2. Cell Electrophysiology Laboratory, Wannan Medical College, Wuhu 241002, China;
3. Neurobiology Laboratory, Wannan Medical College, Wuhu 241002, China
2. 皖南医学院 细胞电生理研究室,安徽 芜湖 241002;
3. 皖南医学院 神经生物学研究室,安徽 芜湖 241002
Anorexia nervosa (AN) is a serious eating disorder characterized by an intense desire to lose body weight or a fear of gaining weight that leads to body image distortion and has the highest mortality rate among all the psychiatric illnesses[1-3]. Data indicated that most of the deaths associated with AN result from starvation-related medical complications, partly due to cardiac complications and infections[1, 4, 5].
The medullary cardiovascular center includes mainly the depressor area of the caudal ventrolateral medulla (CVLM) and the pressor area of the rostral ventrolateral medulla (RVLM). The RVLM is one of the important sites for the generation and maintenance of cardiac sympathetic tone and sympathetic vasoconstrictor tone. Evidence from previous studies shows that the RVLM is an integrated center that receives cardiovascular-related regulatory information from the nucleus tractus solitarii (NTS), CVLM, and paraventricular nucleus (PVN), as well as input information from peripheral cardiovascular activity[6, 7]. These findings raise the hypothesis that acute changes in cardiovascular function in patients with AN may be partly attributed to the change in neural activity of the RVLM.
A diverse collection of stimuli to the NAc are shown to regulate reward-seeking behaviors and food consumption[8, 9]. In particular, the medial shell of the NAc is responsible for the hedonic impact of sensory pleasures and for producing incentive stimuli for food intake, drugs, and other rewards[10, 11]. Therefore, some psychiatric conditions, especially eating disorders, might be associated with the dysfunction of the NAc that regulates food intake activities. This notion is supported by observations that the deactivation of the medial shell of the NAc selectively increases the consumption of certain foods, which elicits the implication of the NAc in the control of feeding behavior[12]. In electrophysiological studies, the NAc neurons in rat models of AN were shown to discharge a high frequency burst of action potentials, indicating an increased activity of neurons in the NAc[13, 14]. Indeed, the NAc is believed to be one of the most vital components that influence the onset and development of AN[15-17].
Here we hypothesized that in patients with AN, a potential link between the NAc and the RVLM affects the mechanisms involved in the regulation of cardiovascular function. To test this hypothesis, we established a rat model of AN with hyperactivity and restricted feeding. To clarify the relation of the NAc with the RVLM, a retrograde tracer was applied to identify the neural pathway between the NAc and the RVLM, and the electrophysiological properties of RVLM neurons and the peripheral physiological indexes of the rats were simultaneously recorded during electric activation of the NAc.
MATERIALS AND METHODS Animal modelingFifty male Sprague-Dawley rats weighing 260-290 g were purchased from Qinglongshan Lab Animal Ltd. (Nanjing, China). All the animal treatments were approved by the Animal Care and Use Committee of Wannan Medical College. Rat models of AN were established following the protocols described in previous reports with minor modifications [18, 19]. Briefly, following a one-week habituation period, the rats were randomly divided into AN model group and control group, all housed in separated cages. The rats in the model group were allowed free access to a running wheel inside the cage all the time but with restricted food intake by giving them access to daily food for 1 h. The rats in the control group were housed without running wheels under the same conditions but were fed ad lib during the whole experiment. The body weight of the rats was measured daily, and the modeling was considered successful when the model rats had a 25% reduction of body weight relative to that of the normally fed rats.
