2. 新乡医学院第三附属医院皮肤科, 河南 新乡 453003
2. Department of Dermatology, Third Affiliated Hospital of Xinxiang Medical College, Xinxiang 453003, China
涎腺肿瘤是头颈部较为常见的一组肿瘤, 组织学类型繁多, 生物学行为各异, 准确的病理学诊断对于评估肿瘤预后和制定合理的治疗方案具有重要的临床价值[1-3]。但是, 由于该组肿瘤细胞起源、分化和组成相对复杂, 在组织学上大多具有上皮-肌上皮细胞的双相性特征, 而采用免疫组化染色(IHC)鉴别上皮-肌上皮细胞是诊断该组肿瘤不可或缺的重要手段[4-6]。目前, 常用于鉴别肌上皮细胞(MECs)的免疫组化标记物有p63、Calponin、CK5/6、S-100、CD10等[7-9]。近来, 作者在研究胸苷酸合成酶(TS)在肿瘤耐药的作用机制过程中, 首次发现TS不仅可作为乳腺肌上皮细胞和前列腺基底细胞特异性标志物[10-11], 还能特异性高表达于涎腺肿瘤中肌上皮细胞胞核内, 并由此提出TS也可作为涎腺肌上皮细胞的特异性分子标志。这一发现尚未见有文献报道。该研究拟采用免疫组化技术通过检测TS在不同类型涎腺病变细胞中的表达, 并与p63、Calponin、CK5/6、S-100等常用特异性肌上皮细胞标志物相比较, 评估TS作为涎腺肌上皮细胞特异性分子标志的特异性和敏感性, 为涎腺肿瘤的诊断和鉴别诊断提供新的分子标志。
1 资料和方法 1.1 临床资料选取西安交通大学第二附属医院病理科2017年12月~2018年12月间手术切除的涎腺病变标本32例, 包括涎腺炎症和非肿瘤性病变切除样本10例、混合瘤样本11例、基底细胞癌样本5例和腺样囊性癌样本6例。患者年龄在45~78岁, 平均56岁。
1.2 方法采用EnVision法免疫组化染色检测TS、p63、Calponin、CK5/6、S-100在上述样本中的表达, 所有操作均按照全自动免疫组化仪Ventana Bench Mark XT (瑞士, 罗氏)操作流程进行; EnVision免疫组化试剂盒(罗氏), 抗-p63、抗-Calponin、抗-CK5/6、抗-S-100抗体(福州迈新生物技术开发有限公司); 抗-TS抗体(克隆号: S106)(广州安必平医药科技股份有限公司)。以非肿瘤性涎腺病变切除的涎腺组织作为阳性对照, PBS代替一抗作为阴性对照。所有样本均经10%福尔马林固定、石蜡包埋、常规HE染色后进行病理组织学诊断。
1.3 结果判读在显微镜下对TS、P63、Calponin、CK5/6、S-100在不同类型涎腺组织中腺上皮细胞、肌上皮细胞和间质细胞中的表达模式、强度进行观察, 根据细胞核、细胞质、细胞膜着色部位分别判定为胞核型、胞质型、胞膜型表达模式, 细胞质、细胞核同时着色者判定为质/核型表达模式, 细胞质、细胞膜同时着色者判定为质/膜型表达模式; 在细胞中呈不同阳性强度着色者判读为阳性(+), 不着色者判读为阴性(-)。
1.4 统计学分析应用列联表、Fisher精确概率法及SPSS 18.0统计软件进行统计学分析, 比较TS、P63、Calponin、CK5/6、S-100在不同类型涎腺组织中表达的差异, 评估TS作为肌上皮细胞特异性免疫标志物的敏感性、特异性、阳性预测值及阴性预测值。P < 0.05为差异有统计学意义。
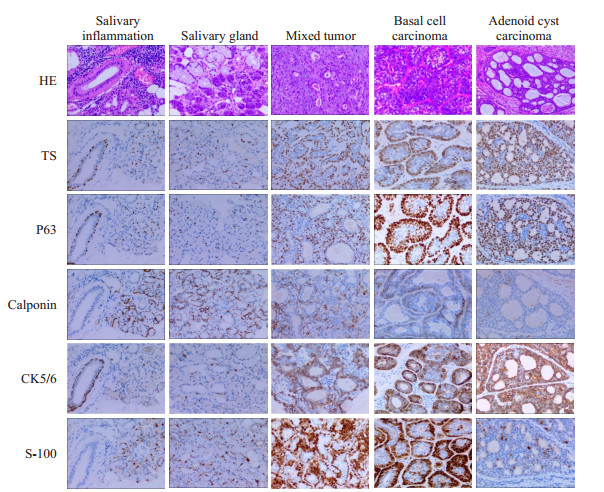
2 结果 2.1 TS、P63、Calponin、CK5/6、S-100蛋白在涎腺组织中的表达免疫组化染色结果显示, TS与P63在涎腺炎症组织和非肿瘤性病变切除组织、混合瘤、基底细胞癌、腺样囊性癌等组织样本的腺泡及导管周围肌上皮细胞中均呈清晰的胞核型强阳性表达, 但在肌上皮细胞胞质、胞膜中呈阴性表达; Calponin、CK5/6、S-100在肌上皮细胞中呈典型的质/膜型阳性表达。同时, TS在少数腺上皮细胞、癌细胞和散在的间质细胞的胞质中呈弱或中等不同强度的阳性着色, 但在细胞核中呈阴性表达。而P63、Calponin、CK5/6、S-100在所有样本的腺上皮细胞、癌细胞及间质细胞中均呈阴性表达(图 1, 表 1)。
|
图 1 TS、P63、Calponin、CK5/6和S-100蛋白在涎腺病变组织中的表达(HE和IHC染色) Fig.1 Expression of TS, P63, Calponin, CK5/6 and S-100 proteins in salivary gland lesions (HE and immunohistochemical staining, original magnification: × 200). TS and P63 show similar expressions in the nuclei of salivary adenoid MECs. Calponin, CK5/6 and S-100 show cytoplasmic/membranous expression in the MECs. S-100 shows cytoplasmic/nucleus immunohistochemical expression patterns in the MECs. |
