, Song SH, et al. Emulsified isoflurane preconditioning protects isolated rat Kupffer cells against hypoxia/ reoxygenation-induced injury[J]. Int J Med Sci, 2013, 10(3): 286-91. DOI:10.7150/ijms.5343 |
| [13] |
Flondor M, Hofstetter C, Boost KA, et al. Isoflurane inhalation after induction of endotoxemia in rats attenuates the systemic cytokine response[J]. Eur Surg Res, 2008, 40(1): 1-6. |
| [14] |
杨立群, 俞卫锋. 麻醉药预处理减轻肝脏缺血再灌注损伤的研究进展[J]. 中华临床医师杂志:电子版, 2012, 6(4): 812-6. |
| [15] |
McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia[J]. Anesth Analg, 2007, 104(5): 1066-77. DOI:10.1213/01.ane.0000260321.62377.74 |
| [16] |
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine[J]. Transplantation, 1993, 55(6): 1265-72. DOI:10.1097/00007890-199306000-00011 |
| [17] |
Zhao JL, Wang F, Zhang YQ, et al. Sevoflurane preconditioning attenuates myocardial ischemia/reperfusion injury via caveolin-3- dependent cyclooxygenase-2 inhibition[J]. Circulation, 2013, 128(11 Suppl 1): S121-9. |
| [18] |
Xu Y, Yao J, Zou C, et al. Asiatic acid protects against hepatic ischemia/reperfusion injury by inactivation of Kupffer cells via PPARγ/NLRP3 inflammasome signaling pathway[J]. Oncotarget, 2017, 8(49): 86339-55. DOI:10.18632/oncotarget.21151 |
| [19] |
Zhao HL, Huang H, Alam A, et al. VEGF mitigates histone-induced pyroptosis in the remote liver injury associated with renal allograft ischemia-reperfusion injury in rats[J]. Am J Transplant, 2018, 18(8): 1890-903. DOI:10.1111/ajt.14699 |
| [20] |
Hua SY, Ma MY, Fei XY, et al. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated kupffer cells pyroptosis[J]. Int Immunopharmacol, 2019, 68: 145-55. DOI:10.1016/j.intimp.2019.01.002 |
| [21] |
Schauvliege R, Vanrobaeys J, Schotte P, et al. Caspase-11 gene expression in response to lipopolysaccharide and interferon-Gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1[J]. J Biol Chem, 2002, 277(44): 41624-30. DOI:10.1074/jbc.M207852200 |
| [22] |
Shi JJ, Zhao Y, Wang YP, et al. Inflammatory caspases are innate immune receptors for intracellular LPS[J]. Nature, 2014, 514(7521): 187-92. DOI:10.1038/nature13683 |
| [23] |
An JS, Kim SH, Hwang D, et al. Caspase-4 disaggregates lipopolysaccharide micelles via LPS-CARD interaction[J]. Sci Rep, 2019, 9(1): 826. |
| [24] |
Hagar JA, Powell DA, Aachoui Y, et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock[J]. Science, 2013, 341(6151): 1250-3. DOI:10.1126/science.1240988 |
| [25] |
Lee BL, Stowe IB, Gupta A, et al. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation[J]. J Exp Med, 2018, 215(9): 2279-88. DOI:10.1084/jem.20180589 |
| [26] |
Takeuchi D, Yoshidome H, Kato A, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice[J]. Hepatology, 2004, 39(3): 699-710. DOI:10.1002/hep.20117 |
| [27] |
Inoue Y, Shirasuna K, Kimura H, et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes[J]. J Immunol, 2014, 192(9): 4342-51. DOI:10.4049/jimmunol.1302039 |
| [28] |
Zhao Y, Shi JJ, Shao F. Inflammatory caspases: activation and cleavage of gasdermin-D in vitro and during pyroptosis[J]. Methods Mol Biol, 2018, 1714: 131-48. |
| [29] |
Smith C, Wang XH, Yin H. Caspases come together over LPS[J]. Trends Immunol, 2015, 36(2): 59-61. |
| [30] |
Deng MH, Tang YT, Li WB, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in Sepsis[J]. Immunity, 2018, 49(4): 740-53.e7. DOI:10.1016/j.immuni.2018.08.016 |
| [31] |
Niu XF, Yao Q, Li WF, et al. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice[J]. Eur J Pharmacol, 2019, 849: 160-9. DOI:10.1016/j.ejphar.2019.01.062 |
 2020, Vol. 40
2020, Vol. 40

