2. 中国科学院大学,北京 100049;
3. 中国科学院上海高等研究院//同步辐射科学中心,上海 201210;
4. 宁波大学物理科学与技术学院,浙江 宁波 315211
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China;
4. School of Physics Science and Technology, Ningbo University, Ningbo 315211, China
细胞外囊泡(EVs)包括外泌体和微囊泡,作为新的一种细胞间通讯方式,是近些年生理、病理、纳米科学等研究的“新星”[1]。细胞外囊泡是由细胞分泌的高度异质性的囊泡状小体,其内容物包含蛋白质、mRNA/ miRNA、DNA、脂质等[2-4]。研究表明,几乎所有的细胞都会释放细胞外囊泡到细胞外部,发挥局部、远程信息交流的作用。细胞外囊泡广泛分布在机体组织液当中,包括血液、淋巴液、脑脊液、尿液、唾液、乳汁、腹水等[5-8]。在不同的生理病理情况下,细胞外囊泡所含内容物有所不同,发挥不同功能,因此细胞外囊泡被认为是丰富、稳定的生物标志物[9]。除此之外,细胞外囊泡具有良好的生物相容性,来源广泛,可以通过血脑屏障等各种生物屏障,被认为是理想的药物载体,被广泛应用于纳米药物递送工程[10-12]。细胞显微成像是研究细胞外囊泡的生物学特征和功能的重要技术基础。但是,由于细胞外囊泡纳米尺寸和其多层次异质性的特征,缺乏合适的成像技术,阻碍了人们对细胞外囊泡生物学特性进一步的研究。
本文概述了细胞外囊泡起源、分类,重点总结了在细胞外囊泡起源、分离、动态摄取和释放过程中的成像方法,讨论了不同方法的优势和不足,为细胞外囊泡相关研究提供了参考。
1 细胞外囊泡简介及成像方法 1.1 细胞外囊泡简介根据膜起源不同,细胞外囊泡被分为3大类:外泌体、微囊泡和凋亡小体[13]。本文着重介绍外泌体和微囊泡(图 1)。外泌体尺寸较小,为30~150 nm,其形成过程相对复杂,先由细胞膜向内凹陷,形成早期胞内体,早期胞内体分选包裹胞内物质后进一步成熟,形成包含腔内体的晚期胞内体,也被称为多泡小体。多泡小体可通过溶酶体降解也可与细胞质膜融合释放形成外泌体[14-15]。目前,外泌体起源过程中胞内各种细胞器与胞内体的交流、物质互换、分选机制等研究尚不清楚,这也导致了相同细胞来源的外泌体内容物的差异[16-17]。微囊泡是由细胞质膜直接出芽产生,尺寸为100~1000 nm[18]。研究发现,在不同的细胞中,除了微囊泡,还存在许多其他由质膜延伸形成的不同形态的质膜来源的细胞外囊泡,如迁移小体,微绒毛来源的囊泡等[19-21]。不同亚型的细胞外囊泡具有类似的生物物理学特征如尺寸、密度、内容物、膜成分等,一旦脱离其起源细胞,现有方法都不能有效地分离不同亚型的细胞外囊泡[22]。严格意义上,不同分离方法得到的只是不同性质的细胞外囊泡亚群。
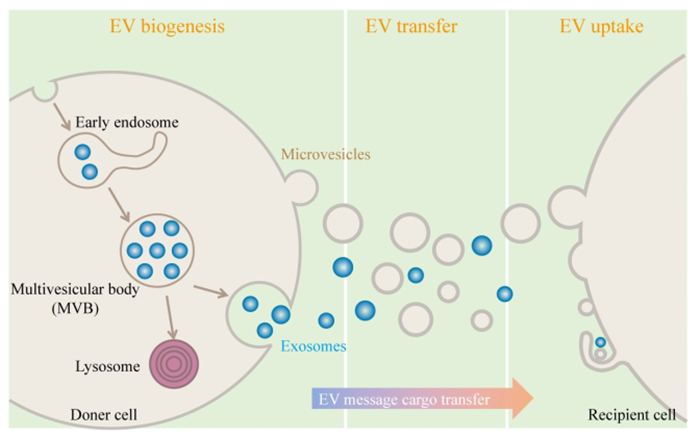
|
图 1 细胞外囊泡产生、传递及靶细胞摄取 Fig.1 Schematic representation of the EV biogenesis, transfer and uptake by target cells. According to the origin of the membrane, EVs are divided into microvesicles budding directly from the plasma membrane (PM) and exosomes that are initially formed intracellularly within the so-called multivesicular bodies (MVBs). EVs or a specific EV subtype taking message cargoes is internalized by recipient cells through the direct or indirect pathway |
细胞外囊泡的不同亚群,都包含蛋白质、脂质、核酸等生物信息物质,可以靶向传递到受体细胞,影响受体细胞表型[23-24]。细胞外囊泡摄取的第一步是与受体细胞细胞膜识别结合。然后,细胞外囊泡通过直接与细胞膜融合将内容物递送到受体细胞,或通过胞吞、胞饮等方式被受体细胞内化降解[25]。不同亚型细胞外囊泡摄取融合的过程和机制尚不明确。细胞外囊泡的起源、传递、摄取等过程机制仍有许多未知,先进可靠的成像方法是研究的基础,对探究细胞外囊泡生物学过程至关重要。
1.2 细胞外囊泡原位成像方法不同亚型细胞外囊泡根据其生物发生过程不同,在起源阶段可以被很好区分。
1.2.1 质膜起源细胞外囊泡的显微成像细胞质膜起源的微囊泡主要分布在细胞表面。扫描电子显微镜(SEM)是目前应用最广泛的细胞表面形貌成像工具。SEM主要利用电子束扫描样品表面,通过电子与样品中的原子相互作用产生反馈信号,提供样品表面元素构成以及高分辨率的三维形貌信息。SEM虽然能够提供微囊泡的高分辨三维信息,但由于样品制备过程必须经过固定、脱水等操作,一定程度影响形貌,无法反应活细胞状态下细胞外囊泡形态以及生物组分等特征(图 2A)[26]。微囊泡的生物发生包括分子物质运输到质膜,骨架蛋白参与的膜脂质重排,以及表面的收缩[27]。免疫荧光方法可以通过标记骨架蛋白如F-actin,膜联蛋白Annexin A1等反映局域膜形貌(图 2B、C),各种亲脂性探针如FM1-43(图 2D)也可被用来示踪微囊泡[28-30]。需要注意的是,目前尚未发现特异性的蛋白标志物,可以标记所有膜起源的细胞外囊泡,因此需要结合多种成像方法原位研究不同膜起源囊泡的调控和功能[31-32]。
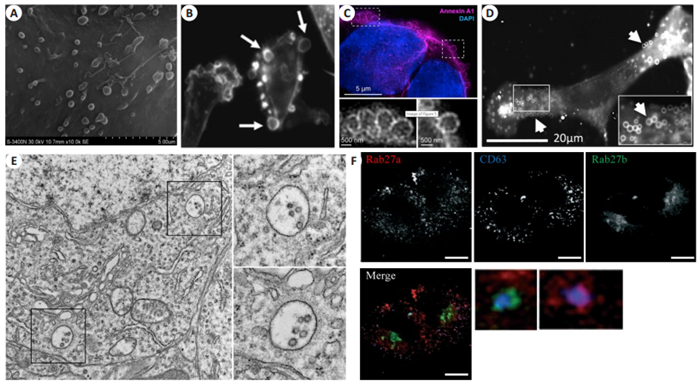
|