| 表 1 TS、P63、Calponin、CK5/6和S-100蛋白在涎腺病变样本肌上皮细胞中的表达 Tab.1 Expression of TS, P63, Calponin, CK5/6 and S-100 protein in MECs of salivary adenoid[n (%)] |
统计分析结果显示, TS在涎腺病变样本肌上皮细胞中的表达结果与P63、Calponin、CK5/6和S-100蛋白的表达结果高度一致(P>0. 05)(表 1、2), 除与CK5/6在涎腺炎和涎腺组组表达比较之外, Kappa>0.75。而且, TS作为涎涎肌上皮细胞标志的敏感性、特异性均为100% (表 3)。
| 表 2 TS与P63、Calponin、CK5/6和S-100蛋白在涎腺病变样本一致性分析 Tab.2 Consistency test of TS with P63, Calponin, CK5/6 and S-100 protein in salivary gland lesions specimens[n (%)] |
| 表 3 TS作为肌上皮细胞标志的敏感性、特异性及阳性、阴性预测值、准确率 Tab.3 Sensitivity, specificity, positive, negative predictive value, and accuracy of TS as a MECs marker (%) |
涎腺又称唾液腺, 属于外分泌腺, 具有典型的双层上皮结构, 由内层的腺上皮细胞和底层的肌上皮细胞构成的腺泡和导管系统是其基本分泌单位[12-14]。由于肌上皮细胞不仅参与了大多数涎腺肿瘤的发生, 而且, 在不同涎腺肿瘤的组织形成及构造过程中处于中心地位, 而肌上皮细胞形态特征和胞外产物的差异是导致涎腺肿瘤呈现异质性特征的重要因素[15-16]。因此, 准确识别肌上皮细胞的有无和形态特征对于诊断涎腺肿瘤起着决定性作用。但是, 由于涎腺肿瘤中肿瘤性肌上皮细胞不同程度的形态变异, 使得在普通的HE染色切片中难以被识别[17-20]。因此, 采用免疫组化染色标记肌上皮细胞成为涎腺肿瘤诊断和鉴别诊断不可或缺的辅助手段[21-24]。
既往研究证实, TS是嘧啶核苷酸合成的关键催化酶, 在DNA的复制和DNA修复过程中发挥基础性作用[25-26]。而且TS蛋白的表达与以氟尿嘧啶类化疗药物的疗效密切相关, 并已作为评估此类药物敏感性的分子靶标而广泛应用于临床[27-31]。近来, 作者研究发现TS具有作为肌上皮细胞和基底细胞特异性标志物的潜在价值[10-11], 并能特异性高表达于多种类型涎腺肿瘤的肌上皮细胞胞核内。因此, 为评估TS作为涎腺肌上皮细胞特异性分子标志的可信性, 该研究采用免疫组化方法通过检测TS在32例不同类型涎腺组织样本中的表达, 并与P63、Calponin、CK5/6、S-100等常用肌上皮细胞特异性标志物相比较[32-33], 全面评估TS作为涎腺肌上皮细胞特异性免疫组化标志物的特异性、敏感性和阳性预测值、阴性预测值、准确率及临床应用价值。
研究结果发现TS和P63在涎腺腺泡和导管的肌上皮细胞、混合瘤和腺样囊性癌中的肌上皮细胞及基底细胞癌细胞中的表达模式和强度与P63的表达模式与强度完全一致, 均呈典型的胞核型强阳性表达。而Calponin、CK5/6、S-100等在上述细胞中则呈质/膜型阳性表达。统计学分析显示TS在涎腺组织、混合瘤、和腺样囊性癌中的肌上皮细胞及基底细胞癌细胞中的表达与P63、Calponin、CK5/6、S-100等肌上皮细胞分子标志在上述细胞中的表达高度一致(P>0.05), 且TS作为肌上皮细胞分子标志的特异性、敏感性和阳性预测值、阴性预测值甚至可高达100%。
同时, 该研究结果还显示, TS除了能特异性表达于正常涎腺组织肌上皮细胞和涎腺混合瘤、腺样囊性癌的肌上皮细胞及基底细胞癌的癌细胞胞核之外, 还在少数散在的腺上皮细胞和间质细胞中呈弱阳性胞质型表达。既往研究业已证实TS在肿瘤细胞胞质中的阳性表达强度与5-FU类化疗药物的耐药性密切相关, 被普遍认为可作为评估肿瘤细胞对5-FU耐药及预后的分子靶标[34-36]。作者在此前的研究中也对于TS在肿瘤细胞胞质中的表达意义进行过较深入的讨论[10-11]。而且, 由于表达模式的不同, TS在腺上皮和癌细胞中呈弱的胞质型表达并不会对TS在肌上皮细胞/基底细胞中强阳性胞核型表达结果的判读产生任何影响。同时, 由于TS在肌上皮细胞和基底细胞中呈典型胞核型表达, 使得TS表达结果具有可清晰、明确计数的特征。因而, 采用TS作为肌上皮细胞特异性的分子标志明显优于CK-H、Calponin、CK5/6、S-100等胞质型分子标志。
总之, 该研究发现并证实TS可作为理想的涎腺肌上皮细胞特异性分子标志物用于涎腺肿瘤的诊断和鉴别诊断。
| [1] |
Skálová A, Stenman G, Simpson RHW, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas[J]. Am J Surg Pathol, 2018, 42(2): e11-e27. DOI:10.1097/PAS.0000000000000980 |
| [2] |
