2. 衡阳市健康危害因子检验检疫新技术研究重点实验室,湖南 衡阳 421001;
3. 南华大学医学院,湖南 衡阳 421001
2. Hengyang Key Laboratory for Health Hazard Factors Inspection and Quarantine, Hengyang 421001, China;
3. School of Medical Sciences, University of South China, Hengyang 421001, China
沙眼衣原体可感染泌尿生殖道导致非淋球菌性尿道炎,并可引起严重的并发症,如不孕、异位妊娠、宫颈鳞状细胞癌、增加HIV感染机率等[1-4]。因此,对沙眼衣原体致病机制的研究变得尤为重要。目前沙眼衣原体鼠生物型现被划分为一新的衣原体种,即鼠衣原体(Cm),由于其引起的鼠泌尿生殖道感染和沙眼衣原体引起的人类泌尿生殖道感染极为相似,故目前Cm鼠生殖道感染模型常被用于探索沙眼衣原体的免疫保护作用以及其病理损伤机制[5-6]。
大量的中性粒细胞在炎症部位聚集是急性衣原体感染的早期特征,涉及多环节诱导产生的免疫介质网络;TNF-α是由活化的巨噬细胞或单核细胞产生的一种前炎症细胞因子,可明显增强中性粒细胞和巨噬细胞的吞噬活性,并且促进抗原提呈细胞成熟,在宿主防御微生物感染中发挥重要作用[7-9]。有研究发现Cm急性感染可诱导小鼠生殖道局部分泌大量TNF-α,并在第7天达高峰[10]。小鼠生殖道感染Cm后,注射外源性TNF-α抗体中和小鼠体内产生的TNF-α或敲除TNF-α基因,均可显著减轻小鼠输卵管病理损伤的严重程度[11]。有文献报道约80%的生殖道或呼吸道感染沙眼衣原体的女性外周血中TNF-α水平显著增高[12]。研究显示,沙眼衣原体生殖道感染者输卵管病理损伤加重与TNF-α水平升高有关,而阻断TNF-α的产生,输卵管粘膜的损伤程度大大减轻[13]。Kamalakaran等[14]发现TNF-α缺失不影响小鼠对衣原体的清除,但可减轻小鼠生殖道病理损伤程度。
虽然TNF-α与衣原体感染的关系已有报道,但均为从TNF-α的表达,用TNF-α中和抗体或使用TNF-α基因敲除小鼠模型进行研究,而TNF-α通过与细胞膜表面的TNF受体(包括TNFR1和TNFR2)特异性结合而发挥生物学功能,若TNF-α受体缺失可阻断TNF-α生物学效应的发挥。那么TNF-αR基因敲除(KO)后对衣原体感染是否会有类似的作用呢?有研究认为,与野生型(WT)小鼠比较,TNFR1、TNFR2和TNFR1/2双敲除后对衣原体清除速率均没有影响,但可显著减轻输卵管的水肿程度[15]。但评价沙眼衣原体对生殖道的损伤主要包括输卵管和子宫的炎性病变和积水水肿。因此,本研究利用TNF-αR KO小鼠,经生殖道感染Cm后,重点评估其对输卵管和子宫的炎性病变和积水水肿损伤程度,以进一步揭示TNF-α在鼠衣原体生殖道感染中的作用,为衣原体感染靶向辅助治疗提供理论依据。
1 材料和方法 1.1 材料 1.1.1 实验动物5~6周龄雌性C57BL/6JWT小鼠及TNF-αR KO小鼠各15只(美国Jackson实验室,Bar Harbor, Maine)。
1.1.2 菌株及试剂C. muridarum(Cm, Nigg株)由实验室传代保存。HeLa细胞(美国标准菌株保存中心,ATCC)。IL-4、IL-5、IL-17、IFN-γ、IL-6、IL-8、IL-1α、IL-1β和TNF-α等细胞因子检测试剂盒(Minneapolis);Depo-provera(Pharmacia)。
1.2 方法 1.2.1 小鼠生殖道衣原体感染模型制备C57BL/6J WT和TNF-αR KO小鼠各15只,均经阴道接种1× 104 IFUs的Cm,初次感染后第56天,每组8只小鼠再次感染同等剂量的Cm。在初次和再次感染前5 d,皮下注射2.5 mg Depo-provera,用于提高小鼠对衣原体感染的敏感程度。在感染后第80天处死小鼠,分离生殖道,并收集脾细胞。
