2. 蚌埠医学院 药学院,安徽 蚌埠 233030;
3. 蚌埠医学院 临床学院,安徽 蚌埠 233030
2. School of Pharmacy, Bengbu Medical College, Bengbu 233030, China;
3. College of Clinical Medicine, Bengbu Medical College, Bengbu 233030, China
鼻咽癌的发生率位于耳鼻咽喉恶性肿瘤之首,我国是鼻咽癌的高发国家之一。全球每年的新发病例中我国约占1/3,且具有南高北低的显著性地域差异[1-2]。虽然鼻咽癌大多为低分化癌,对放疗较敏感,但是接近75%的病人在发现和治疗时,往往已处于Ⅲ和Ⅳ期,预后较差,单一的放疗不能满足治疗的需要[3-5],因此,化疗在鼻咽癌的治疗中亦发挥着非常重要的作用。寻找有效且耐受性好的治疗方案及解决化疗药物耐药对于临床治疗重要而紧迫。
本课题组前期研究结果显示,氯喹可诱导过度的内质网应激,促进鼻咽癌细胞凋亡,并能通过下调MDR1和P-gp的表达,逆转耐药人鼻咽癌细胞HNE1/顺铂的耐药[6]。氯喹是一种公认的自噬抑制剂。研究表明,自噬抑制剂能通过抑制内质网应激诱导的保护性自噬,增强鼻咽癌细胞对放疗和化疗的敏感性[7],但目前对氯喹抑制保护性自噬,增敏顺铂在鼻咽癌中的作用机制尚不十分清楚,进一步阐明氯喹增敏顺铂治疗鼻咽癌作用的机制,对指导其临床治疗具有重要的现实意义。
miRNA是非编码的小分子RNA,已被证实其表达变化与鼻咽癌放化疗敏感性密切相关[7],且miR129在乳突状病毒相关口咽癌中表达异常[9],上调miR129能抑制鼻咽癌细胞增殖和转移能力[10]。氯喹调控多种肿瘤中miRNA的表达,影响化疗药物敏感性的作用也在多种肿瘤研究中被报道[11-15],但氯喹是否通过调控miR129,增敏顺铂治疗鼻咽癌尚未见报道。本研究通过检测人鼻咽癌细胞株HNE1中氯喹对miR129表达的影响,以及通过转染miR129 inhibitor进一步观察氯喹和顺铂联合作用对HNE1细胞增殖、自噬和凋亡的影响,以期为探究和阐明氯喹增强顺铂作用的可能机制提供更多的实验依据。
1 材料和方法 1.1 材料 1.1.1 细胞及动物人鼻咽癌HNE1细胞购于中南大学湘雅医学院,由蚌埠医学院药学院科研中心冻存。BALB/C裸鼠购于南京模式动物中心,饲养于校科研中心SPF屏障系统无菌动物房。
1.1.2 主要试剂RPMI 1640培养基(HyClone),胎牛血清(杭州四季青),MTT(Sigma),凋亡周期试剂盒(上海吉凯),LC3B、p62、Beclin1、β-actin单克隆抗体(CST),miR129 inhibitor(上海吉玛),逆转录试剂盒(上海生工)q- PCR试剂盒(上海翊圣),Trizol(北京天根),Lipofectamine 2000(Thermo Fisher)。
1.2 方法 1.2.1 细胞培养HNE1细胞培养于含10%胎牛血清的1640培养基(内加双抗,青霉素:80 U/mL,链霉素:100 U/mL)中,置于37 ℃,5% CO2培养箱。
1.2.2 MTT法检测细胞存活率将人鼻咽癌HNE1细胞接种于96孔板中,5×103细胞/孔,给予不同浓度的顺铂(2、4、8、16、32 μmol/L)处理24、48、72 h后,加入20 μL MTT(5 mg/mL),于37 ℃,5% CO2培养箱中继续培养4 h后弃上清,加入DMSO 150 μL /孔,37 ℃孵育30 min,置于酶标仪上振荡20 s,使结晶充分溶解,酶标仪检测吸光度A490 nm。细胞存活率(%)= (处理组A490 nm -空白对照组A490nm)(/阴性对照组A490nm -空白对照组A490nm)×100%,以上实验重复3次。
1.2.2 集落克隆实验检测细胞的增殖能力以8×103细胞/孔,接种细胞于6孔板中,37 ℃,5% CO2培养24 h后,给予顺铂(0.35 μmol/L)、氯喹(1 μmol/L)以及氯喹(1 μmol/L)+顺铂(0.35 μmol/L)处理HNE1细胞,置于37 ℃,5% CO2培养箱中继续培养7 d,观察细胞集落形成后,弃去培养基,PBS轻柔清洗2次,加入4%多聚甲醛室温固定30 min,去除固定液后用PBS小心清洗3次,加结晶紫染色25 min,弃去染色液,用双蒸水小心清洗干净,放置干燥后拍照,实验重复3次。
1.2.3 裸鼠成瘤实验BALB/C裸鼠接种0.1 mL细胞悬液与Matrigel胶的混合液(含200 μL 3×106细胞),待肿瘤长至80 mm×80 mm×80 mm时,随机分为4组:对照组、氯喹组、顺铂组、氯喹+顺铂组,给药量为顺铂5 mg/kg、氯喹45 mg/kg,对照组给予0.9%生理盐水,均为腹腔注射,3 d给药1次并测量小鼠体质量,每5 d测量肿瘤体积,给药4周后处死动物剥离肿瘤并称重,肿瘤体积(V)=ab2/2(mm3),a:肿瘤最长径,b:垂直方向最大横径。