Retrograde labeling with FluoroGoldThe retrograde tracer, FluoroGold (FG, Fluorochrome, Inc., Denver, USA), was applied due to the lack of concomitant anterograde transport, its high sensitivity and low uptake by fibers of passage. Based on the protocols in previous reports [20, 21], the rats were anesthetized with an intraperitoneal injection of composite anesthetic agent (14 g urethane, 0.7 g chloralose and 0.7 g borax per 100 mL normal saline). The rat was fixed in a prone position in a stereotaxic frame (Model 68002, Shenzhen Ruiwo De Life Technology Co., Ltd., China), and the skull was exposed through a midline incision for a small craniotomy, which was drilled over the right RVLM (stereotaxic coordinates with respect to the bregma: posterior 12.00-12.36 mm, lateral 1.9-2.4 mm, and vertical 8.9-10.2 mm below the skull) according to the stereotaxic atlas of Paxinos and Watson[22]. A saline solution (0.5 μL) consisting of 4% FG was subsequently injected into the RVLM over a 10-minute period, followed by an additional minute to prevent the backflow. After the microinjection, the incision was filled with gelatin sponge and the incision was closed after disinfection with 75% alcohol. The rats were kept undisturbed for one week until tissue harvesting. For tissue harvesting, the rats were anesthetized and perfused with heparinized saline for 60 min, followed by 4% paraformaldehyde fixative in 0.1 mol/L PBS (pH 7.4). Brain tissues were harvested immediately after perfusion and incubated in cold fixative overnight. The tissues were then treated with sucrose solution of increasing concentrations (20% and 30%) overnight until they sunk to the bottom. A cryostat microtome (Thermo Scientific HM525 NX, Germany) was used to prepare brain tissue slices to identify the sites of retrograde tracer injection and labeling. The FG-labeled neurons in the target regions were examined with a standard fluorescent microscope (Olympus, BX53, Japan). The slices containing the NAc, PVN and RVLM were identified based on their best matched standard stereotaxic plane, as presented in the atlas of Paxinos and Watson for the rat[22].
Synchronous recording of RVLM neuron firing and peripheral physiological indicatorsFollowing the descriptions in a previous study[23], we recorded the physiological hemodynamic parameters and electrocardiogram (ECG) of the rats using an integrated multiple-channel intraoperative monitor (PowerLab system, AD Instruments, Australia), which sent signals through the amplifiers. The rats were anesthetized following the afore-mentioned procedure, fixed in a stereotactic frame, and warmed to 37 ℃ using heating pad. The arterial blood pressure (ABP) was measured by cannulation of the left femoral artery. After an oblique cut was made on the surface of the femoral artery towards the heart, a catheter filled with 0.02% heparinized saline was inserted, bevel up, into the femoral artery for ABP monitoring. The catheter was connected to the amplifier (ML221, AD Instruments, Australia) through a transducer. Standard limb II lead ECG was recorded with the needle electrodes. In addition, a rectal temperature probe was inserted into the rectum of the rat to measure the body temperature. Data acquisition and analysis were performed using LabChart software (AD Instruments).
To record the discharges of RVLM neurons, the rats were fixed in a prone position, and a small craniotomy was drilled over the right NAc (stereotaxic coordinates with respect to the bregma: anterior 1.2-1.7 mm, lateral 1.0-1.7 mm, and vertical 6.0-7.5 mm below the skull) according to the stereotaxic atlas [22]. A stimulating electrode was implanted in the NAc, and another glass recording microelectrode filled with 0.5% sodium acetate containing 2% Chicago sky blue dye was implanted into the RVLM. The resistance of these microelectrodes measured in vivo averaged 5-15 MΩ. A reference electrode was placed on the skull surface. Before detecting the neuronal signals, the electrodes were manually fine-tuned to capture the spontaneous discharge of the RVLM neurons. Neuronal signals were acquired, processed by the amplifier (Model ML408, AD Instruments, Australia), and input to a channel of the PowerLab system. RVLM neuronal signal was recorded simultaneously with peripheral cardiovascular indicators such as ECG and ABP.
Stimulating methodsTo explore the relationship between the NAc and the RVLM neurons, a stimulating electrode was inserted into the NAc to deliver the stimuli. The stimulation parameters were: 200 μA, 100 Hz using 0.2 ms pulse; and 400 μA, 100 Hz using 0.2 ms pulse; each stimulation with the two parameter settings lasted for 0.2 s. The stimuli-induced activities in RVLM neurons and cardiovascular changes were detected using the method as described above.
Data acquisition and analysisRaw signals were captured, amplified, and transferred to PowerLab data acquisition and analysis system. Then LabChart software was used to analyze the data acquired. The results were expressed as Mean±SD. Comparison of the data acquired before and after the electrical stimulation of the NAc was made using one-way analysis of variance (one-way ANOVA). The data of AN model group and the control group were compared using independent-sample t-test, and the body weight changes of the rats in the two groups at days 14 and 21 were compared using paired t-test. A P value less than 0.05 was considered to indicate a statistically significant difference.