1.2.2 小鼠生殖道中衣原体数目检测每组小鼠经阴道感染Cm后,每隔3~4 d收集阴道分泌物,将其置于500 μL SPG中充分混匀,进行梯度稀释,取合适稀释度的悬液感染单层HeLa细胞,在5% CO2培养箱内培养24 h,进行荧光抗体染色。弃培养板中的旧培养基,PBS洗2次;4%多聚甲醛室温固定30 min后,再用2% Saponion处理30 min,室温封闭1 h,兔抗Cm多克隆抗体37 ℃孵育1 h,充分洗涤后,加入二抗混合液(含1:100羊抗兔IgG-Cy3、1:1000 Hoechst细胞核DNA染液),37 ℃避光孵育1 h,PBS洗涤5次,浸泡2 min/次,弃洗液。封片,在荧光显微镜下,随机计数5个视野IFU数量并取平均值,然后换算成log10 IFUs来计算每组小鼠的均数和标准差。
1.2.3 小鼠生殖道病理损伤检测分离小鼠子宫、卵巢和输卵管,10%甲醛固定后制成切片,H & E染色,盲法对其炎症反应和管腔扩张程度进行评分,判定病变情况[16]。
1.2.4 巨噬细胞上清中的细胞因子水平测定小鼠处死后,注射4~5 mL预冷的PBS缓冲溶液于小鼠腹腔中。轻柔按压后慢慢抽取腹腔液。用RPMI 1640培养基(含10%小牛血清、10 μg/mL庆大霉素)调整细胞浓度并接种至48孔板中,5% CO2培养箱孵育2 h,然后用PBS洗去未贴壁细胞,RPMI 1640培养过夜,制备单层巨噬细胞,每孔用感染复数(MOI)为5的Cm进行感染,5%CO2培养箱内培养48 h,取上清,根据试剂盒说明书ELISA法检测IL-6、IL-8、IL-1α、IL-1β和TNF-α等细胞因子水平。
1.2.5 脾细胞上清中细胞因子水平测定无菌配置含10 μg/mL庆大霉素的DMEM(D0)和含10%小牛血清的DMEM(D10)。在操作台中无菌取小鼠脾脏,制备匀浆,并用D0漂洗于10 mL的离心管中,再加5 mL红细胞裂解液,充分混匀,室温静置5 min,1500 r/min离心5 min;去上清,用D10重悬沉淀,1000 r/min离心5 min;弃上清,加3 mL D10,混匀后制成细胞悬液,调整细胞数为1×106/mL,1 mL/孔接种至24孔细胞培养板中,5% CO2培养箱内24 h,细胞贴壁后其中一孔加紫外线灭活的Cm EB,同时设一孔不加衣原体为对照。5% CO2培养箱内培养72 h,取上清,根据试剂盒操作要求,ELISA检测IL-4、IL-5、IL-17和IFN-γ等细胞因子水平。
1.3 统计学分析采用双侧t检验(Two-tailed Student' t test)比较分析两组间的IFU、各细胞因子水平及病理评分结果;用Fisher's exact test比较分析子宫角与输卵管水肿程度统计数据。P < 0.05为差异有统计学意义。
2 结果 2.1 TNF-αR敲除对小鼠生殖道衣原体清除速度的影响检测TNF-αR KO和WT小鼠生殖道中Cm数量,发现TNF-αR KO小鼠生殖道衣原体清除速度与WT小鼠基本一致,与初次感染或再次感染无关。再次感染后,小鼠生殖道中包涵体数量较首次感染均减少1000倍左右,且包涵体存在时间缩短(图 1)。
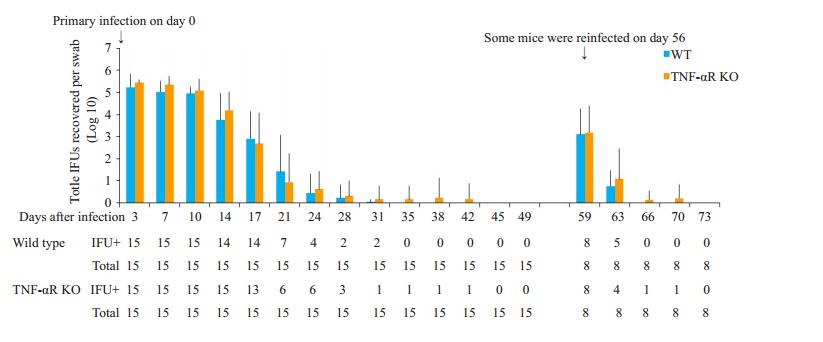
|
图 1 TNF-αR基因敲除对小鼠生殖道衣原体清除速度的影响 Fig.1 Effect of TNF-αR knockout on clearance of Chlamydia muridarum from the urogenital tract of mice. IFU+: Number of mice with detectable IFUs. Total: Number of mice infected with C. muridarum. |
初次感染后第80天分离生殖道组织,甲醛固定,H & E染色后,镜下观察到病变子宫角和输卵管粘膜柱状上皮细胞呈矮柱状且管壁增厚,而病变严重时出现管腔变窄甚至堵塞的现象,部分管腔变大且较多浆细胞和淋巴细胞浸润(图 2A)。对其生殖道组织的管腔扩张以及炎性细胞浸润程度进行半定量分析,结果发现两组小鼠子宫角和输卵管的炎症程度差异没有统计学意义(P>0.05),但TNF-αR KO小鼠输卵管水肿程度明显低于WT组(图 2B,P < 0.05)。