图 2 原位表征微囊泡和外泌体生成 Fig.2 Characterization of microvesicles and exosomes origin in situ. A: Scanning electron microscopy (SEM) showing threedimensional surface topology of microvesicles in mouse astrocytes[26]; B: MDA-MB-231 cells analyzed by immunofluorescent microscopy using rhodamine-conjugated phalloidin to visualize F-actin (arrows indicate the large microvesicles[28]); C: DKO-1 cells stained with Annexin A1 and DAPI to visualize the microvesicles[29]; D: Mouse embryonic fibroblast cells stained with the fluorescent membrane dye FM1-43FX to visualize the microvesicles on the cell surface[30]; E: Transmission electron microscopic (TEM) images of the hippocampus of a wild-type mice (Scale bar: 1 μm). Enlarged insets highlight MVBs containing typical intraluminal vesicles[36]; F: Confocal fluorescence microscopy of HeLa cells co-transfected with mCherry-Rab27a- and GFP-Rab27bencoding plasmids and stained subsequently with anti-CD63 (Scale bar=10 μm[37]) |
与细胞表面质膜来源的囊泡不同,外泌体起源于胞内多泡小体外排,且尺寸更小,原位成像更加困难。透射电子显微镜(TEM)通过电子束透过超薄样品反映样品信息,具有更高的分辨率(< 1 nm),是分析细胞内部超微结构的有力工具。因TEM特有的优势,使其成为目前研究外泌体在胞内形成、发展成熟、内容物分选最直接的成像工具[33-35]。结合免疫胶体金技术,TEM可以在形貌表征同时进一步获得分子生物学特征。图 2E展示的是小鼠海马内细胞外泌体在分泌出细胞前多泡小体的典型TEM图像,呈现单层膜嵌套结构,腔体内为不同尺寸腔内体[36]。然而,受限于仪器,样品制备复杂,视野有限,检测通量低等不足,TEM并不能满足外泌体起源的研究。因此,基于不同结构生物标志物免疫荧光的激光共聚焦显微镜被广泛用于外泌体生物过程研究(图 2F)[37]。但是,受限于光学显微镜衍射极限[38],激光共聚焦显微镜下纳米尺度的胞内体结构只是呈现荧光斑点,并不能反映更加详细的内部信息。此外,胞内外泌体生物发生研究过程中,所选胞内体等细胞器蛋白标志物并不完全具备“特异性”,如常用来表示多泡小体的蛋白标志物CD63在溶酶体中同样高表达[39],因此基于免疫荧光的激光共聚焦显微镜技术并不能准确反应其完整过程。
1.3 分离后细胞外囊泡成像方法目前,细胞外囊泡的分离方法主要包括超速离心、蔗糖密度梯度离心、共沉淀、尺寸排阻色谱法、场流分离等[40-44]。然而,任何方法都无法完全分离不同亚型的细胞外囊泡。例如,最普遍应用的通过100 000 g超速离心获取的沉淀物被称为“外泌体”,实际上包含各种混合的小尺寸细胞外囊泡,其含有的分子标志物互不相同,具有不同的亚细胞起源,严格定义应为小细胞外囊泡[22, 45]。
因分离后的细胞外囊泡尺寸为纳米级,传统光学显微镜分辨率并不能满足其成像需求。目前,具有纳米级分辨能力的TEM和原子力显微镜(AFM)是细胞外囊泡高分辨成像的最有力工具。超速离心获得的小细胞外囊泡在TEM下呈现经典的杯状、茶托样结构,结合免疫胶体金技术,可以对单个细胞外囊泡分子生物学特征进行研究(图 3A)[46]。然而,常规TEM制样需要经过干燥脱水等操作,并不能完整呈现细胞外囊泡天然形貌特征,不同的制样方法也会导致细胞外囊泡形貌差异[47]。同时,常规TEM并不能展现细胞外囊泡内部结构特征。冷冻电子显微镜可以在超低温下(-100 ℃)分析冷冻样品结构,避免了干燥、化学固定剂等对细胞外囊泡的影响[48]。采用冷冻电子显微镜方法不但获得了经典的细胞外囊泡呈现圆形结构(图 3B),也发现了一些异形的大囊泡嵌套小囊泡结构(图 3C)[49-51]。目前尚不清楚这些嵌套样囊泡结构是由细胞天然分泌,还是在细胞外囊泡分离存储过程中相互融合形成。与电子显微镜不同,AFM通过探针与样品接触的力反馈信号成像,既可以对样品形貌高分辨表征,又可以获得样品粘度、软硬等物理性质,是另一种细胞外囊泡分析的有利工具[52-54]。典型的细胞外囊泡AFM图像呈现和TEM类似的杯状结构(图 3D)[55]。这些基于分离后单个囊泡的成像方法,从大小、结构、内容物、粘度、硬度等方面展示了细胞外囊泡高度异质性的特点。
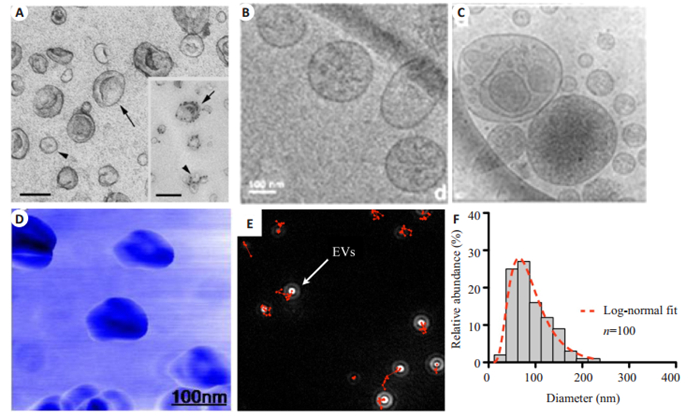
|
图 3 分离后细胞外囊泡的成像 Fig.3 Characterization of isolated EVs. A: Transmission electron microscopic (TEM) observation of EVs purified from mouse dendritic cells. Insert: Immunogold labeling of MHC class Ⅱ molecules (Scale bar: 100 nm[46]); B: Cryo-electron microscopic (Cryo-EM) observation of classical EVs[49]; C: Cryo-EM image of small EVs inserted inside large vesicles[49]; D: Tapping mode topographic AFM image of EVs[55]. EVs from primary human glioblastoma cell line GBM20/3 are counted by nanoparticle tracking analysis (NTA). The onscreen images show a correctly focused EV (E) and the concentration and size distribution of EVs(F)[58] |
