孕烷X受体(PXR)是配体活化的转录因子,属于核受体家族重要成员之一。PXR的主要生物学作用是通过调控下游药物代谢酶和转运蛋白等的表达参与机体的解毒作用[1],除此外PXR还具有调控糖脂代谢、类固醇、胆汁酸胆红素等代谢[2]、免疫炎症[3]和参与肿瘤进展等作用[4-6]。程序性细胞死亡因子(PDCDs)是研究发现的细胞凋亡相关因子,其中PDCD4作为肿瘤抑制基因和抗肿瘤治疗靶点已被广泛认知,PDCD4与多种肿瘤的发生发展密切相关[7-8]。研究证实,PXR在肝癌介导多种化疗药物的耐药性[9-10],此外还能通过调控细胞增殖迁移、凋亡等参与肝癌发生发展过程[11-13],然而,PXR能否通过调控PDCDs参与肝癌进展过程,国内外研究尚不明确。本研究以人肝癌细胞株HepG2为研究模型,探究PXR活化对PDCDs的表达调控作用及PXR调控PDCDs参与肝癌进展新机制,为临床防治肝癌提供新思路。
1 材料和方法 1.1 主要试剂利福平(Sigma)、SR12813(Sigma)、PDCD4抗体(Abcam)、VP16抗体(Abcam)、β-acitn抗体(Santa Cruz)、miRNA21逆转录试剂盒(Takala)、SYBR Green荧光染料(Promega)。
1.2 细胞培养HepG2细胞(取自西安交通大学心血管研究中心)置于含10%胎牛血清(FBS)的DMEM液体培养基中,放入37 ℃,CO2培养箱内培养。
1.3 利福平或SR12813给药刺激PXR是一种核转录因子,活化后可调控下游靶基因CYP3A4和MDR1等的表达,利福平或SR12813均是人源PXR的经典激动剂,在既往研究中发现利福平或SR12813对PXR有很好的激动效应[3, 14],因此选用利福平或SR12813作为激动剂给药刺激。HepG2细胞给予激动剂利福平(10 μmol/L)或SR12813(1 μmol/L)刺激细胞24 h后[15],DMSO作为阴性对照(Control),收集细胞提取RNA和蛋白进行后续检测。
1.4 腺病毒感染实验为了排除配体的非特异性作用,我们采用持续激活型PXR的腺病毒(VP-PXR)(取自西安交通大学心血管研究中心)感染HepG2细胞。该腺病毒编码人全长PXR和转录激活子VP16的融合蛋白,其表达受到转录激活子tTA的控制,四环素作为控制开关,即加入四环素抑制了PXR的表达即为Mock(对照组),不加四环素即为VP-PXR组(图 1)。其感染效率我们也在既往人脐静脉内皮细胞(HUVECs)研究中取得了验证[3]。HepG2细胞给予VP-PXR腺病毒(10 μL VP-PXR、10 μL tTA和1 μL DMSO)或Mock腺病毒(10 μL VP-PXR、10 μL tTA和1 μL四环素)处理36 h后[15],收集细胞提取RNA和蛋白进行后续检测。

|
图 1 四环素介导的Tet-off系统 Fig.1 Tetracycline mediated Tet-off system. |
HepG2细胞给药刺激或VP-PXR腺病毒感染后,提取细胞RNA进行qRT-PCR,以GADPH为内参。所用引物序列如表 1。miRNA21逆转录反应按照反转录试剂盒操作,qRT-PCR检测miRNA21的表达,以U6作为内参。
| 表 1 qRT-PCR引物序列 Tab.1 Primers for qRT-PCR |
培养的6孔板细胞加利福平刺激或VP-PXR感染结束后,提取蛋白。通过Western blot技术检测胞内PDCD4和VP16的蛋白表达水平,以β-acitn作内参。
1.7 生物信息学分析利用生物信息学网站http://genome.ucsc.edu/,查找数据库中人源PDCD4基因启动子区序列。根据比对PXR结合反应元件的核心序列[AG(G/T)TCA]以及同向重复序列DR3、DR4和DR5和反向重复序列ER6和ER8 [16],预测PDCD4的启动子区(nt-3000到nt+150)可能存在的PXREs。
1.8 统计处理应用SPSS16.0统计软件对数据进行处理分析,各指标正态分布的数据用均数±标准差表示。采用两独立样本t检验或两独立样本非参数秩和检验进行组间比较。实验数据来自至少3次以上的重复结果,以P < 0.05为差异有统计学意义。