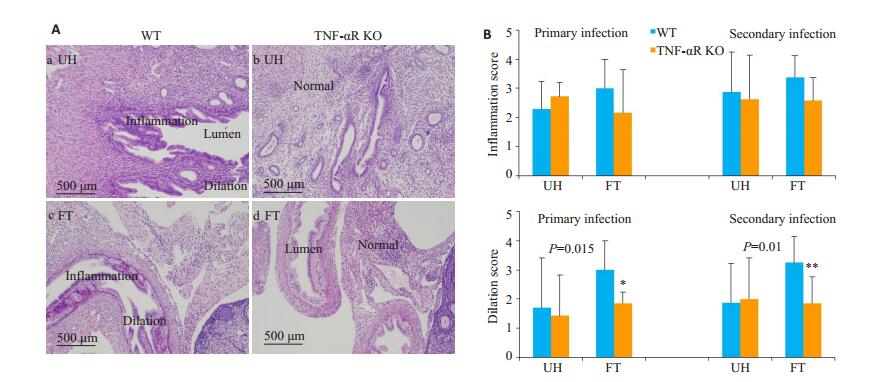
|
图 2 TNF-αR基因敲除对生殖道病理损伤的影响 Fig.2 Effect of TNF-αR knockout on uterine horn and fallopian tube pathology in mice following intravaginal C. muridium infection. A: HE staining of the urogenital tract tissues (Original magnification: ×200). a and c show extensive infiltration by mononuclear cells and dilatation of the fallopian tube and uterine horn cavity in WT mice. b and d show the uterine horns or fallopian tubes of TNF-αR KO mice; B: Semi-quantitative scores for inflammation and lumen dilatation of both the uterine horns and fallopian tubes. *P < 0.05, **P < 0.01 vs WT. |
取小鼠腹腔巨噬细胞,MOI=5的Cm感染后,ELISA检测结果显示,两组小鼠的IL-6、IL-8、IL-1α和IL-1β水平差异无统计学意义(P>0.05),而TNF-αR KO小鼠产生的TNF-α水平高于WT小鼠(P < 0.01,图 3)。
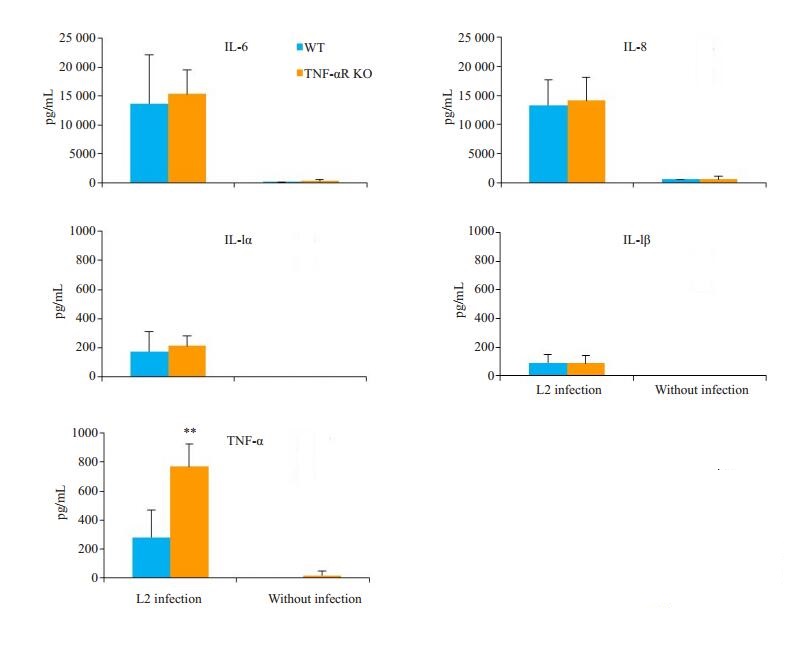
|
图 3 TNF-αR基因敲除对巨噬细胞细胞因子产生的影响 Fig.3 Cytokine production by cultured peritoneal macrophages collected from wild-type and TNF-αR knockout mice 24 h after urogenital C. muridarum infection. The concentrations of the cytokines IL-6, IL-8, IL-1α, IL-1β and TNF-α in the culture supernatant were detected using ELISA. The level of TNF-α produced by the macrophages was significantly higher in TNF-αR KO mice than in WT mice (**P < 0.01 vs WT). |
无论是初次感染还是再次感染,经衣原体再次刺激后,两组小鼠脾细胞均产生较高水平的IFN-γ,差异无统计学意义(P>0.05);WT小鼠产生较高水平的IL-17,明显高于TNF-αR KO小鼠(P < 0.05);IL-4未检测到(图 4)。
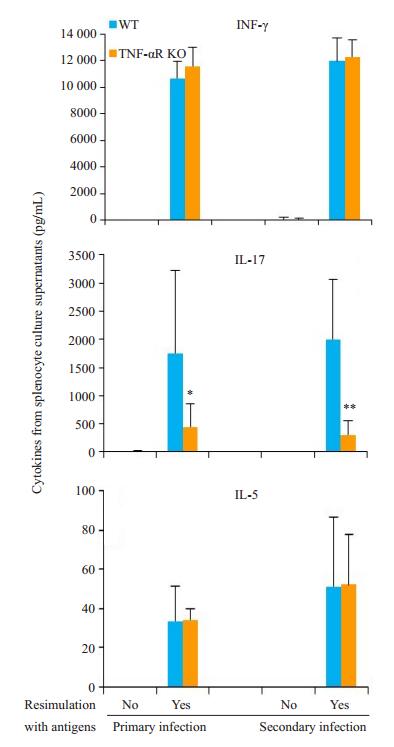
|
图 4 TNF-αR基因敲除对脾细胞细胞因子产生的影响 Fig.4 Cytokine production in response to UV-inactivated chlamydial stimulation in vitro for 72 h in the splenocytes collected from WT and TNF-αR KO mice 80 days after intravaginal infection with C. muridarum. IFN-γ, IL-17, IL-4 and IL-5 in the culture supernatants were measured by ELISA. The levels of IL-17 in spleen lymphocytes are higher in WT mice than in TNF-αR KO mice regardless of the primary or a second infection. IL-4 could not be detected. *P < 0.05, **P < 0.01 vs WT mice. |