Fonseca FP, Sena Filho M, Altemani A, et al. Molecular signature of salivary gland tumors:potential use as diagnostic and prognostic marker[J]. J Oral Pathol Med, 2016, 45(2): 101-10. DOI:10.1111/jop.12329 |
| [3] |
De Cecio R, Cantile M, Fulciniti F, et al. Salivary epithelialmyoepithelial carcinoma:clinical, morphological and molecular features[J]. Pathologica, 2017, 109(1): 1-8. |
| [4] |
Zhu S, Schuerch C, Hunt J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors[J]. Arch Pathol Lab Med, 2015, 139(1): 55-66. DOI:10.5858/arpa.2014-0167-RA |
| [5] |
Tanya D, Russell, Sonali Jindal, et al. Myoepithelial cell differentiation markers in ductal carcinoma in situ progression[J]. Am J Pathol, 2015, 185(11): 3076-89. DOI:10.1016/j.ajpath.2015.07.004 |
| [6] |
Toshitaka Nagao, Eiichi Sato, Rie Inoue, et al. Immunohistochemical analysis of salivary gland tumors:application for surgical pathology Practice[J]. Acta Histochem Cytochem, 2012, 45(5): 269-82. |
| [7] |
Abdulrahman SS, Mohammad DN, Hamied, Marwa Abdul-Salam, et al. Immunohistochemical evaluation of salivary gland tumors differentiation and proliferation by using calponin and telomerase[J]. Samdi Dental J, 2019, 31(1): 105-14. |
| [8] |
Patrón-Bolaños C, Acosta-Torres L, Tenorio-Rocha F, et al. Immunohistochemical patterns in different stromal variants of pleomorphic adenomas:literature review[J]. Histol Histopathol, 2016, 31(3): 239-48. |
| [9] |
Triantafyllou A, Mikkelsen LH, Gnepp DR, et al. Salivary myoepithelial cells:an addendum[J]. Ultrastruct Pathol, 2018, 42(6): 465-76. DOI:10.1080/01913123.2018.1551259 |
| [10] |
Guo R, Tian Y, Jin X, et al. Thymidylate Synthase, a New Myoepithelial Biomarker for Breast Lesions[J]. Int J Surg Pathol, 2019, 27(8): 852-8. DOI:10.1177/1066896919858403 |
| [11] |
郭睿, 田怡, 金雪媛, 等. 胸苷酸合成酶免疫组化染色在标记前列腺基底细胞中的应用[J]. 山西医科大学学报, 2019, 50(5): 658-63. |
| [12] |
Redman RS. Myoepithelium of salivary glands[J]. Microsc Res Tech, 1994, 27(1): 25-45. DOI:10.1002/jemt.1070270103 |
| [13] |
Dardick I, Thomas MJ, van Nostrand AW. Myoepithelioma——new concepts of histology and classification:a light and electron microscopic study[J]. Ultrastruct Pathol, 1989, 13(2-3): 187-224. DOI:10.3109/01913128909057442 |
| [14] |
