2. Department of Ultrasound, Wuhan Central Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430014, China
2. 武汉市中心医院超声诊断科, 湖北 武汉 430014
Infliximab (IFX), a monoclonal antibody against tumor necrosis factor alpha (TNF-α), has become an established therapy for inducing and maintaining remission in inflammatory bowel disease (IBD) and is routinely used for treatment of moderate and severe Crohn's disease (CD)[1-3]. For treatment of CD, the patients initially receive 3 infusions at the dose of 5 mg/kg at 0, 2 and 6 weeks, and if the patients show a positive response, maintenance IFX treatment is decided at an 8-week interval. Unfortunately, a large proportion of patients who initially respond to IFX subsequently suffer a disease relapse[4], or loss of response (LOR). The mechanism of LOR remains poorly understood, but mounting evidence suggest that the formation of antidrug antibodies (ADA) of IFX and pharmacokinetic variations in the individual patients play an important role [4-6].
Some prospective studies of patients in clinical remission have been conducted to identify potential biomarkers that may help predict the occurrence of LOR to IFX. Patients with IBD who have low IFX trough concentrations and significant amounts of ADA are found to have largely poor clinical outcomes in terms of a low rate of clinical response and poor mucosal healing[4-6]. But considering the technical difficulty and high cost of detecting plasma IFX concentrations and ADA of IFX, researchers attempted to find a more cost-effective predictor for LOR in CD patients. In particular, a prospective study showed that Crohn's patients with a C-reactive protein (CRP) level above 20 mg/L had a relative risk of short-term relapse of 8.0 as compared with the patients with a lower CRP level (≤20 mg/L)[7].
The neutrophil-lymphocyte ratio (NLR) is a convenient and effective marker of subclinical inflammation, and can be indicative of inflammatory and neoplastic diseases in the biliary tract[8]and bladder[9]as well as breast cancer[10]. Several studies have reported the association between NLR and the activity of IBD[11, 12]. In regard of IFX, a study by Wlodarczyk et al[13]showed that for patients with CD who received a 52-week therapy of IFX, NLR at 14 weeks was a promising predictor of a sustained response. We therefore hypothesized that NLR at 14 weeks predicts LOR to IFX in patients with CD receiving IFX maintenance therapy and conducted this retrospective analysis of 54 patients receiving IFX therapy for CD to test this hypothesis.
PATIENTS AND METHODS PatientsThis study was conducted under approval by the Medical Ethics Committee of Wuhan University Zhongnan Hospital. Due to the retrospective nature of this study and data collection from the patients on an anonymous basis, written informed consent from the patients were waived.
We collected data from 54 patients with CD undergoing a 52-week treatment with IFX, who achieved positive response to the induction treatment in Zhongnan Hospital between January, 2012 and December, 2016. In all the cases included, the diagnosis of CD was established based on the clinical, endoscopic and histopathological findings according to diagnostic criteria from second European Crohn's and Colitis Organization (ECCO) Consensus on the diagnosis and management of CD[14]. The patients with a history of cardiovascular disease, hemopathy, malignant tumor, connective tissue disease or clinical evidence suggesting an ongoing infection, which could affect the result of NLR determination, were excluded from the study.
LOR to IFX was characterized by a clinical relapse requiring infliximab dose optimization or secondary alternative therapies among the responders during the maintenance period[1], whereas a sustained response to IFX was defined as the absence of LOR over the follow-up period among the responders. Responders were defined as the patients who achieved a clinical response at the end of the induction period (week 14) without secondary alternative therapies such as tacrolimus, corticosteroids, or proctocolectomy. Clinical response was defined as a decrease by ≥70 points as well as a ≥25% reduction from the baseline Crohn's Disease Activity Index (CDAI).
Laboratory testsWe collected the laboratory data concerning NLR, C-reactive protein (CRP), albumin (ALB), hemoglobin (HGB), and mean platelet volume (MPV) from the patients at the 14th week of IFX therapy and assessed the disease activity every 8 weeks from the 14th week to the 52th week based on the clinical records. The differential WBC count was analyzed using a DXH-80 hematology analyzer, as per the manufacture's protocol. In each case, the NLR of a blood sample was calculated by dividing the absolute neutrophil count with the absolute lymphocyte count.