生殖道衣原体感染急性期炎症反应过程中可诱导促炎细胞因子产生和释放,清除病原体同时可能导致病理损伤[17-18]。TNF-α作为机体重要的细胞因子,不仅可以上调黏附分子,协助白细胞血管渗出,还可以促进IL-6、IL-8等细胞因子表达,与组织纤维化以及瘢痕的形成有一定关系[19-21]。TNF-α通过与细胞膜表面的TNF受体特异性结合而发挥生物学功能。本研究显示,TNF-αR KO和WT小鼠均能在初次感染后28 d或再次感染后1周左右清除衣原体,带菌时间无明显差异,说明抵御生殖道Cm感染,不完全依赖TNF-α介导的免疫反应,与文献报道的结果一致[14]。TNF-α可促进宿主细胞凋亡,对衣原体的生长发育有一定的干扰作用,在机体清除衣原体中起关键作用[22-24]。而本文结果显示,清除Cm感染不依赖于TNF-α,可能与衣原体的清除还依赖于其他细胞因子协同发挥作用有关。如文献报道,IFN-γ、IL-17等细胞因子在宿主防御衣原体感染的过程中也发挥着重要作用[25-27]。
虽然TNF-αR与小鼠生殖道清除Cm感染无关,但生殖道病理反应结果显示,TNF-αR基因敲除后对子宫角和输卵管的炎症程度无明显影响,却可显著降低小鼠输卵管的水肿程度。这一结果与其他文献报道一致[11, 14-15]。原因可能为衣原体感染可引起宿主细胞产生TNF-α,通过刺激组织成纤维细胞增生,加快释放胶原酶,引起组织病理损伤,而由于TNF-αR缺失,TNF-α介导的免疫损伤无法正常发挥作用。
文献报道IFN-γ是抗衣原体感染的主要细胞因子之一[25-26, 28-29],本研究两组小鼠脾细胞体外经UV-Cm EB再刺激后,均产生较高水平的IFN-γ,差异无统计学意义,与两组小鼠清除衣原体的速度没有区别相呼应。而WT小鼠脾细胞产生的IL-17细胞因子水平明显高于TNF-αR KO小鼠。IL-17主要由辅助性Th17细胞分泌,除具有保护性免疫功能外,高表达的IL-17还可以诱导炎症细胞因子(IL-6、IL-8、TNF-α)和趋化因子到达炎症部位,引起炎性细胞浸润和组织破坏,导致组织病理损伤[30-32]。TNF-αR KO小鼠病理损伤程度减轻,可能也与IL-17依赖的TH17型免疫反应水平较低有关。
综上所述,本文通过鼠衣原体感染小鼠模型,体内实验进一步证实了TNF-α在衣原体感染过程中的作用,发现抗衣原体感染不完全依赖TNF-α介导的免疫反应,但TNF-α可介导Cm引起的生殖道病理损伤。
| [1] |
张莉, 陈超群. 沙眼衣原体感染动物模型的研究及应用[J]. 微生物学免疫学进展, 2016, 44(5): 80-5. |
| [2] |
Karim S, Souho T, Benlemlih M, et al. Cervical cancer induction enhancement potential of chlamydia trachomatis: a systematic review[J]. Curr Microbiol, 2018, 75(12): 1667-74. DOI:10.1007/s00284-018-1439-7 |
| [3] |
Pietro MD, Filardo S, Porpora MG, et al. HPV/chlamydia trachomatis co-infection: metagenomic analysis of cervical microbiota in asymptomatic women[J]. SIVIM, 2018, 41(1): 32-43. |
| [4] |
Barbara SS, Carlos NO, Oliver K, et al. Chlamydia trachomatis fails to protect its growth niche against pro-apoptotic insults[J]. Cell Death Differ, 2019, 26(12): 1485-500. |