目前,纳米粒子追踪分析(NTA)是最常用的细胞外囊泡定量方法[56-57]。它是一种光学粒子跟踪方法,用于检测溶液中粒子的浓度及大小分布。细胞外囊泡在NTA视野下呈现散射光斑(图 3E),通过追踪单个囊泡布朗运动轨迹,根据Strokes-Einstein方程可以计算出溶液下样品粒径及浓度分布(图 3F)[58]。值得注意的是,NTA是非特异性检测,并不能将细胞外囊泡同溶液中纳米气泡、蛋白聚集物、不溶性颗粒等杂质区分开[59],且检测准确度受限于人为设定的检测参数[60]。
1.4 细胞外囊泡动态成像方法受限于细胞外囊泡纳米尺寸和多层次异质性的特征,其形成、释放、循环、摄取、内容物递送等动力学过程一直是该领域未解决的重要问题[61]。缺乏完善的细胞外囊泡标记技术和高时空分辨率的成像方法一直是限制其发展的重要原因。目前,细胞外囊泡标记技术主要分为基于亲脂性荧光探针的标记技术和基于荧光蛋白标签的标记技术。成像方法以激光共聚焦显微镜和小动物成像仪为主。
基于亲脂性荧光探针的标记技术主要用于细胞外囊泡分离后标记示踪,具有操作简便、标记效率高、信号强、寿命长等特点。这种标记策略主要用于细胞外囊泡功能研究中的细胞摄取、纳米载药工程中体内分布成像[62-63]。常用的亲脂性荧光探针主要包括,PKH家族如PKH26、PKH67等,Dil、Dio、DiR等,以及其他间接偶联发光基团的方法(图 4A)[64-66]。图 4B是细胞外囊泡偶联发光基团或标记PKH26、Dil后所做的细胞摄取实验[67],图 4C为PKH26标记细胞外囊泡后体内注射后在各个器官的分布情况[68]。值得注意的是,任何基于荧光探针的标记策略不可避免的都会因染料聚集、人为外源性污染物引入、细胞碎片等杂质造成非特异性标记信号,引起实验结果的偏差[69]。
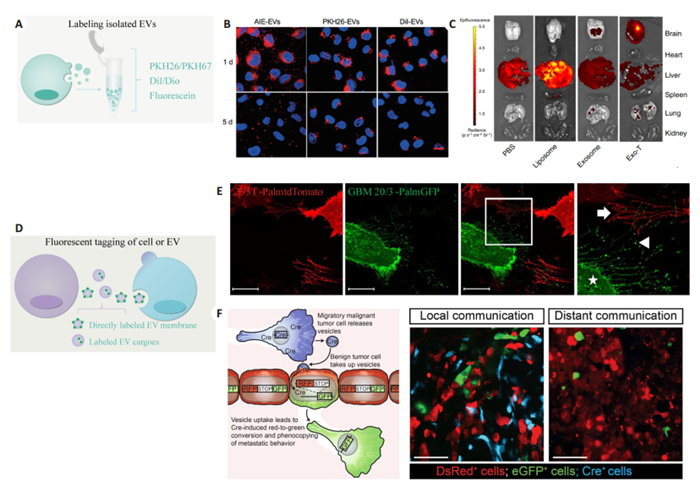
|
图 4 细胞外囊泡动态形成、释放、摄取过程成像 Fig.4 Visualization of EV formation, release and uptake. A: Labelling isolated EVs based on lipophilic dye or fluorescein; B: Internalization images of EVs labeled with DPA-SCP (an aggregation-induced emission luminogen), PKH26, or DiI for 1 and 5 days[67]; C: Tissue distribution analyses shown that EVs labeled with PKH26[68]; D: Strategy for EV labelling via fluorescent tagging of membrane or EV cargo; E: Stably transduced 293T-PalmtdTomato and primary GBM-PalmGFP cells were co-cultured and imaged with confocal microscopy for cellular interactions and EV exchanged between the cells[70]; F: Cartoon showing the use of Cre-LoxP system to report the transfer of Cre recombinase (Cre) activity. A red-to-green color switch was induced in reporter+ cells (right) upon the transfer of Cre activity derived from CFP+ Cre+ cells[73] |
另一种标记策略是通过荧光蛋白标签标记细胞外囊泡,这种策略主要用于原位研究细胞外囊泡起源,释放、内容物递送等生物学过程。主要分为直接标记细胞外囊泡膜和标记细胞外囊泡内容物(图 4D)。例如,研究人员将融合荧光蛋白EGFP或tdTomato插入棕榈酰化序列以此标记细胞质膜,通过荧光信号记录细胞外囊泡的释放、交换等生物过程(图 4E)[70]。比如,利用多泡小体不同成熟阶段腔内pH不同,通过融合pH敏感的荧光蛋白连接到腔内膜上跨膜蛋白CD63,在TIRF显微镜下实时观测单细胞多泡小体与质膜融合释放,以此定量单细胞外泌体释放[71-72]。相比于直接膜标记,融合荧光蛋白还可以特定标记细胞外囊泡内容物,如特定RNA,以此追踪细胞外囊携带内容物在细胞间的传递[70]。基于融合荧光蛋白的优势是可以在模式生物中进行不同疾病模型研究。例如,研究人员对细胞外囊泡供体细胞和受体细胞分别标记不同颜色,细胞外囊泡通过传递Cre mRNA进入受体细胞利用Cre-LoxP系统将受体细胞转变为绿色,体内证明了细胞外囊泡介导的肿瘤细胞原位和远端侵袭行为(图 4F)[73]。然而,同其他基于荧光蛋白标签标记的在体研究一样,这项研究中阳性细胞较少,这是因为荧光蛋白标记效率较低,都只能标记特定物质,由于细胞外囊泡高度异质性,其他未被标记的细胞外囊泡会被人为“忽视”。同样,由于荧光蛋白标记效率低,半衰期短的特点,后期成像尤其是在体研究变得很困难[69]。在体研究细胞外囊泡动态学过程中模式动物的选择同样重要,斑马鱼因为其体积小组织层薄等特点,可能是在体可视化研究细胞外囊泡的很好选择[74-75]。
除了传统荧光显微镜成像外,研究人员还开发了其他成像技术,如基于光学显微镜的非线性成像系统、光代谢成像等,用无标记成像探究细胞外囊泡在活细胞或组织中的动态过程[76-77]。其他用于动物中细胞外囊泡分布成像的单光子发射计算机断层成像术、正电子发射断层成像术和磁共振成像等,侧重于分析检测,并不能提供单个囊泡形态特征,在这里不详尽描述[78-79]。
2 总结和展望细胞外囊泡作为新的一种细胞间信息传递媒介,在不同的生理、病理情况中发挥着重要作用。尽管人们对这一新兴领域的研究兴趣逐年提高,但对细胞外囊泡生物发生、内容物分选、亚型分析、体液传递、靶细胞摄取、内容物递送等基本情况认知十分有限。缺乏高时空分辨、特异性的成像技术是限制细胞外囊泡精准研究的重要因素。目前,细胞外囊泡动态示踪成像主要是基于传统的光学显微镜,受限于光学显微镜的分辨率,所有细胞外囊泡在视野下都是“荧光点”。然而,细胞外囊泡大小是高度异质性的,为30~1000 nm,作为研究重点的外泌体尺寸更是小于150 nm,低于光学显微镜分辨率。因此,小尺寸的细胞外囊泡都无法“成像”展示,尤其是在活细胞水平对单囊泡行为的成像分析。