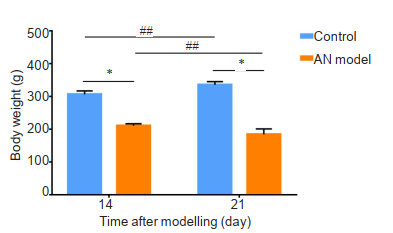
RESULTS Body weight monitoringIn AN model group, the rats showed a significant decrease in body weight, whereas the rats in the control group continued to gain weight (P < 0.01). On days 14 and 21, the rats with restricted feeding demonstrated a significant body weight reduction compared with that of the control rats (P < 0.05, Fig. 1). At the end of the 3-week experiment, the AN model rats had a 25% lower body weight than the control rats, indicating successful establishment of AN rat models.
|
Fig.1 Changes in body weight of the rats in AN model group (n=12) and control group (n=15) during the 3-week experiment. Data are presented as Mean ± SD. *P < 0.05 (independent-sample t-test), ##P < 0.01 (paired t-test). |
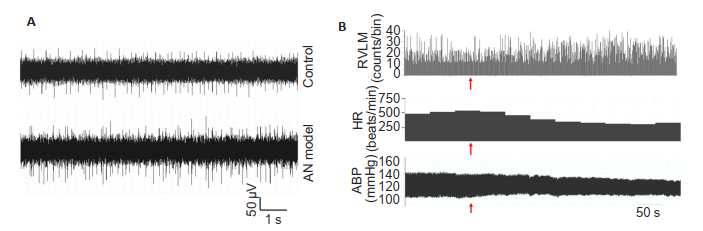
Compared with the control rats, the AN model rats showed significant reductions in the cardiovascular parameters including systolic blood pressure (SBP), mean arterial pressure (MAP), and heart rate (HR) (P < 0.05). A significant increase was noted in the single-unit discharge frequency of RVLM neurons in the model rats (P < 0.05, Tab. 1, Fig. 5A).
| Tab.1 Comparison of cardiovascular parameters and discharge frequency of RVLM neurons between AN model and control groups (Mean±SD) |
|
Fig.5 Representative raw diagrams of the RVLM neuron electrical activity (A), tracings of ABP, HR and the histogram of RVLM neuron firing before and after electrical stimulation of the NAc (B). The electrical stimulation time was indicated by the arrows. The tracing of HR is based on ECG histogram. |
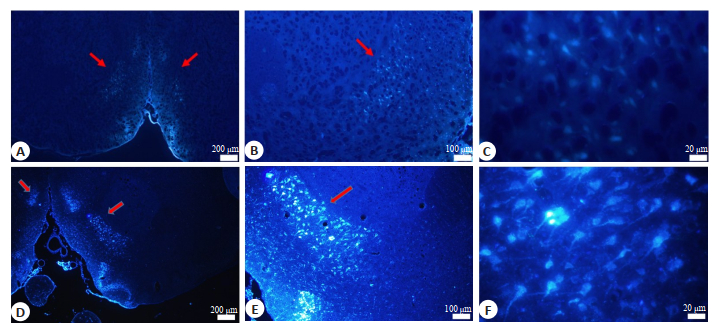
To investigate whether NAc neurons directly project to the RVLM, the retrograde tracer, FluoroGold, was injected into the RVLM. At 1 week after tracer injection, coronal sections of the brain (50 μm) were prepared for observation of FG labeling. Fluorescent analysis of these sections revealed the presence of retrogradely filled neurons in the NAc of both normal rats and AN model rats (Fig. 2), confirming the projection from NAc to the RVLM.