2 结果 2.1 PXR激动剂上调HepG2细胞内PDCD4的基因表达培养HepG2细胞,给予PXR激动剂利福平或SR12813刺激,采用qRT- PCR检测HepG2细胞内PDCD2、PDCD4、PDCD5和PDCD6的基因表达变化。利福平与对照组相比,PDCD2表达下调(t=-2.875,P < 0.05),PDCD4的表达显著上调(t=4.209,P < 0.01),而PDCD5和PDCD6的表达无明显差异(图 2)。SR12813与对照组相比,PDCD4表达明显升高(t=4.574,P < 0.01),PDCD2、PDCD5和PDCD6则无明显变化。利福平与SR12813组相比,PDCD2、PDCD4、PDCD5和PDCD6的表达均无明显改变。检测PXR下游经典靶基因MDR1的表达,利福平、SR12813组与对照组相比,MDR1的表达均明显上调(P < 0.05)。
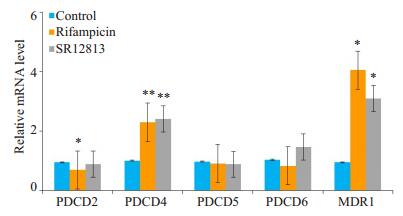
|
图 2 利福平或SR12813上调HepG2细胞内PDCD4的基因表达 Fig.2 Rifampicin and SR12813 increase the gene expression of PDCD4 in HepG2 cells. HepG2 cells were treated with rifampicin (10 μmol/L) or SR12813 (1 μmol/L) or vehicle control for 24 h. The mRNA levels of PDCD2, PDCD4, PDCD5, PDCD6 and MDR1 (PXR target gene) were quantified by qRT-PCR. Data are shown as Mean±SE of at least 3 independent experiments, expressed as fold change after normalization to GAPDH. *P < 0.05, **P < 0.01 vs control group. |
为了排除激动剂的非特异性,采用VP-PXR腺病毒感染HepG2细胞,Mock为对照组,检测PDCD2、PDCD4和PDCD6的基因表达改变。VP-PXR与Mock组相比,PDCD4的表达显著升高(t=3.343,P < 0.05),但PDCD2和PDCD6的表达无明显差异(图 3A)。VP-PXR组与Mock组相比,MDR1的表达则显著升高(t=3.343,P < 0.01),Western blot结果显示VP16的表达在过表达组显著升高(图 3B)。
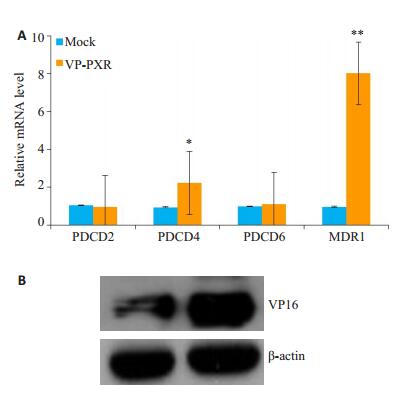
|
图 3 过表达VP-PXR上调PDCD4的基因表达 Fig.3 VP-PXR promotes expression of PDCD4 mRNA in HepG2 cells. A: HepG2 cells were infected with Ad-VP-PXR or with mock infection for 36 h. The mRNA levels of PDCD2, PDCD4, PDCD6 and MDR1 were quantified by qRT-PCR; B: Protein level of VP16 detected with Western blotting. Data are shown as Mean±SE of at least three independent experiments (expressed as fold change after normalization to GAPDH). *P < 0.05, **P < 0.01 vs Mock. |
由图 2,3得出,PXR活化上调了PDCD4的基因水平,PDCD5和PDCD6没有明显改变,PDCD2只在利福平刺激后出现下调现象,而SR12813处理以及VP-PXR腺病毒感染并未出现下调趋势,因此我们判断利福平下调PDCD2为非特异性现象,可能并不依赖于PXR。随后我们采用Western blot检测了PDCD4蛋白水平变化,结果显示利福平刺激后与对照组相比,HepG2细胞内PDCD4的蛋白表达显著上调(图 4A),灰度扫描统计结果证实其上调有统计学意义(t=2.779,P < 0.05,图 4B)。过表达VP-PXR腺病毒与Mock组相比,PDCD4的蛋白表达也明显升高(图 4C),且差异也有统计学意义(t= 3.066,P < 0.05,图 4D)。