1.2.4 q-PCR检测HNE1细胞中miR129的表达将细胞收集后用Trizol提取细胞中的总RNA,逆转录试剂盒进行反转录合成cDNA,按照根据All-in One miRNA qRT-PCR Detection kit操作说明在PCR仪上进行扩增,miR129定量分析以循环阈值(Ct)进行比较,数据用2-△△Ct相对定量公式进行计算。
1.2.5 Westernblot法检测LC3B、p62、Beclin1、P-gp等蛋白的表达细胞处理后,用预冷的PBS洗涤3次,加入80~100 μL RIPA Lysis Buffer(含相应蛋白酶、磷酸酶抑制剂),冰上充分裂解,反复冻融后提取细胞总蛋白,BCA法检测蛋白浓度。SDS-PAGE聚丙烯酰胺凝胶电泳进行蛋白分离,20 mA恒流转移60 min(置于冰上)转至PVDF上,5%脱脂牛奶4 ℃封闭过夜。TPBS洗膜2次后,加入相应一抗抗体4 ℃过夜,辣根过氧化物酶标记的二抗37 ℃孵育反应2 h。按照ECL显影试剂盒说明,用Bio-Rad凝胶成像系统检测和成像,实验重复3次。
1.2.6 Annexin V/PI双染,流式细胞仪检测细胞凋亡细胞接种过夜后,加入顺铂(3.5 μmol/L)、氯喹(10 μmol/L)以及氯喹(10 μmol/L)+顺铂(3.5 μmol/L)处理HNE1细胞24 h后,用预冷的PBS洗涤2遍,所有冲洗液收集至相应离心管中,做好标记,每孔加入适量胰酶消化,1000 r/min离心5 min,收集处理后的各组细胞,PBS洗2次,每管加入100 μL Binding Buffer重悬细胞,相应管中加入5 μLAnnexin V-FITC,4 ℃避光孵育15 min后,在相应管中加5 μL PI,4 ℃避光孵育5 min。加入300 μL BindingBuffer,1h内流式细胞仪上机检测,实验重复3次。
1.2.7 统计学分析采用SPSS 22.0软件分析,数据用均数±标准差表示,两组之间的数据比较采用独立样本的t检验,多组之间的数据比较采用单因素方差分析,P < 0.05为差异有统计学意义。
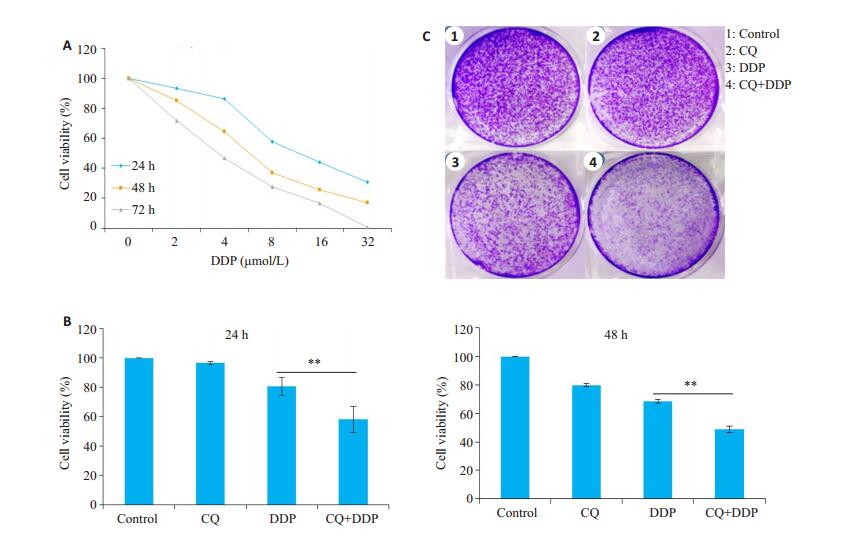
2 结果 2.1 氯喹增强顺铂对HNE1细胞的增殖抑制作用顺铂(2、4、8、16、32 μmol/L)对HNE1在24、48、72 h的增殖抑制作用呈现时间和浓度依赖性(图 1A),接下来我们选择了顺铂的亚适浓度3.5 μmol/L与对细胞无明显抑制作用的氯喹(10 μmol/L)联合使用,结果显示,在24 h和48 h,顺铂(3.5 μmol/L)+氯喹(10 μmol/L)联合给药组,较顺铂(3.5 μmol/L)单独给药组,显著降低了HNE1细胞的细胞存活率(P < 0.01,图 1B)和集落克隆形成能力(图 1C)。
|
图 1 氯喹增强顺铂对HNE1细胞的增殖抑制作用 Fig.1 Chloroquine enhances the inhibitory effect of cisplatin on HNE1 cell proliferation. A: Viability of HNE1 cells after treatment with cisplatin at different concentrations (2, 4, 8, 16, and 32 μmol/L); B: Viability of HNE1 cells treated by chloroquine combined with cisplatin at 24 and 48 h; C: Colony formation of HNE1 cells with combined treatment with chloroquineand cisplatin. Data are presented as (Mean±SD, n=3). **P < 0.01 vs cisplatin group. CQ: Chloroquine; DDP: Cisplatin. |
氯喹(45 mg/kg)+顺铂(5 mg/kg)联合给药组,较顺铂(5 mg/kg)单独给药组,小鼠的体质量有增加趋势(图 2A)。氯喹+顺铂组小鼠的肿瘤体积较顺铂组,从15 d开始显著降低(P < 0.05,图 2B)。给药4周后,氯喹+顺铂组小鼠的瘤重较顺铂组显著降低(P < 0.01,图 2C)。