Statistical analysisAll data analyses were performed using IBM SPSS statistical software package (version 17.0). The continuous variables are presented as the median with interquartile range (IQR). Receiver-operating characteristics (ROC) curves were plotted to calculate the area under the ROC curve (AUC) and comparison of the AUCs was performed using DeLong analysis. The clinical characteristics of the patients were compared using Chi-square test for categorical variables, and Mann-Whitney U test was used for continuous variables. The predictive factors of LOR were evaluated based on the cumulative relapse-free rate (illustrated with a Kaplan-Meier plot). The differences in the survival curves of the patients were assessed with the log-rank test. Univariate and multivariate analyses were performed using the Cox regression model. The variables with a P value less than 0.15 in univariate analysis were analyzed in multivariate analysis, and the data are presented as hazard ratios (HR) with 95% confidence intervals (95% CI). A P value less than 0.05 was considered to indicate a statistically significant difference.
RESULTS Demographic and baseline clinical data of the patientsThe demographic and baseline clinical data of the patients are presented in Tab. 1. The median age of the patients at the start of IFX therapy was 26 years (IQR 20-36 years). At the time of infliximab induction, concomitant therapies with mesalamine, corticosteroids, immunomodulators were used in 70.3%, 7.4%%, and 31.5% of the patients, respectively. During the 52-week follow-up, 15 of the patients on maintenance therapy lost response to IFX.
| Tab.1 Baseline demographic and clinical data of the patients |
Tab. 2 shows the comparison of the demographic variables between patients with a sustained response and those with LOR. Laboratory tests at the 14th week revealed that patients in LOR group had significantly higher NLR (P=0.00), CRP level (P=0.00) and MPV (P= 0.04).
| Tab.2 Comparison of the demographic variables between patients with a sustained response and those with LOR |
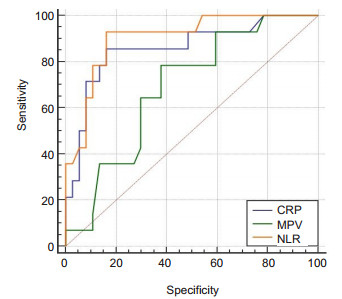
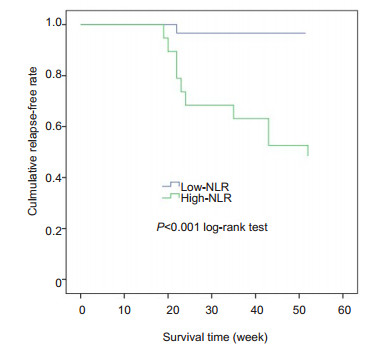
We constructed ROC curves to assign the optimal cut-off values of NLR, CRP and MPV at 14 weeks, which were the variables potentially associated with LOR to IFX. NLR had the highest predictive value, with an AUC of 0.903; CRP and MPV at 14 weeks had lower predictive value, with AUCs of 0.859 and 0.688, respectively (Fig. 1), but the difference in the AUCs was not statistically significant between NLR and CRP. ROC curve analysis showed that a cut-off value of NLR greater than 2.75 resulted in a sensitivity of 93.33% and a specificity of 84.62% for predicting LOR, and we therefore set 2.75 as the cut-off value for NLR, according to which the patients were classified into high NLR group (n=20) and low NLR group (n=34). As shown in Fig. 2, the relapse-free survival rate was significantly lower in the high NLR group than in the low NLR group (P=0.00).
|
Fig.1 Receiver-operating characteristic curve (ROC) comparing the neutrophils-lymphocytes ratio (NLR), C-reactive protein (CRP) and mean platelet volume (MPV) at 14 weeks for predicting LOR to infliximab in patients with Crohn's disease. The area under the ROC curve was 0.903 for NLR, 0.859 for CRP, and 0.688 for MPV. |
|
Fig.2 Relapse-free survival of the patients with different baseline neutrophil-lymphocyte ratios (NLR) during infliximab therapy. |
To minimize the influences by confounding variables, we searched PubMed and Embase databases for studies published in the years from 2010 to 2015 to identify the clinical variables that were related to LOR in CD patients[4, 5, 7, 13, 15-19]and were also documented in the clinical records of the patients. Tab. 3 and Tab. 4 show univariate and multivariate Cox regression analyses of these variables, among which NLR at 14 weeks was identified as an independent prognostic factor for LOR with a HR of 1.851 (95% CI: 1.096-3.026, P=0.021).
| Tab.3 Univariate Cox regression analysis of the factors for predicting loss of response after induction with infliximab in the patients |
| Tab.4 Multivariate Cox regression analysis of risk for loss of response during follow-up after induction of infliximab |
We found that patients who lost response to maintenance IFX treatment after successful induction therapy had a significantly higher NLR at 14 weeks than those who maintained the response (3.51 vs 1.77, P= 0.000). NLR at 14 weeks showed an AUC of 0.903 for predicting LOR to IFX maintenance treatment, and at the cut-off value of 2.75, NLR at 14 weeks had a sensitivity of 93.33% and a specificity of 84.62% for predicting LOR.