|
Fig.2 Fluorescent microscopic images of the neurons in the NAc labeled with FluoroGold injected in the RVLM of normal rats (A, B and C) and AN model rats (D, E and F). Blue fluorescence represents the neurons filled with FluoroGold. Arrows indicate the NAc. |
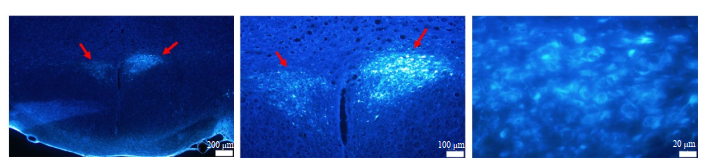
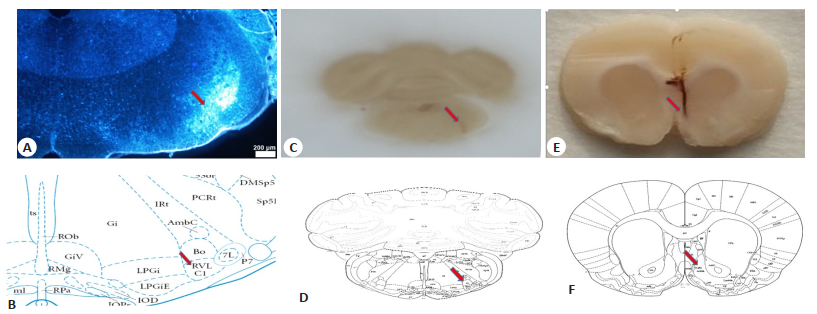
We also examined the PVN on the sections to validate the injection site and the retrograde tracing ability of FG. The PVN neurons were obviously stained with FG, demonstrating that FG was correctly injected into the RVLM (Fig. 3). In addition, the injection site and the diffusion area of FG were visualized and mapped onto standard brainstem atlas for the rat[22], and the results demonstrated that the tracer injection site and the diffusion maps closely matched the coordinates of the RVLM (Fig. 4A, C).
|
Fig.3 Fluorescent microscopic images of the neurons in the PVN (arrows) labeled with FluoroGold injected in the RVLM. Blue fluorescence represents the neurons filled with FluoroGold. |
|
Fig.4 Identification of retrograde tracer injection site and the stimulation site. A: Diffusion area of FluoroGold (arrow) close to the region of RVLM; B, D: Diagrams showing the location of the RVLM at Bregma -12.12 mm (adapted from Paxinos and Watson's rat brain atlas); C: Injection site of the retrograde tracer (arrow); E: Electrical stimulation site of the NAc (arrow); F: Diagram showing the location of the NAc at Bregma 1.42 mm (adapted from the Paxinos and Watson's rat brain atlas). |
In normal rats, electrical stimulation (200 μA) of the NAc resulted in significantly lowered SBP (P < 0.05), which was further decreased following stimulation with 400 μA current (P < 0.01); the heart rate was significantly decreased (P < 0.05) and the discharge frequency of the RVLM neurons was significantly increased in response to 400 μA current stimulation (P < 0.01, Tab. 2, Fig. 5B). In AN model rats, the cardiovascular parameters and the discharge frequency of RVLM neurons did not exhibit significant changes in response to 200 μA electrical stimulation; stimulation of the NAc with 400 μA current significantly reduced SBP and heart rate and obviously increased the discharge frequency of RVLM neurons (P < 0.05). Compared with the normal rats, the AN model rats showed more obvious reduction in DBP and MAP (P < 0.05) and a slightly greater increase in the discharge frequency of RVLM neurons following 400 μA electric stimulation (Tab. 3). The electrical stimulation sites in the NAc were shown in Fig. 4.
| Tab.2 Changes in cardiovascular parameters and discharge frequency of RVLM neurons in normal rats after electrical stimulation (Mean±SD) |
| Tab.3 Changes in cardiovascular parameters and discharge frequency of RVLM neurons in AN model rats after electrical stimulation (Mean±SD) |
The findings of our investigation confirm the presence of a neuronal pathway connecting the NAc and the RVLM, which is associated with the regulation of cardiovascular functions in a rat model of AN. The activity-based anorexia model we used is a typical model to investigate AN, although its limitations should be recognized, including the absence of psychosocial and interpersonal factors in the modeling[2]. In addition, the rats did not lose weight on a voluntarily basis as in human anorexic patients, and began to regain weight when allowed free access to food[24]. But this model is so far the best animal model to mimic AN in humans because of its two most important features, physical activity and limited food intake, which are typical of human patients with AN[25, 26].
We show that electrical stimulation of the NAc causes changes in cardiovascular functions and RVLM neuron activities, suggesting potential impairment of cardiovascular functions in the AN model rats. These alterations raise the question over whether the NAc and the RVLM are involved in the regulatory mechanism underlying the cardiovascular abnormalities of AN patients.