|
图 2 氯喹增强顺铂对BALB/C裸小鼠的肿瘤生长的抑制作用 Fig.2 Chloroquine enhances the inhibitory effect of cisplatin on tumor growth in BALB/C nude mice. A: Weight of nude mice from day 1 to day 30 in all the groups; B: Tumor volume in different groups; C: Tumor weight in different groups on day 30. Data are presented as (Mean±SD, n=6). *P < 0.05, **P < 0.01 vs cisplatin group. CQ: Chloroquine; DDP: Cisplatin. |
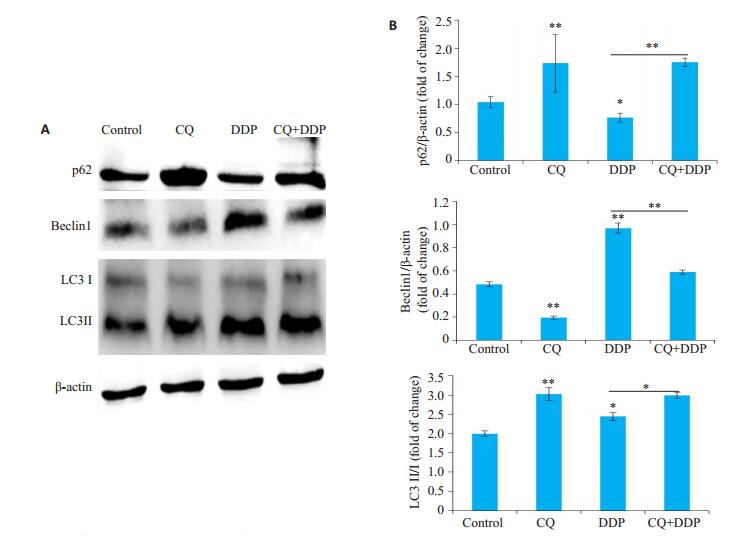
进一步检测顺铂对HNE1细胞自噬的作用,以及氯喹对顺铂作用的HNE1细胞自噬的影响。顺铂(3.5 μmol/L)能明显降低p62的表达(P < 0.05),显著增加Beclin1(P < 0.01)和LC3BII(P < 0.05)的表达,促进HNE1细胞自噬。氯喹(10 μmol/L)显著上调了顺铂作用后细胞内p62的表达(P < 0.01)和LC3BII(P < 0.05)的表达,明显下调了Beclin1的表达(P < 0.01),抑制了HNE1细胞自噬(图 3)。
|
图 3 氯喹抑制顺铂诱导的HNE1细胞自噬 Fig.3 Chloroquine inhibits cisplatin-induced autophagy in HNE1 cells. A, B: Expression of p62, Beclin1, and LC3 in different groups. Data are presented as (Mean±SD, n=3). *P < 0.05, **P < 0.01 vs control group. CQ: Chloroquine; DDP: Cisplatin. |
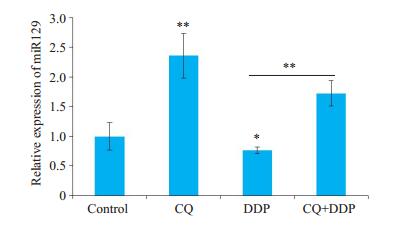
氯喹能够显著上调HNE1细胞中miR129的表达(P < 0.01),顺铂明显下调了HNE1细胞中miR129的表达(P < 0.05),联合用药后,氯喹显著减弱了顺铂对HNE1细胞中miR129表达的抑制作用(P < 0.01,图 4)。
|
图 4 氯喹上调顺铂处理后HNE1细胞中miR129的表达 Fig.4 Chloroquine up-regulates the expression of miR129 in HNE1 cells treated with cisplatin (Mean ± SD, n=3). *P < 0.05, **P < 0.01 vs control group. CQ: Chloroquine; DDP: Cisplatin. |
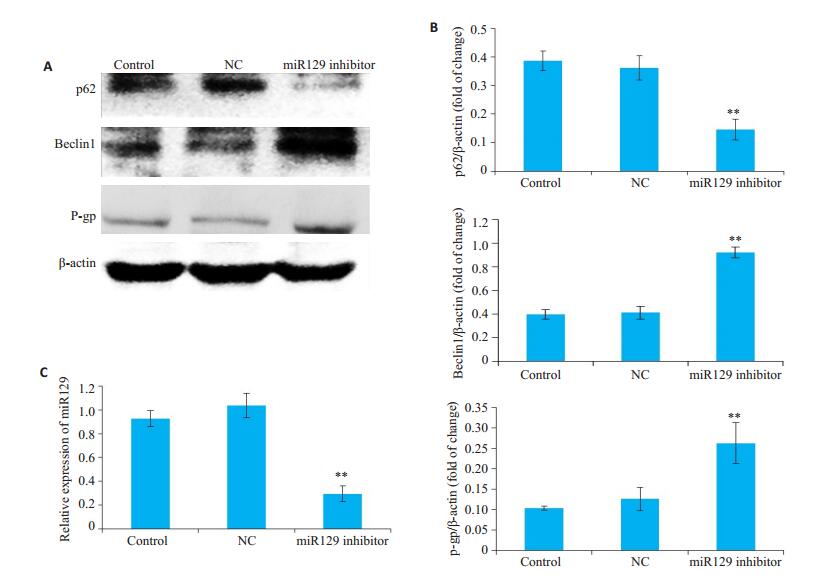
我们使用了miR129抑制剂,发现miR129抑制剂显著下调了p62的表达(P < 0.01),上调了Beclin1(P < 0.01)和P-gp(P < 0.01)的表达(图 5)。
|
图 5 抑制miR129促进HNE1细胞自噬,上调HNE1细胞中P-gp的表达 Fig.5 Inhibition of miR129 promotes autophagy and the expression of P-gp in HNE1 cells. A, B: Expression of p62, Beclin1and P-gp in different groups; C: Expression of miR129 in HNE1 cells in different groups. Data are presented as (Mean±SD, n=3). *P < 0.05, **P < 0.01 vs control group. |
为了进一步证实氯喹是否通过上调miR129增强顺铂对HNE1细胞的增殖抑制作用,我们观察了使用miR129抑制剂前后氯喹与顺铂联合给药对HNE1细胞存活率的影响,使用miR129抑制剂下调miR129表达后,显著增加了氯喹与顺铂联合给药后HNE1的细胞存活率(P < 0.05,图 6)。
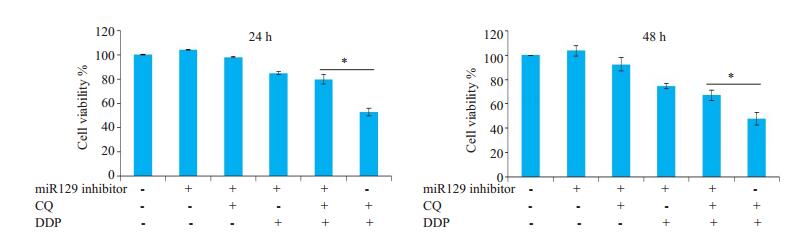
|