细胞外囊泡领域的发展,需要结合不同学科优势,开发新的成像分析技术,进行单细胞、单囊泡水平的动态研究,由此加深对细胞外囊泡起源、动态过程以及生物学功能的理解。
| [1] |
El Andaloussi S, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities[J]. Nat Rev Drug Discov, 2013, 12(5): 347-57. DOI:10.1038/nrd3978 |
| [2] |
Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth[J]. Nature, 2015, 527(7576): 100-4. DOI:10.1038/nature15376 |
| [3] |
Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nat Cell Biol, 2007, 9(6): 654-9. |
| [4] |
Simpson RJ, Lim JW, Moritz RL, et al. Exosomes: proteomic insights and diagnostic potential[J]. Expert Rev Proteomics, 2009, 6(3): 267-83. DOI:10.1586/epr.09.17 |
| [5] |
Kalra H, Adda CG, Liem M, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma[J]. Proteomics, 2013, 13(22): 3354-64. DOI:10.1002/pmic.201300282 |
| [6] |
Saunderson SC, Dunn AC, Crocker PR, et al. CD169 mediates the capture of exosomes in spleen and lymph node[J]. Blood, 2014, 123(2): 208-16. DOI:10.1182/blood-2013-03-489732 |
| [7] |
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine[J]. Proc Natl Acad Sci USA, 2004, 101(36): 13368-73. DOI:10.1073/pnas.0403453101 |
| [8] |
Hock A, Miyake H, Li B, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth[J]. J Pediatr Surg, 2017, 52(5): 755-9. DOI:10.1016/j.jpedsurg.2017.01.032 |
| [9] |
Nawaz M, Camussi G, Valadi H, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers[J]. Nat Rev Urol, 2014, 11(12): 688-701. DOI:10.1038/nrurol.2014.301 |
| [10] |
Morad G, Carman CV, Hagedorn EJ, et al. Tumor-Derived extracellular vesicles breach the intact blood-brain barrier via transcytosis[J]. ACS Nano, 2019, 13(12): 13853-65. DOI:10.1021/acsnano.9b04397 |
| [11] |
Vader P, Mol EA, Pasterkamp G, et al. Extracellular vesicles for drug delivery[J]. Adv Drug Deliv Rev, 2016, 106(Pt A): 148-56. |
| [12] |
Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles[J]. Biomaterials, 2016, 105(2): 195-205. |
| [13] |
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends[J]. J Cell Biol, 2013, 200(4): 373-83. DOI:10.1083/jcb.201211138 |
| [14] |
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes[J]. J Cell Biol, 1983, 97(2): 329-39. DOI:10.1083/jcb.97.2.329 |
| [15] |
Pan BT, Teng K, Wu C, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes[J]. J Cell Biol, 1985, 101(3): 942-8. DOI:10.1083/jcb.101.3.942 |
| [16] |
Lauwers E, Wang YC, Gallardo R, et al. Hsp90 mediates membrane deformation and exosome release[J]. Mol Cell, 2018, 71(5): 689-702. DOI:10.1016/j.molcel.2018.07.016 |
| [17] |
Bobrie A, Colombo M, Krumeich S, et al. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation[J]. J Extracell Vesicles, 2012, 1(1): 18397-108. DOI:10.3402/jev.v1i0.18397 |