Shah AA, Mulla AF, Mayank M. Pathophysiology of myoepithelial cells in salivary glands[J]. J Oral Maxillofac Pathol, 2016, 20(3): 480-90. DOI:10.4103/0973-029X.190952 |
| [15] |
Chitturi RT, Veeravarmal V, Nirmal RM, et al. Myoepithelial Cells (MEC) of the Salivary Glands in Health and Tumours[J]. J Clin Diagn Res, 2015, 9(3): ZE14-8. |
| [16] |
Savera AT, Zarbo RJ. Defining the role of myoepithelium in salivary gland neoplasia[J]. Adv Anat Pathol, 2004, 11(2): 69-85. DOI:10.1097/00125480-200403000-00001 |
| [17] |
Zarbo RJ. Salivary gland neoplasia:a review for the practicing pathologist[J]. Mod Pathol, 2002, 15(3): 298-323. DOI:10.1038/modpathol.3880525 |
| [18] |
Skálová A, Gnepp DR, Lewis JS Jr, et al. Newly Described Entities in Salivary Gland Pathology[J]. Am J Surg Pathol, 2017, 41(8): e33-e47. DOI:10.1097/PAS.0000000000000883 |
| [19] |
Balachander N, Masthan KM, Babu NA, et al. Myoepithelial cells in pathology[J]. J Pharm Bioallied Sci, 2015, 7(Suppl 1): S190-3. |
| [20] |
Simpson RH, Skálová A, Di Palma S, et al. Recent advances in the diagnostic pathology of salivary carcinomas[J]. Virchows Arch, 2014, 465(4): 371-84. DOI:10.1007/s00428-014-1639-x |
| [21] |
Foschini MP, Scarpellini F, Gown AM, et al. Differential Expression of Myoepithelial Markers in Salivary, Sweat and Mammary Glands[J]. Int J Surg Pathol, 2000, 8(1): 29-37. DOI:10.1177/106689690000800108 |
| [22] |
Zarbo RJ, Prasad AR, Regezi JA, et al. Salivary gland basal cell and canalicular adenomas:immunohistochemical demonstration of myoepithelial cell participation and morphogenetic considerations[J]. Arch Pathol Lab Med, 2000, 124(3): 401-5. |
| [23] |
Mărgăritescu C, Mercuţ V, Mogoantă L, et al. Salivary gland Basal cell adenomas immunohistochemical evaluation of four cases and review of the literature[J]. Rom J Morphol Embryol, 2005, 46(1): 29-40. |
| [24] |
Ogawa Y. Immunocytochemistry of myoepithelial cells in the salivary glands[J]. Prog Histochem Cytochem, 2003, 38(4): 343-426. DOI:10.1016/S0079-6336(03)80001-3 |
| [25] |
Montfort WR, Weichsel A. Thymidylate synthase:structure, inhibition, and strained conformations during catalysis[J]. Pharmacol Ther, 1997, 76(1-3): 29-43. DOI:10.1016/S0163-7258(97)00099-5 |
| [26] |
Donner DB, Nakakura EK, Venook AP, et al. High thymidylate synthase gene expression predicts poor outcome after resection of hepatocellular carcinoma[J]. PLoS One, 2019, 14(7): e0219469. DOI:10.1371/journal.pone.0219469 |