图 6 下调miR129显著增加氯喹与顺铂联合给药后HNE1的细胞存活率 Fig.6 Down-regulation of miR129 significantly increases the cell survival rate of HNE1 cells after combined treatment with chloroquine and cisplatin. The viability of HNE1 cells at 24 h and 48 h is shown. Data are presented as (Mean±SD, n=3). *P < 0.05 vs chloroquine+cisplatin group. CQ: Chloroquine; DDP: Cisplatin. |
接下来我们观察了miR129抑制剂作用前后,氯喹与顺铂联合给药对HNE1细胞自噬程度的影响,结果显示miR129抑制剂显著增强了氯喹与顺铂联合给药后HNE1细胞的自噬水平(图 7)。
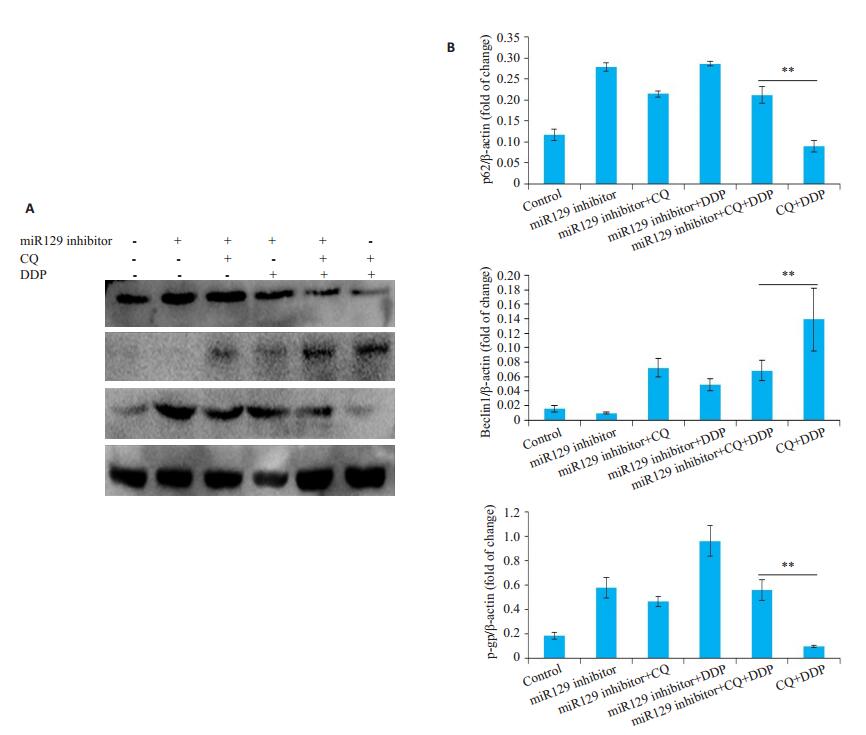
|
图 7 氯喹通过上调miR129抑制HNE1细胞自噬 Fig.7 Chloroquine inhibits autophagy in HNE1 cells by up-regulating miR129. A, B: Expression of p62, Beclin1and P-gp in different groups. Data are presented as (Mean±SD, n=3). *P < 0.05, **P < 0.01 vs chloroquine+cisplatin group. CQ: Chloroquine; DDP: Cisplatin. |
最后,我们通过抑制miR129,观察氯喹与顺铂联合给药对HNE1细胞凋亡的影响,结果发现抑制miR129表达后,氯喹与顺铂联合给药组HNE1细胞凋亡显著减少(图 8)。
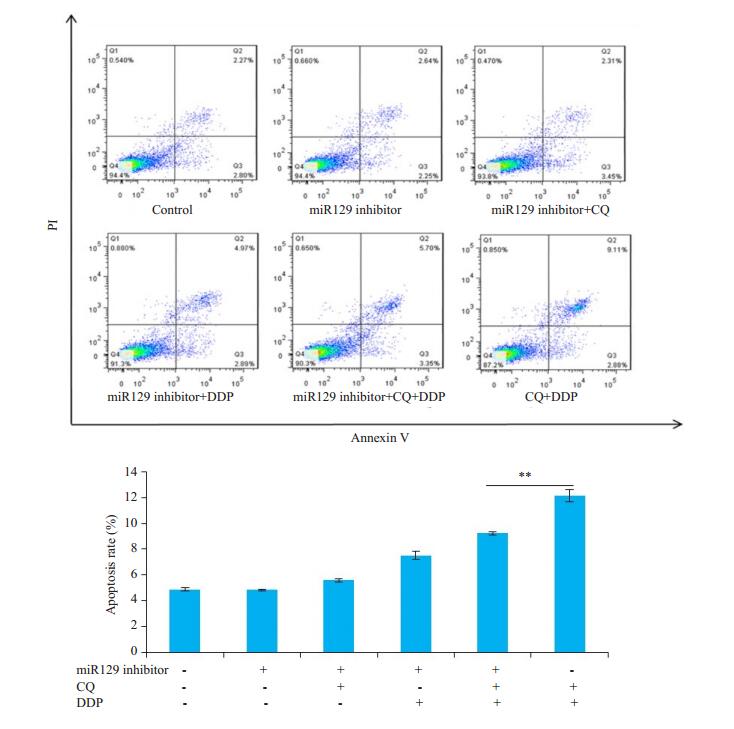
|
图 8 氯喹通过上调miR129促进HNE1细胞凋亡 Fig.8 Chloroquine promotes apoptosis in HNE1 cells by up-regulating miR129. The apoptosis rate of each group was measured by flow cytometry. Data are presented as Mean ± SD (n=3). **P < 0.05 vs chloroquine+cisplatin group. CQ: Chloroquine; DDP: Cisplatin. |
目前已较为公认,肿瘤细胞对放化疗耐受与其自噬增加密切相关,抑制自噬可以改善某些肿瘤的化疗效果[16-17]。氯喹是经典的抗疟疾药物,能够靶向细胞溶酶体,抑制晚期自噬。研究报道,氯喹能增强化疗药物治疗多种肿瘤如皮肤癌、鼻咽癌等的敏感性[18-19]。多位研究者也报道了氯喹通过抑制自噬,在鼻咽癌放疗中的增敏作用[20-22]。顺铂可通过诱导非小细胞肺癌细胞的保护性自噬而导致化疗耐药,雷帕霉素(自噬诱导剂)能使细胞的耐药性增强[23]。因此,我们猜想氯喹是否能抑制自噬增敏顺铂对鼻咽癌的治疗作用。我们体内外的研究结果表明,氯喹与顺铂联合能明显抑制鼻咽癌的生长,并能抑制顺铂治疗过程中细胞的保护性自噬的发生。肿瘤细胞在化疗药物作用下,会通过增加自噬,促进活性氧和过氧化物的清除,增强肿瘤细胞在应激状态下的适应性,促进肿瘤细胞存活。有报道,自噬抑制剂(3-MA)可在一定程度上逆转白血病细胞对化疗药物的抵抗[24],提示调控肿瘤细胞内自噬信号通路可能是解决肿瘤治疗耐药的途径和潜在靶标。
miRNA是由18~25个核苷酸组成的,非编码小分子RNA,在动物和植物中广泛表达。每个miRNA可通过对靶基因的精细调控,参与生理和病理过程。miRNA能够影响自噬、氧化应激和凋亡过程,参与包括肿瘤在内的多种免疫失调性疾病[25-27]。miR129是一种多功能抑癌基因,其在肾癌、神经母细胞瘤、前列腺癌等多种肿瘤的发生发展中发挥着重要的抑制作用[28-30]。研究发现miR129是通过下调Beclin1抑制自噬过程[31]。此外,高迁移率族蛋白B1也是miR129的靶基因,miR129能通过负性调控HMGB1抑制自噬增加乳腺癌细胞MCF-7对紫杉醇的敏感性[32]。已有报道miR129能抑制鼻咽癌细胞增殖和转移能力[10]。我们的研究发现氯喹能上调HNE1细胞中miR129的表达,抑制miR129不但能增强HNE1细胞自噬,而且减弱氯喹增敏顺铂抑制HNE1细胞增殖、上调细胞中耐药蛋白P-gp的表达。这些结果表明氯喹是通过上调miR129抑制HNE1细胞发生自噬和耐药,发挥增敏顺铂的抗鼻咽癌作用的。