| [18] |
Muralidharan-Chari V, Clancy JW, Sedgwick A, et al. Microvesicles: mediators of extracellular communication during cancer progression[J]. J Cell Sci, 2010, 123(Pt 10): 1603-11. |
| [19] |
Ma L, Li Y, Peng J, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration[J]. Cell Res, 2015, 25(1): 24-38. DOI:10.1038/cr.2014.135 |
| [20] |
Kim HR, Mun Y, Lee KS, et al. T cell microvilli constitute immunological synaptosomes that carry messages to antigenpresenting cells[J]. Nat Commun, 2018, 9(1): 3630-41. DOI:10.1038/s41467-018-06090-8 |
| [21] |
Atkin-Smith GK, Tixeira R, Paone S, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-astring membrane structure[J]. Nat Commun, 2015, 6(11): 7439-50. |
| [22] |
Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles[J]. Philos Trans R Soc Lond B Biol Sci, 2018, 373(1737): 20160479-90. DOI:10.1098/rstb.2016.0479 |
| [23] |
Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery[J]. Leukemia, 2006, 20(5): 847-56. DOI:10.1038/sj.leu.2404132 |
| [24] |
Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers[J]. Nat Cell Biol, 2008, 10(12): 1470-6. |
| [25] |
van Niel G, D'angelo G, raposo G. shedding light on the cell biology of extracellular vesicles[J]. Nat Rev Mol Cell Biol, 2018, 19(4): 213-28. DOI:10.1038/nrm.2017.125 |
| [26] |
Wang K, Ye L, Lu H, et al. TNF-α promotes extracellular vesicle release in mouse astrocytes through glutaminase[J]. J Neuroinflammation, 2017, 14(1): 87-98. DOI:10.1186/s12974-017-0853-2 |
| [27] |
Tricarico C, Clancy J, D'souza-Schorey C. Biology and biogenesis of shed microvesicles[J]. Small GTPases, 2017, 8(4): 220-32. DOI:10.1080/21541248.2016.1215283 |
| [28] |
Antonyak MA, Li B, Boroughs LK, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells[J]. Proc Natl Acad Sci USA, 2011, 108(12): 4852-7. DOI:10.1073/pnas.1017667108 |
| [29] |
Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition[J]. Cell, 2019, 177(2): 428-45. DOI:10.1016/j.cell.2019.02.029 |
| [30] |
Kreger BT, Dougherty AL, Greene KS, et al. Microvesicle cargo and function changes upon induction of cellular transformation[J]. J Biol Chem, 2016, 291(38): 19774-85. DOI:10.1074/jbc.M116.725705 |
| [31] |
He C, Hu X, Weston TA, et al. Macrophages release plasma membrane-derived particles rich in accessible cholesterol[J]. Proc Natl Acad Sci USA, 2018, 115(36): E8499-508. DOI:10.1073/pnas.1810724115 |
| [32] |
Wu D, Xu Y, Ding T, et al. Pairing of integrins with ECM proteins determines migrasome formation[J]. Cell Res, 2017, 27(11): 1397-400. DOI:10.1038/cr.2017.108 |
| [33] |
Wang K, Wei Y, Liu W, et al. Mechanical stress-dependent autophagy component release via extracellular nanovesicles in tumor cells[J]. ACS Nano, 2019, 13(4): 4589-602. DOI:10.1021/acsnano.9b00587 |