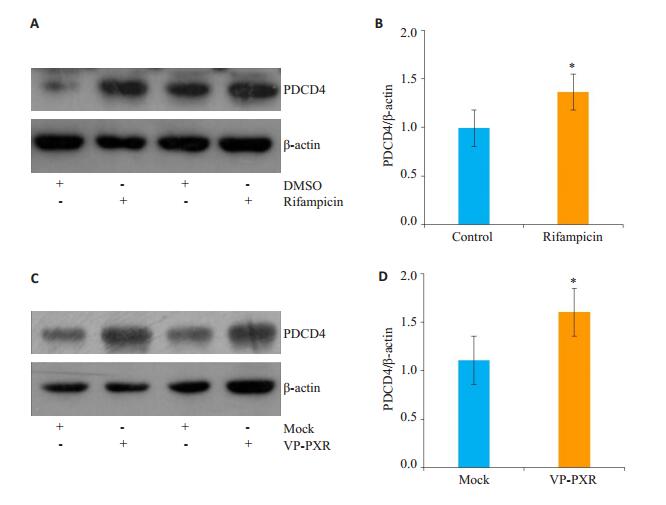
|
图 4 PXR活化上调PDCD4的蛋白表达 Fig.4 PXR activation increases the protein level of PDCD4. HepG2 cells were treated with rifampicin (10 μmol/L) for 24 h (A) or Ad-VP-PXR for 36 h (C), and the protein level of PDCD4 was detected with Western blotting; B, D: Bar graphs showing quantitative results of Western blots. Data shown are as Mean±SE of at least three independent experiments expressed as fold change after normalization to β- actin. *P < 0.05 vs control or mock. |
为了探究PXR上调PDCD4表达的分子机制,对PDCD4上游的调控因子进行了分析,目前已明确PDCD4在多种肿瘤细胞是已知的miRNA21的靶基因[17-20]。我们进一步探究PXR活化上调PDCD4的基因和蛋白表达是否与miRNA21有关,应用qRT-PCR技术检测PXR活化后miRNA21的相对表达。利福平或VP-PXR腺病毒与各自对照组相比,miRNA21表达均无明显变化(P>0.05)。
2.5 PDCD4可能是PXR的下游靶基因深入探究PXR活化上调PDCD4的分子机制,利用生物信息学网站对人源PDCD4基因启动子区序列进行分析,发现PDCD4的启动子区存在潜在的PXREs(表 2)。
| 表 2 人源PDCD4基因启动子区存在的PXREs序列 Tab.2 Sequences of putative PXREs in human PDCD4 gene promoter |
肝癌是我国人群中高发的,危害极大的恶性肿瘤,居我国恶性肿瘤死亡原因的第2位,寻求肝癌作用靶点和新机制己成为治疗肝癌和改善患者预后的关键。研究发现多种化疗药物都可激活核受体PXR,PXR通过对下游靶基因的调控,进而影响肝癌细胞的化疗敏感性,如化疗药物索拉非尼能激活原发性肝癌细胞系内PXR上调CYP3A4和P-gp的表达,增强其化疗耐药性[9]。大豆中的植物雌激素染料木素会激活PXR上调HepG2细胞内P-gp和MRP2的表达,进而介导索拉非尼的耐药性[21]。TGF-β通过与其受体结合启动下游SMAD非依赖的ERK通路激活ETS1转录因子,最终促进PXR的表达而参与肝癌细胞的化疗耐药性[22]。除此外,PXR还能通过调控细胞增殖迁移、凋亡等参与肝癌发生发展过程,PXR活化能直接抑制肿瘤肝癌凋亡,人和小鼠肝细胞中PXR的过表达可以促进Bcl-2和Bcl-xL等抗凋亡因子的表达,进而抑制Fas途径诱导的细胞凋亡[13]。研究发现原发性肝癌细胞系过表达PXR后,细胞增殖迁移速度显著降低,同时证明维生素K2通过激活PXR抑制肝癌细胞增殖[11]。此外,PXR及其下游相关解毒基因在肝癌组织的表达显著降低,PXR在肝癌组织的高表达则可以有效降低其致瘤潜能[12]。采用人肝癌HepG2细胞株、人原代肝细胞及PXR基因敲除小鼠等模型,证实抗真菌药酮康唑可通过阻断AF-2活化区抑制PXR活性,从而逆转利福平诱导的紫杉醇代谢加快,使其血药浓度显著升高,最终起到抗肿瘤作用[23]。利福平激活HepG2细胞内PXR活化可以拮抗LPS-诱导的细胞增殖标记物Ki67的表达,使HepG2阻滞在G0/G1期,起到抗增殖作用[24]。综上,PXR在肝癌中起着错综复杂的作用,其具体机制仍需深入探究。
PDCDs是机体重要的凋亡相关因子,参与多种肿瘤细胞的凋亡过程。其中PDCD4被认为是已知的肿瘤抑制基因,具有抑制肿瘤细胞生长、侵袭、转移和诱导凋亡等多种功能[7-8],PDCD4在多种恶性肿瘤中表达出现下调和缺失,其中在肝癌组织中的表达显著低于癌旁组织[25]。PDCD2在不同癌症组织的作用尚有争议,在胃癌和骨肉瘤被认为是肿瘤抑制因子[26-27],而在人急性白血病细胞中,PDCD2则促进肿瘤细胞增殖[28]。PDCD5作为促凋亡基因,能诱导多种肿瘤细胞发生凋亡[29],其中PDCD5在肝癌的表达显著降低且发挥抗肿瘤作用[30-31]。PDCD6在不同肿瘤中则可能作用相反,PDCD6能促进T细胞受体,Fas-糖皮质激素诱导的细胞凋亡过程发挥抗肿瘤作用,然而其在乳腺癌中则促进肿瘤细胞的侵袭和转移[32]。