让我们感兴趣的是在前期的研究中,我们发现氯喹抑制鼻咽癌细胞异常增殖与促进其凋亡有关[33]。两项研究也分别在肾癌和神经胶质母细胞瘤中证实了上调miR129表达能促进细胞凋亡[28, 34]。我们发现抑制miR129后,不仅显著抑制了自噬,而且减弱了氯喹促进顺铂诱导的细胞凋亡。干扰Beclin1自噬相关基因表达后,发现细胞凋亡率明显增加,这可能与肿瘤细胞由于自噬被抑制营养物质进一步缺乏,促进细胞凋亡有关[35]。也有文献报道,上调miR129本身可以诱导肿瘤细胞的凋亡,miR129能激活钙调蛋白依赖性蛋白激酶IV,抑制肝癌细胞的增殖、迁移和侵袭[36]。在前列腺癌研究中发现,miR129通过下调前B细胞白血病同源盒基因3的表达,抑制前列腺癌细胞的增殖和迁移[37]。这些结果提示,氯喹增强顺铂的作用可能主要与其上调miR129抑制自噬,增强顺铂诱导HNE1细胞凋亡有关,此外,也可能通过上调miR129直接促进鼻咽癌细胞的凋亡。
综上所述,本研究证实了氯喹可能通过上调miR129从两方面增强顺铂抗鼻咽癌作用。一方面是通过上调miR129发挥自噬抑制,减轻顺铂单用诱导的细胞保护性自噬和耐药;另一方面是通过上调miR129发挥促进顺铂诱导的细胞凋亡。氯喹作为一种老药,低毒安全和其化疗增敏作用或许是提高鼻咽癌临床治疗效果的一个重要方向,我们的研究为临床提高鼻咽癌的化疗增敏治疗和减少耐药提供了实验基础和理论依据。
| [1] |
Chattopadhyay NR, Das P, Chatterjee K, et al. Higher incidence of nasopharyngeal carcinoma in some regions in the world confers for interplay between genetic factors and external stimuli[J]. Drug Discov Ther, 2017, 11(4): 170-80. DOI:10.5582/ddt.2017.01030 |
| [2] |
Zong JF, Huang QT, Guo Q, et al. Evolution of the Chinese staging system for nasopharyngeal carcinoma[J]. 临床肿瘤学杂志, 2016, 5(2): 19-19. |
| [3] |
Jin T, Zhang Q, Luo DH, et al. Concurrent chemoradiotherapy with or without induction chemotherapy for patients with stage Ⅱ nasopharyngeal carcinoma: an update[J]. Transl Oncol, 2020, 13(1): 25-31. |
| [4] |
Wang ZQ, Mei Q, Li JB, et al. The long-term survival of patients with Ⅲ-IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis[J]. BMC Cancer, 2019, 19(1): 1122. DOI:10.1186/s12885-019-6156-5 |
| [5] |
Wu SG, Zhang WW, Jun W, et al. The 1-year mortality after radiotherapy for nasopharyngeal carcinoma: a population-based analysis[J]. Future Oncol, 2019, 15(29): 3357-65. DOI:10.2217/fon-2019-0371 |
| [6] |
张浩轩, 孙小锦, 孙一鸣, 等. 氯喹逆转人鼻咽癌细胞HNE1/DDP的耐药作用[J]. 南方医科大学学报, 2015, 35(5): 687-91. |
| [7] |
宋乐乐, 马琳艳, 陈根德, 等. 自噬抑制剂3-甲基腺嘌呤增强鼻咽癌细胞对放射和化学治疗的敏感性[J]. 中南大学学报:医学版, 2016, 41(1): 9-18. |
| [8] |