| [34] |
Minakaki G, Menges S, Kittel A, et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype[J]. Autophagy, 2018, 14(1): 98-119. DOI:10.1080/15548627.2017.1395992 |
| [35] |
Villarroya-Beltri C, Baixauli F, Mittelbrunn M, et al. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins[J]. Nat Commun, 2016, 7(1): 13588-97. |
| [36] |
Song L, Tang S, Han X, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a[J]. Nat Commun, 2019, 10(1): 1639-49. DOI:10.1038/s41467-019-09720-x |
| [37] |
Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway[J]. Nat Cell Biol, 2010, 12(1): 19-30. DOI:10.1038/ncb2000 |
| [38] |
van der Pol E, Hoekstra AG, Sturk A, et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes[J]. J Thromb Haemost, 2010, 8(12): 2596-607. DOI:10.1111/j.1538-7836.2010.04074.x |
| [39] |
Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63[J]. Exp Cell Res, 2009, 315(9): 1584-92. DOI:10.1016/j.yexcr.2008.09.020 |
| [40] |
Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response[J]. Nature, 2018, 560(7718): 382-93. DOI:10.1038/s41586-018-0392-8 |
| [41] |
Leidal AM, Huang HH, Marsh T, et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles[J]. Nat Cell Biol, 2020, 22(2): 187-99. DOI:10.1038/s41556-019-0450-y |
| [42] |
Alvarez ML, Khosroheidari M, Ravi RK, et al. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers[J]. Kidney Int, 2012, 82(9): 1024-32. DOI:10.1038/ki.2012.256 |
| [43] |
Kreimer S, Ivanov AR. Rapid isolation of extracellular vesicles from blood plasma with size-exclusion chromatography followed by mass spectrometry-based proteomic profiling[J]. Methods Mol Biol, 2017, 1660(3): 295-302. |
| [44] |
Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization[J]. Nat Protoc, 2019, 14(4): 1027-53. DOI:10.1038/s41596-019-0126-x |
| [45] |
Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes[J]. Proc Natl Acad Sci USA, 2016, 113(8): E968-77. DOI:10.1073/pnas.1521230113 |
| [46] |
Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids[J]. Curr Protoc Cell Biol, 2006, 19(3): 322-34. |
| [47] |
Rikkert LG, Nieuwland R, Terstappen LWMM, et al. Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent[J]. J Extracell Vesicles, 2019, 8(1): 1555419-31. DOI:10.1080/20013078.2018.1555419 |
| [48] |
Yuana Y, Koning RI, Kuil ME, et al. Cryo-electron microscopy of extracellular vesicles in fresh plasma[J]. J Extracell Vesicles, 2013, 2(1): 21494-105. DOI:10.3402/jev.v2i0.21494 |
| [49] |
Tatischeff I, Larquet E, Falcón-Pérez JM, et al. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and raman tweezers microspectroscopy[J]. J Extracell Vesicles, 2012, 1(1): 19179-90. DOI:10.3402/jev.v1i0.19179 |