本研究关注PXR在肝癌中的作用,通过HepG2细胞加入PXR激动剂利福平或SR12813以及过表达VP-PXR腺病毒等方式激活PXR,探究PXR参与肝癌进展是否与PDCDs有关。研究结果显示:利福平或SR12813可以上调PDCD4基因的表达,但对PDCD2、PDCD5和PDCD6无影响(图 2)。采用VP-PXR腺病毒感染HepG2细胞,发现VP-PXR也上调了PDCD4的基因表达,但对其它PDCDs的表达无明显变化(图 3)。为了验证PXR激动剂以及VP-PXR对PXR的激活情况,我们也检测了PXR下游靶基因MDR1以及转录激活子VP16表达情况,结果显示在利福平、SR12813刺激或VP-PXR感染细胞后MDR1的基因表达均出现上调,VP-PXR感染后VP16的蛋白表达也升高(图 2,3),证明激动剂和VP-PXR腺病毒均激活了PXR。随后Western blot结果证实利福平和VP-PXR同样上调PDCD4的蛋白水平(图 4)。我们得出HepG2细胞PXR活化上调抑癌基因PDCD4的表达,这可能是PXR参与调控肝癌进展的一个新机制。
研究证明PDCD4的表达受多种转录因子的负性调控和甲基化表观遗传学等调控,目前已明确PDCD4在多种肿瘤细胞是已知的miRNA21的靶基因,miRNA21可以特异性的结合到PDCD4 mRNA的3'-UTR区促进PDCD4 mRNA的降解,降低PDCD4的表达,影响肿瘤细胞的增殖和迁移。已有证据证明miRNA 21可通过PDCD4调控肝癌细胞的生长和侵袭[19, 25, 33]。我们进一步探究了PXR上调PDCD4的表达是否与miRNA21有关,结果显示利福平或VP-PXR腺病毒对miRNA21的表达均无明显影响,得出PXR上调PDCD4的表达不依赖于miRNA21。PXR发挥活性时主要通过和RXR形成异源二聚体,结合到靶基因上特异的重复序列[AG(G/T)TCA]PXRE上,启动对下游基因的调控。常见的PXRE包括同向重复序列(DR3、DR4和DR5)和反向重复序列(ER6和ER8)[16]。为了进一步探究PXR上调PDCD4的分子机制,我们通过生物信息学技术分析了PDCD4的启动子区,发现若干PXR结合反应元件(PXREs)(表 2),除此外我们也分析了大鼠和小鼠PDCD4的启动子区,同样存在若干PXREs,提示PDCD4可能是PXR的下游靶基因,后续我们还需通过免疫共沉淀、PDCD4启动子区荧光素酶报告基因等实验进行验证。
综上所述,HepG2细胞激活PXR上调PDCD4的表达,机制研究发现PXR上调PDCD4并不依赖于miRNA21,PDCD4在HepG2细胞是潜在的PXR靶基因。阐明了PXR可能通过调控抑癌基因PDCD4参与肝癌进展,为PXR在肿瘤发展中的作用提供新思路。
| [1] |
He LQ, Zhou XH, Niu H, et al. Functions of pregnane X receptor in self-detoxification[J]. Amino Acids, 2017, 49(12): 1999-2007. DOI:10.1007/s00726-017-2435-0 |
| [2] |
Oladimeji PO, Chen TS. PXR: more than just a master xenobiotic receptor[J]. Mol Pharmacol, 2018, 93(2): 119-27. DOI:10.1124/mol.117.110155 |
| [3] |
Wang SL, Lei T, Kang Z, et al. Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells[J]. J Biol Chem, 2014, 289(43): 30075-81. DOI:10.1074/jbc.M114.578781 |
| [4] |
Sergio CC, William CW, Chen TS. Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer[J]. Med Res Rev, 2019, 28: Online ahead of print.