| [5] |
Sun X, Tian Q, Wang L, et al. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract[J]. Microbes Infect, 2017, 19(8): 536-45. |
| [6] |
陈丽丽, 吴移谋, 周洲, 等. MyD88在鼠衣原体生殖道感染过程中的作用[J]. 微生物学通报, 2010, 37(6): 937-42. |
| [7] |
Lausen M, Christiansen G, Bouet G, et al. Immunobiology of monocytes and macrophages during Chlamydia trachomatis infection[J]. Microbes Infect, 2019, 21(2): 73-84. DOI:10.1016/j.micinf.2018.10.007 |
| [8] |
Baxter AE, Kaufmann D. Tumor-necrosis factor is a master of T cell exhaustion[J]. Nat Immunol, 2016, 17(5): 476-8. DOI:10.1038/ni.3436 |
| [9] |
陈玉姣, 欧阳晴晴, 王然, 等. 肿瘤坏死因子-α对骨髓源性肥大细胞表达MMP-3、MMP-9、IL-17的影响[J]. 南方医科大学学报, 2015, 35(11): 1633-7. |
| [10] |
陈凡, 张宁洁, 余平, 等. 沙眼衣原体感染诱导小鼠生殖道局部促炎性细胞因子的表达情况[J]. 微生物学杂志, 2013, 33(6): 70-4. DOI:10.3969/j.issn.1005-7021.2013.06.013 |
| [11] |
Murthy AK, Li W, Chaganty BK, et al. Tumor necrosis factor alpha production from CD8 + T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection[J]. Infect Immun, 2011, 79(7): 2928-35. DOI:10.1128/IAI.05022-11 |
| [12] |
Jordan SJ, Gupta K, Ogendi B, et al. The predominant CD4 + Th1 cytokine elicited to chlamydia trachomatis infection in women is tumor necrosis factor alpha and not interferon gamma[J]. Clin Vaccine Immunol, 2017, 24(4): e00010-7. |
| [13] |
李海燕, 梁占光. 沙眼衣原体感染输卵管性不孕患者输卵管液中肿瘤坏死因子α和白细胞介素6的测定[J]. 中华妇产科杂志, 2000, 35(7): 26-7. |
| [14] |
Kamalakaran S, Chaganty KR, Gupta R, et al. Vaginal chlamydial clearance following primary or secondary infection in mice occurs independently of TNF-α[J]. Front Cell Infect Microbiol, 2013, 36(3): 11-23. |
| [15] |
Manam S, Thomas JD, Li W, et al. Tumor necrosis factor(TNF) receptor superfamily member 1b on CD8 +T cells and TNF receptor superfamily member 1a on Non-CD8+T cells contribute significantly to upper genital tract pathology following chlamydial infection[J]. J Infect Dis, 2015, 211(12): 2014-22. DOI:10.1093/infdis/jiu839 |