The number of neutrophils increases during acute inflammatory response as the first line of defense against invading pathogens[20]. The role of neutrophils in the pathology of CD remains unclear. Functional impairment of the neutrophils results in lowered capacity of bacterial clearance and fuels the ongoing chronic inflammatory response. Within epithelial crypts and in the intestinal lumen, the accumulation of neutrophils directly correlates with the clinical disease activity and epithelial injury [21]. Strong evidence from previous studies has shown that in patients with IBD, lymphocyte function is abnormal at both the peripheral and mucosal levels[22]. In this context, NLR can serve as a universally available and cost-effective biomarker that integrates two white blood cell (WBC) subtypes, and can be easily calculated from the differential WBC counts and is less prone to influences by conditions.
A few previous studies demonstrated the value of NLR in patients with IBD[11-13, 23]. According to Gao et al[11] and Wlodarczyk et al[13], the value of NLR was associated with the severity of CD, and a NLR higher than 3.667 at 14 weeks during the 52-week IFX therapy could predict LOR with a 67% sensitivity and a 80% specificity[13]. But as the study by Wlodarczyk et al did not control the variables that could potentially affect NLR (such as cardiovascular disease), the cut-off value of NLR they proposed appeared much higher than that in our study. We assessed the potential factors affecting LOR in the patients who achieved clinical remission through IFX therapy. Comparison of the clinical parameters between the patients with sustained response and those with LOR indicated that both NLR and CRP were risk factors for LOR, but NLR had a greater AUC than CRP (0.903 vs 0.859), although this differences was not statistically significant. The result of multivariate analysis showed that only NLR at 14 weeks was an independent factor for predicting LOR in the responders.
Currently the mechanism of LOR to IFX is not fully understood. Researchers suggest that serum trough level of IFX and the formation of IFX antibody are associated with the occurrence of LOR. The correlation between NLR and LOR, based on the fact that IFX clearance is related with an immunoglobulin G1 monoclonal antibody, involves Fc-γ-receptor-mediated phagocytosis by cells such as neutrophils [24]. Nishida et al [25]hypothesized that patients with a higher NLR had a potentially higher IFX clearance ability to result in a lower IFX concentration, which could be a possible explanation for the relationship between NLR and LOR in patients with CD.
This study also has some limitations. First, we did not detect serum trough IFX levels and IFX antibodies in all the patients, so that the relationship between NLR and serum trough level of IFX could not be evaluated. Second, this study is of a retrospective nature and involves a relatively small cohort. Further prospective studies with larger sample sizes and integrating serum trough IFX level in the analysis are needed to verify our conclusion that NLR at 14 weeks during a 52-week IFX therapy is a key predictor for LOR in patients with CD.
| [1] |
Taxonera C, Olivares D, Mendoza JL, et al. Need for infliximab dose intensification in Crohn's disease and ulcerative colitis[J]. World J Gastroenterol, 2014, 20(27): 9170-7. |
| [2] |
Baumgart DC. The diagnosis and treatment of Crohn's disease and ulcerative colitis[J]. Dtsch Arztebl Int, 2009, 106(8): 123-33. |
| [3] |
Panaccione R, Fedorak RN, Aumais G, et al. Review and clinical perspectives for the use of infliximab in ulcerative colitis[J]. Can J Gastroenterol, 2008, 22(3): 261-72. DOI:10.1155/2008/493405 |
| [4] |
Sono K, Yamada A, Yoshimatsu Y, Takada N, et al. Factors associated with the loss of response to infliximab in patients with Crohn's disease[J]. Cytokine, 2012, 59(2): 410-6. DOI:10.1016/j.cyto.2012.04.026 |
| [5] |
Juillerat P, Sokol H, Froehlich F, et al. Factors associated with durable response to infliximab in Crohn's disease 5 years and beyond:a multicenter international cohort[J]. Inflamm Bowel Dis, 2015, 21(1): 60-70. |
| [6] |