|
| [5] |
Robbins D, Chen TS. Tissue-specific regulation of pregnane X receptor in cancer development and therapy[J]. Cell Biosci, 2014, 4(1): 17. DOI:10.1186/2045-3701-4-17 |
| [6] |
Satyanarayana RP, Sridhar M. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response[J]. Cancer Lett, 2013, 328(1): 1-9. DOI:10.1016/j.canlet.2012.08.030 |
| [7] |
Matsuhashi S, Manirujjaman M, Hamajima H, et al. Control mechanisms of the tumor suppressor PDCD4: expression and functions[J]. Int J Mol Sci, 2019, 20(9): 2304. DOI:10.3390/ijms20092304 |
| [8] |
Qing W, Hsin-Sheng Y. The role of Pdcd4 in tumour suppression and protein translation[J]. Biology of the Cell, 2018, 110(8): 169-77. DOI:10.1111/boc.201800014 |
| [9] |
Fan F, Jiang QY, Shuang C, et al. Pregnane X receptor mediates sorafenib resistance in advanced hepatocellular carcinoma[J]. Biochim Biophys Acta Gen Subj, 2018, 1862(4): 1017-30. DOI:10.1016/j.bbagen.2018.01.011 |
| [10] |
Shao ZY, Li YB, Dai WJ, et al. ETS-1 induces Sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR[J]. Pharmacol Res, 2018, 135(8): 188-200. |
| [11] |
Azuma K, Urano T, Ouchi Y, et al. Vitamin K2 suppresses proliferation and motility of hepatocellular carcinoma cells by activating steroid and xenobiotic receptor[J]. Endocr J, 2009, 56(7): 843-9. DOI:10.1507/endocrj.K09E-108 |
| [12] |
Deepak K, Jaiswal B, Ghose S, et al. Role of PXR in hepatic cancer: its influences on liver detoxification capacity and cancer progression[J]. PLoS One, 2016, 11(10): e0164087. DOI:10.1371/journal.pone.0164087 |
| [13] |
Nathalie Z, Sousa GD, Bailly-Maitre B, et al. Regulation of Bcl- 2 and Bcl-xL anti-apoptotic protein expression by nuclear receptor PXR in primary cultures of human and rat hepatocytes[J]. Biochim Biophys Acta Gen Subj, 2005, 1745(1): 48-58. DOI:10.1016/j.bbamcr.2005.02.005 |
| [14] |
Wang SL, Xie XY, Lei T, et al. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-Dependent mechanism[J]. Mol Pharmacol, 2017, 92(3): 256-64. DOI:10.1124/mol.116.108100 |
| [15] |
Wang X, Fang X, Zhou J, et al. Shear stress activation of nuclear receptor PXR in endothelial detoxification[J]. Pro Nat Acad Sci, 2013, 110(32): 13174-9. DOI:10.1073/pnas.1312065110 |
| [16] |
Hariparsad N, Chu XY, Yabut J, et al. Identification of pregnane-X receptor target genes and coactivator and corepressor binding to promoter elements in human hepatocytes[J]. Nucleic Acids Res, 2009, 37(4): 1160-73. DOI:10.1093/nar/gkn1047 |
| [17] |
Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR- 21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer[J]. Oncogene, 2008, 27(15): 2128-36. DOI:10.1038/sj.onc.1210856 |
| [18] |
Talotta F, Cimmino A, Matarazzo MR, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation[J]. Oncogene, 2009, 28(1): 73-84. DOI:10.1038/onc.2008.370 |
| [19] |