We found that injection of FG in the RVLM resulted in obvious FG staining of the neurons within the NAc, indicating a potential projection from the NAc to the RVLM. This observation partly confirms our hypothesis that this neural connection participates in the regulation of cardiovascular functions in AN patients. We noted that following FG injection in the RVLM, FG staining occurred also in another nucleus, the PVN, which plays a crucial role in the hypothalamus as the so-called cardiovascular integration center [27-29], confirming the correct procedure and the accurate site of tracer injection.
To characterize the connection between the NAc and the RVLM, we used the retrograde tracer FG in this experiment. The major constituent of FG is slightly basic hydroxystilbamidine, which exhibits a broad spectrum of fluorescence when excited by ultraviolet light. For instance, FG emits a golden yellow light at a physiological pH, while a blue light with acidic buffer[21, 30]. More importantly, FG emits intense fluorescence without uptake by intact healthy fibers of passages or diffusion from the filled neurons, and has a long labeling period. These merits make this retrograde tracer the golden standard of neuronal tracing techniques for rodent brain connectivity investigation. When combined with other tracers, FG can be more efficient for labeling target neurons and mapping the neuronal connections.
FG was used to investigate the neuronal connectivity between different central parts that regulate food intake and gastric motility [31]. Various reciprocal neural connections exist in many parts of the hypothalamus, including the arcuate nucleus, the lateral hypothalamic area, the ventromedial nucleus and the PVN[32-34]. The NAc is thought to participate in different aspects of cognitive processing of feeding reward and motivation[35, 36], and the pathways originating from the medial shell of the NAc have the ability to control the activity of food intake[37].
Van et al showed that electrical stimulation of the medial shell of the NAc contributed to an increase in the food intake of rats[12]. This finding, combined with our observation, suggest that electrical stimulation of the NAc directly activates the neurons in the NAc to generate action potential that propagates along the axon to a variety of regions including the RVLM, which evokes the release of neurotransmitters. This explains the observation that electrical stimulation elicited an increase in the discharge frequency of the neurons in the RVLM, which increased the secretion of inhibitory amino acid neurotransmitters or down-regulated the level of excitatory neurotransmitters, causing consequently slowed heart rate and decreased blood pressure. Our observation that 400 μA electrical stimulation significantly lowered DBP and MAP and slightly increase the discharge frequency of RVLM neurons in the model rats further confirms that the neural pathway between NAc and RVLM is involved in the regulation of cardiovascular functions. In addition, we also assume that many other neural pathways and transmitters participate in this functional regulation. The NAc has been shown to project to the hippocampus and deliver various transmitters including some amino acids and orexin, which influence taste preferences, food preferences, and food choices[35, 38]. Collectively, these results show the complex role of the NAc in neural regulation.
Based on previous research and our observation, we propose that AN is triggered by a disruption of the balance in the neuronal network connecting multiple nuclei, including the NAc, RVLM and other parts of the hippocampus, rather than the damage of one single region of the brain. Devising treatment strategies for AN that target to comprehensively regulate neuronal activity throughout the brain may prove beneficial, such as modulation of neuronal excitability in both the feeding center and the satiety center, promoting the secretion of ghrelin (which increases appetite), and inhibiting the release of leptin and its binding with leptin receptor. But a large variety of biochemical factors can influence the onset and development of AN, and many of them still remain unknown or are difficult to control, typically exemplified by blood glucose level that involves the balance between insulin and glucagon. Other factors, including social attitudes toward body appearance, family support, depression and obsessive-compulsive habits, all contribute to the development and maintenance of AN.
In summary, we demonstrate that neurons in the medial shell of the NAc project to the RVLM, and electrical stimulation of the NAc increases the frequency of neural discharge in the RVLM and lowers SBP and heart rate in a rat model of AN. These results support our hypothesis that the neural connection between the NAc and the RVLM participates in the modulation of cardiovascular functions in AN.