| [50] |
Smith ZJ, Lee C, Rojalin T, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content[J]. J Extracell Vesicles, 2015, 4(1): 28533-45. DOI:10.3402/jev.v4.28533 |
| [51] |
Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, et al. Size-Exclusion chromatography-based isolation minimally alters extracellular vesicles' characteristics compared to precipitating agents[J]. Sci Rep, 2016, 6(11): 33641-52. |
| [52] |
Kim SY, Khanal D, Kalionis B, et al. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonanceenhanced atomic force microscopy infrared spectroscopy[J]. Nat Protoc, 2019, 14(2): 576-93. DOI:10.1038/s41596-018-0109-3 |
| [53] |
Sorkin R, Huisjes R, Bošković F, et al. Nanomechanics of extracellular vesicles reveals vesiculation pathways[J]. Small, 2018, 14(39): e1801650-61. DOI:10.1002/smll.201801650 |
| [54] |
Vorselen D, van Dommelen SM, Sorkin R, et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis[J]. Nat Commun, 2018, 9(1): 4960-71. |
| [55] |
Sharma S, Rasool HI, Palanisamy V, et al. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy[J]. ACS Nano, 2010, 4(4): 1921-6. DOI:10.1021/nn901824n |
| [56] |
Ma Y, Wang K, Pan J, et al. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways[J]. Neurobiol Dis, 2019, 124(2): 322-34. |
| [57] |
Tannetta D, Dragovic R, Alyahyaei Z, et al. Extracellular vesicles and reproduction-promotion of successful pregnancy[J]. Cell Mol Immunol, 2014, 11(6): 548-63. DOI:10.1038/cmi.2014.42 |
| [58] |
Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy[J]. Nat Med, 2012, 18(12): 1835-40. DOI:10.1038/nm.2994 |
| [59] |
Wang Y, Shen Z, Guo Z, et al. Effects of nanobubbles on peptide self-assembly[J]. Nanoscale, 2018, 10(42): 2007-12. |
| [60] |
Gardiner C, Ferreira YJ, Dragovic RA, et al. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis[J]. J Extracell Vesicles, 2013, 2(1): 19671-82. DOI:10.3402/jev.v2i0.19671 |
| [61] |
Mathieu M, Martin-Jaular L, Lavieu GA. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication[J]. Nat Cell Biol, 2019, 21(1): 9-17. DOI:10.1038/s41556-018-0250-9 |
| [62] |
Reshke R, Taylor JA, Savard A, et al. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone[J]. Nature Biomed Engineer, 2020, 4(1): 52-68. DOI:10.1038/s41551-019-0502-4 |
| [63] |
Tian T, Wang Y, Wang H, et al. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy[J]. J Cell Biochem, 2010, 111(2): 488-96. DOI:10.1002/jcb.22733 |
| [64] |
Pužar Dominkuš P, Stenovec M, Sitar S, et al. PKH26 labeling of extracellular vesicles: characterization and cellular internalization of contaminating PKH26 nanoparticles[J]. Biochim Biophys Acta-Biomembran, 2018, 1860(6): 1350-61. DOI:10.1016/j.bbamem.2018.03.013 |
| [65] |
Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cellderived exosomes as communicators in drug resistance in multiple myeloma cells[J]. Blood, 2014, 124(4): 555-66. DOI:10.1182/blood-2014-03-562439 |
| [66] |
Tadokoro H, Umezu T, Ohyashiki K, et al. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells[J]. J Biol Chem, 2013, 288(48): 34343-51. DOI:10.1074/jbc.M113.480822 |
| [67] |
Cao H, Yue Z, Gao H, et al. In vivo real-time imaging of extracellular vesicles in liver regeneration via aggregation-induced emission luminogens[J]. ACS Nano, 2019, 13(3): 3522-33. DOI:10.1021/acsnano.8b09776 |
| [68] |
Yang Z, Shi J, Xie J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation[J]. Nature Biomed Engineer, 2020, 4(1): 69-83. DOI:10.1038/s41551-019-0485-1 |
| [69] |
Russell AE, Sneider A, Witwer KW, et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop[J]. J Extracell Vesicles, 2019, 8(1): 1684862-73. DOI:10.1080/20013078.2019.1684862 |
| [70] |
Lai CP, Kim EY, Badr CE, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters[J]. Nat Commun, 2015, 6(12): 7029-40. |
| [71] |
Bebelman MP, Bun P, Huveneers S, et al. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells[J]. Nat Protoc, 2020, 15(1): 102-21. DOI:10.1038/s41596-019-0245-4 |
| [72] |
Verweij FJ, Bebelman MP, Jimenez CR, et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling[J]. J Cell Biol, 2018, 217(3): 1129-42. |
| [73] |
Zomer A, Maynard C, Verweij FJ, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior[J]. Cell, 2015, 161(5): 1046-57. DOI:10.1016/j.cell.2015.04.042 |
| [74] |
Hyenne V, Ghoroghi S, Collot M, et al. Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo[J]. Dev Cell, 2019, 48(4): 554-72. DOI:10.1016/j.devcel.2019.01.014 |
| [75] |
Verweij FJ, Hyenne V, Van Niel G, et al. Extracellular vesicles: catching the light in zebrafish[J]. Trends Cell Biol, 2019, 29(10): 770-6. DOI:10.1016/j.tcb.2019.07.007 |
| [76] |
Sun Y, You S, Tu H, et al. Intraoperative visualization of the tumor microenvironment and quantification of extracellular vesicles by label-free nonlinear imaging[J]. Science Advances, 2018, 4(12): eaau5603-14. DOI:10.1126/sciadv.aau5603 |
| [77] |
You S, Barkalifa R, Chaney EJ, et al. Label-free visualization and characterization of extracellular vesicles in breast cancer[J]. Proc Natl Acad Sci USA, 2019, 116(48): 24012-8. DOI:10.1073/pnas.1909243116 |
| [78] |
Varga Z, Gyurkó I, Pálóczi K, et al. Radiolabeling of extracellular vesicles with (99m)Tc for quantitative in vivo imaging studies[J]. Cancer Biother Radiopharm, 2016, 31(5): 168-73. DOI:10.1089/cbr.2016.2009 |
| [79] |
Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation[J]. Anal Biochem, 2014, 448(2): 41-9. |
 2020, Vol. 40
2020, Vol. 40