| [16] |
Chen L, Lei L, Zhou Z, et al. Contribution of interleukin-12 p35(IL- 12p35)and IL-12p40 to protective immunity and pathology in mice infected with Chlamydia muridarum[J]. Infect Immun, 2013, 81(8): 2962-71. DOI:10.1128/IAI.00161-13 |
| [17] |
Cheong HC, Yap P, Chong CW, et al. Diversity of endocervical microbiota associated with genital Chlamydia trachomatis infection and infertility among women visiting obstetrics and gynecology clinics in Malaysia[J]. PLoS One, 2019, 14(11): e0224658-67. DOI:10.1371/journal.pone.0224658 |
| [18] |
Agrawal SK, Rawre J, Khanna N, et al. Increase in chlamydia trachomatis genital and extra-genital infections in Indian males[J]. Ind J Med Microbiol, 2019, 37(2): 285-6. DOI:10.4103/ijmm.IJMM_19_7 |
| [19] |
Fang S, Akash HV, Amy V, et al. Combined blockade of TNF-α and IL-17A alleviates progression of Collagen-Induced arthritis without causing serious infections in mice[J]. J Immunol, 2019, 202(7): 2017-26. DOI:10.4049/jimmunol.1801436 |
| [20] |
Maria P, Berenice CM, Gary MW. TNF-α contributes to lymphoid tissue disorganization and germinal center B cell suppression during intracellular bacterial infection[J]. J Immunol, 2019, 203(9): 2415-24. DOI:10.4049/jimmunol.1900484 |
| [21] |
Zhao X, Zhu D, Ye J, et al. The potential protective role of the combination of IL-22 and TNF-α against genital tract Chlamydia trachomatis infection[J]. Cytokine, 2015, 73(1): 66-73. DOI:10.1016/j.cyto.2015.01.027 |
| [22] |
Smith-Norowitz T, Chotikanatis K, Weaver D, et al. Chlamydia pneumoniae- induced tumour necrosis factor alpha responses are lower in children with asthma compared with non-asthma[J]. BMJ Open Respir Res, 2018, 5(1): e000239-50. DOI:10.1136/bmjresp-2017-000239 |