| [27] |
Rode W, Leś A. Molecular mechanism of thymidylate synthasecatalyzed reaction and interaction of the enzyme with 2-and/or 4-substituted analogues of dUMP and 5-fluoro-dUMP[J]. Acta Biochim Pol, 1996, 43(1): 133-42. DOI:10.18388/abp.1996_4524 |
| [28] |
Pereira MA, Ramos MFKP, Dias AR, et al. Immunohistochemical expression of thymidylate synthase and prognosis in gastric cancer patients submitted to fluoropyrimidine-based chemotherapy[J]. Chin J Cancer Res, 2018, 30(5): 526-36. DOI:10.21147/j.issn.1000-9604.2018.05.06 |
| [29] |
Sakatani A, Sonohara F, Goel A. Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells[J]. Carcinogenesi, 2019, 40(3): 422-31. DOI:10.1093/carcin/bgy186 |
| [30] |
Anderson KS. Understanding the molecular mechanism of substrate channeling and domain communication in protozoal bifunctional TS-DHFR[J]. Protein Eng Des Sel, 2017, 30(3): 253-61. |
| [31] |
Karunaratne K, Luedtke N, Quinn DM, et al. Flavin-dependent thymidylate synthase:N5 of flavin as a Methylene carrier[J]. Arch Biochem Biophys, 2017, 632: 11-9. DOI:10.1016/j.abb.2017.08.011 |
| [32] |
Teixeira LN, Janner ÉC, Teixeira T, et al. Comparison of p63/p40 Expression With Myoepithelial Markers in Minor Salivary Gland Tumors[J]. Int J Surg Pathol, 2019, 27(4): 360-71. DOI:10.1177/1066896918813678 |
| [33] |
P .C. Anila Namboodiripad. A review:Immunological markers for malignant salivary gland tumors[J]. J Oral Biol Craniofac Res, 2014, 4(2): 127-34. DOI:10.1016/j.jobcr.2014.05.003 |
| [34] |
Hernando-Cubero J, Matos-García I, Alonso-Orduña V, et al. The role of fluoropirimidines in gastrointestinal tumours:from the bench to the bed[J]. J Gastrointest Cancer, 2017, 48(2): 135-47. DOI:10.1007/s12029-017-9946-5 |
| [35] |
Chon J, Stover PJ, Field MS. Targeting nuclear thymidylate biosynthesis[J]. Mol Aspects Med, 2017, 53: 48-56. DOI:10.1016/j.mam.2016.11.005 |
| [36] |
Antosiewicz A, Jarmuła A, Przybylska D, et al. Human dihydrofolate reductase and thymidylate synthase form a complex in vitro and colocalize in normal and cancer cells[J]. J Biomol Struct Dyn, 2017, 35(7): 1474-90. DOI:10.1080/07391102.2016.1186560 |
 2020, Vol. 40
2020, Vol. 40