| [1] |
Arcelus J, Mitchell A J, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies[J]. Arch Gen Psychiatry, 2011, 68(7): 724-31. DOI:10.1001/archgenpsychiatry.2011.74 |
| [2] |
Zipfel S, Giel KE, Bulik CM, et al. Anorexia nervosa: aetiology, assessment, and treatment[J]. Lancet Psychiatry, 2015, 2(12): 1099-111. DOI:10.1016/S2215-0366(15)00356-9 |
| [3] |
Giovinazzo S, Sukkar S, Rosa G, et al. Anorexia nervosa and heart disease: a systematic review[J]. Eat Weight Disord, 2019, 24(2): 199-207. |
| [4] |
Fichter MM, Quadflieg N. Mortality in eating disorders-results of a large prospective clinical longitudinal study[J]. Int J Eat Disord, 2016, 49(4): 391-401. |
| [5] |
Le LK, Barendregt JJ, Hay P, et al. Prevention of eating disorders: a systematic review and meta-analysis[J]. Clin Psychol Rev, 2017, 53: 46-58. DOI:10.1016/j.cpr.2017.02.001 |
| [6] |
Kumagai H, Oshima N, Matsuura T, et al. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure[J]. Hypertens Res, 2012, 35(2): 132-41. |
| [7] |
Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics[J]. Compr Physiol, 2014, 4(2): 825-50. |
| [8] |
Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting"[J]. J Neurosci, 2014, 34(12): 4239-50. DOI:10.1523/JNEUROSCI.4458-13.2014 |
| [9] |
Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY[J]. Am J Physiol Regul Integr Comp Physiol, 2007, 292(1): R217-26. DOI:10.1152/ajpregu.00852.2005 |
| [10] |
Resendez SL, Dome M, Gormley G, et al. μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms[J]. J Neurosci, 2013, 33(21): 9140-9. DOI:10.1523/JNEUROSCI.4123-12.2013 |
| [11] |
Michaelides M, Anderson SA, Ananth M, et al. Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks[J]. J Clin Invest, 2013, 123(12): 5342-50. DOI:10.1172/JCI72117 |
| [12] |
van der Plasse G, Schrama R, van Seters SP, et al. Deep brain stimulation reveals a dissociation of consummatory and motivated behaviour in the medial and lateral nucleus accumbens shell of the rat[J]. PLoS One, 2012, 7(3): e33455. DOI:10.1371/journal.pone.0033455 |
| [13] |
Lipsman N, Woodside DB, Giacobbe P, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial[J]. Lancet, 2013, 381(9875): 1361-70. DOI:10.1016/S0140-6736(12)62188-6 |
| [14] |
Lipsman N, Lam E, Volpini M, et al. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial[J]. Lancet Psychiatry, 2017, 4(4): 285-94. DOI:10.1016/S2215-0366(17)30076-7 |
| [15] |
Fladung AK, Gron G, Grammer K, et al. A neural signature of anorexia nervosa in the ventral striatal reward system[J]. Am J Psychiatry, 2010, 167(2): 206-12. DOI:10.1176/appi.ajp.2009.09010071 |
| [16] |
Oudijn M S, Storosum J G, Nelis E, et al. Is deep brain stimulation a treatment option for anorexia nervosa?[J]. BMC Psychiatry, 2013, 13: 277. DOI:10.1186/1471-244X-13-277 |
| [17] |
Selleck R A, Baldo B A. Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens[J]. Psychopharmacology (Berl), 2017, 234(9-10): 1439-49. DOI:10.1007/s00213-016-4522-4 |
| [18] |
Routtenberg A, Kuznesof A W. Self-starvation of rats living in activity wheels on a restricted feeding schedule[J]. J Comp Physiol Psychol, 1967, 64(3): 414-21. DOI:10.1037/h0025205 |
| [19] |
Scharner S, Prinz P, Goebel-Stengel M, et al. Activity-based anorexia reduces body weight without inducing a separate food intake microstructure or activity phenotype in female rats-mediation via an activation of distinct brain nuclei[J]. Front Neurosci, 2016, 10: 475. |
| [20] |
Cádiz-Moretti B, Otero-García M, Martínez-García F, et al. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse[J]. Brain Struct Funct, 2016, 221(2): 1033-65. DOI:10.1007/s00429-014-0954-y |
| [21] |
Yao F, Zhang E, Gao Z, et al. Did you choose appropriate tracer for retrograde tracing of retinal ganglion cells? The differences between cholera toxin subunit B and Fluorogold[J]. PLoS One, 2018, 13(10): e0205133. DOI:10.1371/journal.pone.0205133 |
| [22] |
Paxinos G, Watson C. The rat brain in stereotaxic coordinates[M]. 5th ed. Burlington, MA: Elsevier Academic Press, 2004.