| [23] |
Sixt BS, Núñez-Otero C, Kepp O, et al. Chlamydia trachomatis fails to protect its growth niche against pro-apoptotic insults[J]. Cell Death Differ, 2019, 26(8): 1485-500. DOI:10.1038/s41418-018-0224-2 |
| [24] |
Waguia KC, Tzivelekidis T, Gentle IE, et al. Infection of epithelial cells with Chlamydia trachomatis inhibits TNF-induced apoptosis at the level of receptor internalization while leaving non-apoptotic TNF-signalling intact[J]. Cell Microbiol, 2016, 18(11): 1583-95. DOI:10.1111/cmi.12598 |
| [25] |
Poston TB, O'connell CM, Girardi J, et al. T cell-independent gamma interferon and B cells cooperate to prevent mortality associated with disseminated Chlamydia muridarum genital tract infection[J]. Infect Immun, 2018, 86(7): e00118-43. |
| [26] |
陈雨晴, 刘安元, 吴移谋. 抗衣原体感染Th1/Th2细胞免疫应答的研究进展[J]. 细胞与分子免疫学杂志, 2018, 34(12): 1136-40. |
| [27] |
Li Y, Wei C, Xu H, et al. The immunoregulation of Th17 in host against intracellular bacterial infection[J]. Mediators Inflamm, 2018, 51(20): 6587296-308. |
| [28] |
Dong X, Liu Y, Chang X, et al. Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection[J]. Infect Immun, 2014, 82(9): 1833-9. |
| [29] |
杨晓玉, 李忠玉. 沙眼衣原体感染与相关细胞因子的研究进展[J]. 现代免疫学, 2014, 34(4): 340-3. |
| [30] |
Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases[J]. Int J Mol Sci, 2019, 20(14): 3394-405. DOI:10.3390/ijms20143394 |
| [31] |
Kim BS, Park YJ, Chung Y. Targeting IL-17 in autoimmunity and inflammation[J]. Arch Pharm Res, 2016, 39(11): 1537-47. DOI:10.1007/s12272-016-0823-8 |
| [32] |
陈晨, 王明永. Th17细胞在自身免疫性疾病中的研究进展[J]. 中国免疫学杂志, 2018, 34(7): 1089-94, 1101. DOI:10.3969/j.issn.1000-484X.2018.07.026 |
 2020, Vol. 40
2020, Vol. 40