Ebert EC, Das KM, Mehta V, Rezac C. Non-response to infliximab may be due to innate neutralizing anti-tumor necrosis factor-alpha antibodies[J]. Clin Exp Immunol, 2008, 154(3): 325-31. DOI:10.1111/j.1365-2249.2008.03773.x |
| [7] |
Consigny Y, Modigliani R, Colombel JF, et al. A simple biological score for predicting low risk of short-term relapse in Crohn's disease[J]. Inflamm Bowel Dis, 2006, 12(7): 551-7. DOI:10.1097/01.ibd.0000225334.60990.5b |
| [8] |
Tang H, Lu W, Li B, Dong J, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers:a systematic review and meta-analysis[J]. Oncotarget, 2017, 8(22): 36857-68. DOI:10.18632/oncotarget.16143 |
| [9] |
Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-tolymphocyte ratio as a prognostic marker in bladder cancer[J]. Dis Markers, 2016. DOI:10.1155/2016/8345286 |
| [10] |
Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer:a systematic review and meta-analysis[J]. Breast Cancer Res, 2017, 19(1): 2. |
| [11] |
Gao SQ, Huang LD, Dai RJ, et al. Neutrophil-lymphocyte ratio:a controversial marker in predicting Crohn's disease severity[J]. Int J Clin Exp Pathol, 2015, 8(11): 14779-85. |
| [12] |
Torun S, Tunc BD, Suvak B, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis:a promising marker in predicting disease severity[J]. Clin Res Hepatol Gastroenterol, 2012, 36(5): 491-7. DOI:10.1016/j.clinre.2012.06.004 |
| [13] |
Wlodarczyk MK, Sobolewska AE, Stec-Michalska K, et al. Neutrophillymphocyte ratio in Crohn's disease patients predicts sustained response to infliximab 52-week therapy[J]. J Gastrointestin Liver Dis, 2015, 24(1): 127-8. |
| [14] |
Sadowski DC, Bernstein CN, Bitton A, et al. Canadian Association of Gastroenterology Clinical Practice Guidelines:the use of tumour necrosis factor-alpha antagonist therapy in Crohn's disease[J]. Can J Gastroenterol, 2009, 23(3): 185-202. DOI:10.1155/2009/201430 |
| [15] |
Nishida N, Sasaki M, Kurihara M, et al. Changes of energy metabolism, nutritional status and serum cytokine levels in patients with Crohn's disease after anti-tumor necrosis factor-alpha therapy[J]. J Clin Biochem Nutr, 2013, 53(2): 122-7. DOI:10.3164/jcbn.13-18 |
| [16] |
Kamata N, Oshitani N, Watanabe K, et al. Efficacy of concomitant elemental diet therapy in scheduled infliximab therapy in patients with Crohn's disease to prevent loss of response[J]. Dig Dis Sci, 2015, 60(5): 1382-8. DOI:10.1007/s10620-014-3493-8 |
| [17] |
Sobolewska A, Wlodarczyk M, Stec-Michalska K, et al. Mean Platelet Volume in Crohn's disease patients predicts sustained response to a 52-week infliximab therapy:a pilot study[J]. Dig Dis Sci, 2016, 61(2): 542-9. DOI:10.1007/s10620-015-3894-3 |
| [18] |
Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment[J]. Haematologica, 2010, 95(2): 199-205. DOI:10.3324/haematol.2009.009985 |
| [19] |
Mitchell RA, Shuster C, Shahidi N, et al. The utility of infliximab therapeutic drug monitoring among patients with inflammatory bowel disease and concerns for loss of response:a retrospective analysis of a real-world experience[J]. Can J Gastroenterol Hepatol, 2016. DOI:10.1155/2016/5203898 |
| [20] |
Segal AW. How neutrophils kill microbes[J]. Annu Rev Immunol, 2005, 23: 197-223. DOI:10.1146/annurev.immunol.23.021704.115653 |
| [21] |
Wera O, Lancellotti P, Oury C. The dual role of neutrophils in inflammatory bowel diseases[J]. J Clin Med, 2016, 5(12): 118-42. DOI:10.3390/jcm5120118 |
| [22] |
Ten HT, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease[J]. Gut, 2002, 50(2): 206-11. DOI:10.1136/gut.50.2.206 |
| [23] |
Feng JR, Qiu X, Wang F, et al. Diagnostic value of neutrophil-tolymphocyte ratio and platelet-to-lymphocyte ratio in Crohn's disease[J]. Gastroenterol Res Pract, 2017. DOI:10.1155/2017/3526460 |
| [24] |
Chen K, Nishi H, Travers R, et al. Endocytosis of soluble immune complexes leads to their clearance by FcgammaRIIIB but induces neutrophil extracellular traps via FcgammaRIIA in vivo[J]. Blood, 2012, 120(22): 4421-31. DOI:10.1182/blood-2011-12-401133 |
| [25] |
Nishida Y, Hosomi S, Yamagami H, et al. Neutrophil-to-lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis[J]. Plos One, 2017, 12(1): 1-14. |
 2020, Vol. 40
2020, Vol. 40