|
| [23] |
Zhang HH, Wang YJ, Zheng C, et al. Apelin in the hypothalamic paraventricular nucleus improves cardiac function in surgical trauma rats[J]. Sheng Li Xue Bao, 2018, 70(2): 99-105. |
| [24] |
Ratnovsky Y, Neuman P. The effect of pre-exposure and recovery type on activity-based anorexia in rats[J]. Appetite, 2011, 56(3): 567-76. DOI:10.1016/j.appet.2011.01.027 |
| [25] |
Gutierrez E. A rat in the labyrinth of anorexia nervosa: contributions of the activity-based anorexia rodent model to the understanding of anorexia nervosa[J]. Int J Eat Disord, 2013, 46(4): 289-301. DOI:10.1002/eat.22095 |
| [26] |
Carrera O, Fraga A, Pellon R, et al. Rodent model of activity-based anorexia[J]. Curr Protoc Neurosci, 2014, 67: 9. |
| [27] |
Kirouac G J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior[J]. Neurosci Biobehav Rev, 2015, 56: 315-29. DOI:10.1016/j.neubiorev.2015.08.005 |
| [28] |
Qin C, Li J, Tang K. The paraventricular nucleus of the hypothalamus: development, function, and human diseases[J]. Endocrinology, 2018, 159(9): 3458-72. DOI:10.1210/en.2018-00453 |
| [29] |
Millan EZ, Ong Z, McNally GP. Paraventricular thalamus: Gateway to feeding, appetitive motivation, and drug addiction[J]. Prog Brain Res, 2017, 235: 113-37. DOI:10.1016/bs.pbr.2017.07.006 |
| [30] |
Wessendorf M W. Fluoro-Gold: composition, and mechanism of uptake[J]. Brain Res, 1991, 553(1): 135-48. |
| [31] |
Russo C, Russo A, Pellitteri R, et al. Hippocampal Ghrelin-positive neurons directly project to arcuate hypothalamic and medial amygdaloid nuclei. Could they modulate food-intake?[J]. Neurosci Lett, 2017, 653: 126-31. DOI:10.1016/j.neulet.2017.05.049 |
| [32] |
Li ZL, Xu L, Sun XR, et al. Central nesfatin-1 influences the excitability of ghrelin-responsive gastric distension neurons in the arcuate nucleus and reduces gastric motility in rats[J]. Eur J Neurosci, 2013, 38(11): 3636-43. DOI:10.1111/ejn.12366 |
| [33] |
Xu L, Wang Q, Guo F, et al. Nesfatin-1 signaling in the basom edial amygdala modulates the gastric distension-sensitive neurons discharge and decreases gastric motility via melanocortin 3/4 receptors and modified by the arcuate nucleus[J]. Eur J Pharmacol, 2015, 764: 164-72. DOI:10.1016/j.ejphar.2015.07.002 |
| [34] |
Guo FF, Xu L, Gao SL, et al. The effects of nesfatin-1 in the paraventricular nucleus on gastric motility and its potential regulation by the lateral hypothalamic area in rats[J]. J Neurochem, 2015, 132(3): 266-75. DOI:10.1111/jnc.12973 |
| [35] |
Salgado S, Kaplitt MG. The nucleus accumbens: a comprehensive review[J]. Stereotact Funct Neurosurg, 2015, 93(2): 75-93. DOI:10.1159/000368279 |
| [36] |
de Macedo IC, de Freitas JS, da Silva Torres I L. The influence of palatable diets in reward system activation: a mini review[J]. Adv Pharmacol Sci, 2016, 2016: 7238679. |
| [37] |
Durst M, Konczol K, Balazsa T, et al. Reward-representing D1-type neurons in the medial shell of the accumbens nucleus regulate palatable food intake[J]. Int J Obes (Lond), 2019, 43(4): 917-27. DOI:10.1038/s41366-018-0133-y |
| [38] |
Gotoh K, Masaki T, Chiba S, et al. Nesfatin-1, corticotropin-releasing hormone, thyrotropin- releasing hormone, and neuronal histamine interact in the hypothalamus to regulate feeding behavior[J]. J Neurochem, 2013, 124(1): 90-9. DOI:10.1111/jnc.12066 |
 2020, Vol. 40
2020, Vol. 40