Katherine TL, Juan-King T, Alfred KL, et al. MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: A critical review[J]. Crit Rev Oncol Hematol, 2016, 103: 1-9. DOI:10.1016/j.critrevonc.2016.04.006 |
| [9] |
Ren SL, Gaykalova D, Wang J, et al. Discovery and development of differentially methylated regions in human papillomavirus-related oropharyngeal squamous cell carcinoma[J]. Int J Cancer, 2018, 143(10): 2425-36. DOI:10.1002/ijc.31778 |
| [10] |
齐力, 耿彬, 宋丽华. miR-129抑制鼻咽癌细胞系CNE2增殖[J]. 基础医学与临床, 2019, 39(8): 1178-82. DOI:10.3969/j.issn.1001-6325.2019.08.020 |
| [11] |
Huang HJ, Jie T, Lei Z, et al. miR-874 regulates multiple-drug resistance in gastric cancer by targeting ATG16L1[J]. Int J Oncol, 2018, 53(6): 2769-79. |
| [12] |
Wei DM, Jiang MT, Lin P, et al. Potential ceRNA networks involved in autophagy suppression of pancreatic cancer caused by chloroquine diphosphate: A study based on differentially-expressed circRNAs, lncRNAs, miRNAs and mRNAs[J]. Int J Oncol, 2019, 54(2): 600-26. |
| [13] |
Huang HJ, Jie T, Lei Z, et al. miR-874 regulates multiple-drug resistance in gastric cancer by targeting ATG16L1[J]. Int J Oncol, 2018, 53(6): 2769-79. |
| [14] |
Peng-Hsu C, Ann-Jeng L, Kuo-Hao H, et al. microRNA-199a/b-5p enhance imatinib efficacy via repressing WNT2 signaling-mediated protective autophagy in imatinib-resistant chronic myeloid leukemia cells[J]. Chem Biol Interact, 2018, 291(8): 144-51. |
| [15] |
Satishkumar VS, Dakhole AN, Deogharkar A, et al. Restoration of miR-30a expression inhibits growth, tumorigenicity of medulloblastoma cells accompanied by autophagy inhibition[J]. Biochem Biophys Res Commun, 2017, 491(4): 946-52. DOI:10.1016/j.bbrc.2017.07.140 |
| [16] |
Li M, Choy E, Raphael EP, et al. Autophagy as a potential target for sarcoma treatment[J]. Biochim Biophys Acta Rev Cancer, 2017, 1868(1): 40-50. DOI:10.1016/j.bbcan.2017.02.004 |
| [17] |
Zhao H, Li Z, Chen ZB, et al. Stress management by autophagy: Implications for chemoresistance[J]. Int J Cancer, 2016, 139(1): 23-32. DOI:10.1002/ijc.29990 |
| [18] |