Liu CZ, Jia Y, Yu SN, et al. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma[J]. J Hepatol, 2010, 53(1): 98-107. DOI:10.1016/j.jhep.2010.02.021 |
| [20] |
Wang Y, Gao X, Wei F, et al. Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis[J]. Gene, 2014, 533(1): 389-97. DOI:10.1016/j.gene.2013.09.038 |
| [21] |
Rigalli JP, Ciriaci N, Arias A, et al. Regulation of multidrug resistance proteins by genistein in a hepatocarcinoma cell line: impact on sorafenib cytotoxicity[J]. PLoS One, 2015, 10(3): e0119502. DOI:10.1371/journal.pone.0119502 |
| [22] |
Bhagyaraj E, Ahuja N, Kumar S, et al. TGF-β induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR[J]. Cell Cycle, 2019, 18(24): 3589-602. DOI:10.1080/15384101.2019.1693120 |
| [23] |
Ekins S, Cheng C, Sridhar M, et al. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites[J]. Mol Pharmacol, 2007, 72(3): 592-603. DOI:10.1124/mol.107.038398 |
| [24] |
Ye NH, Hang W, Li QL, et al. Activation of PXR inhibits LPSinduced NF-κB activation by increasing IκBα expression in HepG2 cells[J]. Mol Cell Toxicol, 2018, 14(1): 93-104. DOI:10.1007/s13273-018-0012-6 |
| [25] |
印滇, 杨莉, 张亮, 等. miR-21通过PDCD4调控肝癌细胞的生长和侵袭[J]. 临床与实验病理学杂志, 2017, 33(4): 412-6. |
| [26] |
Jian Z, Wei W, Jin HC, et al. Programmed cell death 2 protein induces gastric cancer cell growth arrest at the early S phase of the cell cycle and apoptosis in a p53-dependent manner[J]. Oncol Rep, 2015, 33(1): 103-10. |
| [27] |
Yang Y, Jin Y, Du W. Programmed cell death 2 functions as a tumor suppressor in osteosarcoma[J]. Int J Clin Exp Pathol, 2015, 8(9): 10894-900. |
| [28] |
Barboza N, Minakhina S, Daniel JM, et al. PDCD2 functions in cancer cell proliferation and predicts relapsed leukemia[J]. Cancer Biol Ther, 2013, 14(6): 546-55. DOI:10.4161/cbt.24484 |
| [29] |
Wei W, Song XW, Zhao CH. Roles of programmed cell death protein 5 in inflammation and cancer (Review)[J]. Int J Oncol, 2016, 49(5): 1801-6. DOI:10.3892/ijo.2016.3706 |
| [30] |
Fu DZ, Ying C, Hui H, et al. PDCD5 expression predicts a favorable outcome in patients with hepatocellular carcinoma[J]. Int J Oncol, 2013, 43(3): 821-30. DOI:10.3892/ijo.2013.1993 |
| [31] |
Fu DZ, Ying C, Hui H, et al. Recombinant human PDCD5 exhibits an antitumor role in hepatocellular carcinoma cells via clathrindependent endocytosis[J]. Mol Med Rep, 2015, 12(6): 8135-40. DOI:10.3892/mmr.2015.4489 |
| [32] |
Hashemi M, Bahari G, Markowski J, et al. Association of PDCD6 polymorphisms with the risk of cancer: Evidence from a metaanalysis[J]. Oncotarget, 2018, 9(37): 24857-68. |
| [33] |
Damania P, Sen B, Dar SB, et al. Hepatitis B virus induces cell proliferation via HBx-induced microRNA-21 in hepatocellular carcinoma by targeting programmed cell death protein4 (PDCD4) and phosphatase and tensin homologue (PTEN)[J]. PLoS One, 2014, 9(3): e91745. DOI:10.1371/journal.pone.0091745 |
 2020, Vol. 40
2020, Vol. 40