Ou CS, Liu HX, Ding ZH, et al. Chloroquine promotes gefitinib induced apoptosis by inhibiting protective autophagy in cutaneous squamous cell carcinoma[J]. Mol Med Rep, 2019, 20(6): 4855-66. |
| [19] |
Aga T, Endo K, Tsuji A, et al. Inhibition of autophagy by chloroquine makes chemotherapy in nasopharyngeal carcinoma more efficient[J]. Auris Nasus Larynx, 2019, 46(3): 443-50. DOI:10.1016/j.anl.2018.10.013 |
| [20] |
Liang ZG, Lin GX, Yu BB, et al. The role of autophagy in the radiosensitivity of the radioresistant human nasopharyngeal carcinoma cell line CNE-2R[J]. Cancer Manag Res, 2018, 25(10): 4125-34. |
| [21] |
Makowska A, Eble M, Prescher K, et al. Chloroquine sensitizes nasopharyngeal carcinoma cells but not nasoepithelial cells to irradiation by blocking autophagy[J]. PLoS One, 2016, 11(11): e0166766. DOI:10.1371/journal.pone.0166766 |
| [22] |
Li BX, Cao XF, Weng CY, et al. The regulatory effects of autophagy to the CNE2 cells radio-sensitization[J]. Cell Mol Biol, 2016, 62(13): 12. DOI:10.14715/cmb/2016.62.13.3 |
| [23] |
Yang XY, Yin HB, Zhang YZ, et al. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1α activation[J]. Int J Oncol, 2018, 53(1): 215-24. |
| [24] |
Piya S, Andreeff M, Borthakur G. Targeting autophagy to overcome chemoresistance in acute myleogenous leukemia[J]. Autophagy, 2017, 13(1): 214-5. DOI:10.1080/15548627.2016.1245263 |
| [25] |
Xiang CC, Han SC, Nao J, et al. MicroRNAs dysregulation and metabolism in multiple system atrophy[J]. Front Neurosci, 2019, 13(3): 1103. |
| [26] |
Eapen MS, Mcalinden KD, Myers S, et al. microRNAs are key regulators in chronic lung disease: exploring the vital Link between disease progression and lung cancer[J]. J Clin Med, 2019, 8(11): 1986. DOI:10.3390/jcm8111986 |
| [27] |
Balzano F, Campesi I, Cruciani S, et al. Epigenetics, stem cells, and autophagy: exploring a path involving miRNA[J]. Int J Mol Sci, 2019, 20(20): 5091. DOI:10.3390/ijms20205091 |
| [28] |
Qian L, Yang L, Lv WL, et al. UCA1 promotes cell proliferation and invasion and inhibits apoptosis through regulation of the miR129 SOX4 pathway in renal cell carcinoma[J]. Onco Targets Ther, 2018, 28(11): 2475-87. |
| [29] |
Wang XQ, Jing L, Xiao X, et al. miR-129 inhibits tumor growth and potentiates chemosensitivity of neuroblastoma by targeting MYO10[J]. Biomed Pharmacother, 2018, 103(11): 1312-8. |
| [30] |
Song X, Yi XM, Zhang ZY, et al. miR-129 predicts prognosis and inhibits cell growth in human prostate carcinoma[J]. Mol Med Rep, 2016, 14(6): 5025-32. DOI:10.3892/mmr.2016.5859 |
| [31] |
Zhao KC, Zhang YK, Liang K, et al. Methylation of microRNA-129- 5P modulates nucleus pulposus cell autophagy by targeting Beclin-1 in intervertebral disc degeneration[J]. Oncotarget, 2017, 8(49): 86264-76. |
| [32] |
Ying S, Gong WH, Lu L, et al. Upregulation of miR-129-5p increases the sensitivity to Taxol through inhibiting HMGB1-mediated cell autophagy in breast cancer MCF-7 cells[J]. Braz J Med Biol Res, 2019, 52(11): e8657. DOI:10.1590/1414-431x20198657 |
| [33] |
张浩轩, 肖明. 氯喹对鼻咽癌细胞凋亡的影响[J]. 蚌埠医学院学报期刊, 2013, 38(11): 1396-401. |
| [34] |
Kouhkan F, Naser M, Soufi-Zomorrod M, et al. MicroRNA-129- 1 acts as tumour suppressor and induces cell cycle arrest of GBM cancer cells through targeting IGF2BP3 and MAPK1[J]. J Med Genet, 2016, 53(1): 24-33. DOI:10.1136/jmedgenet-2015-103225 |
| [35] |
Gao P, Hao F, Dong X, et al. The role of autophagy and Beclin-1 in radiotherapy-induced apoptosis in thyroid carcinoma cells[J]. Int J Clin Exp Pathol, 2019, 12(3): 885-92. |
| [36] |
Li ZZ, Lu JY, Guang Z, et al. MiR-129-5p inhibits liver cancer growth by targeting Calcium calmodulin-dependent protein kinase IV (CAMK4)[J]. Cell Death Dis, 2019, 10(11): 789. DOI:10.1038/s41419-019-1923-4 |
| [37] |
Qiu ZS, Wang XC, Shi YP, et al. miR-129-5p suppresses proliferation, migration, and induces apoptosis in pancreatic cancer cells by targeting PBX3[J]. Acta Biochim Biophys Sin (Shanghai), 2019, 51(10): 997-1007. DOI:10.1093/abbs/gmz096 |
 2020, Vol. 40
2020, Vol. 40

